INTRODUCTION
To date only a few Trypanosoma species have been isolated from Australian native fauna and little is known of their epidemiology and impact. The first record of Australian trypanosomes in mammals was made by T. L. Bandcroft in 1888 with the discovery of T. lewisi in rats (Mackerras, Reference Mackerras1959). Since then a number of other species have been identified including: T. pteropi from the flying fox (Pteropus sp.), T. hipposideri from the dusky horseshoe-bat (Hipposideros bicolor albanensis) (Mackerras, Reference Mackerras1959), T. binneyi from the platypus (Ornithorhynchus anatinus) (McMillan and Bancroft, Reference McMillan and Bancroft1973), T. thylacis from the northern brown bandicoot (Isoodon macrourus) (Mackerras, Reference Mackerras1959; Mackerras and Mackerras, Reference Mackerras and Mackerras1960), T. chelodina from the Brisbane River turtle (Emydura signata), the saw-shelled tortoise (Elseya latisternum) and the eastern snake-necked tortoise (Chelodina longicollis) (Jakes et al. Reference Jakes, O'Donoghue and Adlard2001). More recently novel Trypanosoma spp. have been identified from the southern brown bandicoot (Isoodon obesulus) (Bettiol et al. Reference Bettiol, Jakes, Le, Goldsmid and Hocking1998), the eastern grey kangaroo (Macropus giganteus), common wombat (Vombatus ursinus) (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999, Reference Noyes, Stevens, Teixeira, Phelan and Holz2000), swamp wallaby (Wallabia bicolor) (Hamilton et al. 2004), chuditch (Dasyrus geoffroiii) and brush-tailed bettongs (Bettongia penicillata), or woylies in Western Australia (Smith et al. Reference Smith, Clark, Averis, Lymbery, Wayne, Morris and Thompson2008).
Recently a Trypanosoma sp. was identified (McConnell, 2001 unpublished observations), along with 2 other haemoparasites (an intraerythrocytic piroplasm (Clark et al. Reference Clark, Adlard, Spratt and Clark2004) and microfilaria), by light microscopy within blood films of several individuals of Australia's most endangered marsupial, the Gilbert's potoroo (Potorous gilbertii), during haematological assessment of the health status of the animals. The Gilbert's potoroo was abundant in the 19th century in the vicinity of King George's Sound near Albany, Western Australia. By the early 1900s the species had declined dramatically and was thought to be extinct until its rediscovery in 1994 on the Mount Gardner headland at Two Peoples Bay Nature Reserve, near Albany (Sinclair et al. Reference Sinclair, Danks and Wayne1996). It is considered unlikely that more than 30 individuals currently exist in the wild (Courtenay and Friend, Reference Courtenay and Friend2004). Current threats to the existing population include fire, feral predators, habitat fragmentation by clearing for agriculture and plant dieback disease (Courtenay and Friend, Reference Courtenay and Friend2004). The discovery of trypanosomes in Gilbert's potoroo prompted investigation of native marsupial species sharing the same habitat, namely the quokka (Setonix brachyurus).
MATERIALS AND METHODS
Study site and sample collection
A total of 7 Gilbert's potoroos (Potorous gilbertii), and 3 quokkas (Setonix brachyurus), were trapped at Two Peoples Bay (34°58'S, 118°11'E) near Albany, Western Australia during the month of November. Albany has a Mediterranean-type climate with generally warm summers (December–February) and cool, wet winters (June–August). The potoroos were anaesthetized with isoflurane and approximately 200 μl of blood was collected by venepuncture of the lateral caudal vein. The blood was mixed with EDTA in commercial tubes (SARSTEDT, Australia) as an anticoagulant and stored at 4°C for a maximum of 10 days. Thin-blood smears were prepared using blood from the Gilbert's potoroos and quokkas.
Ectoparasites including 19 fleas and 13 ticks were collected from the Gilbert's potoroo and placed into 1·5 ml microcentrifuge tubes containing 70% ethanol. Samples were identified to the species level with the aid of the Dunnet and Mardon (Reference Dunnet and Mardon1974) key and Australian ticks (Roberts, Reference Roberts1970) key respectively.
Detection of trypanosomes in whole blood
Preparation of blood films
Wet-smear preparations of the buffy coat from each potoroo blood sample were made by cutting blood-filled heparinized capillary tubes after centrifugation at 12 000 g for 12 min approximately 1–2 mm below the red cell/buffy coat interface and expelling the buffy coat onto a glass microscope slide. A cover-slip was placed over the buffy coat and the preparation examined microscopically under 200× magnification for the presence of motile trypanosomes. Thin-blood smears were stained using Modified Wright's stain using an automated slide stainer (Hematek, Bayer).
Morphological measurement
Digital images of trypanosomes in blood films and in vitro cultures were taken microscopically at ×1000 magnification and measurements of key morphological features (total length, breath, PK, KN, NA and FF as described in Table 1) performed using Image Pro Express version 5.1 (Media Cybernetics Inc., USA). The morphological features used were based on parameters described by Hoare (Reference Hoare1972) and Mackerras (Reference Mackerras1959). A total of 36 individual trypanosomes identified in blood smears of the Gilbert's potoroo, 12 trypanosomes from blood smears of quokkas were measured.
Table 1. Mean dimensions and standard error (s.e.) of morphological features of Trypanosoma copemani n. sp. isolated from blood-stream forms from the Gilbert's potoroo (Potorous gilbertii) and quokka (Setonix brachyurus)
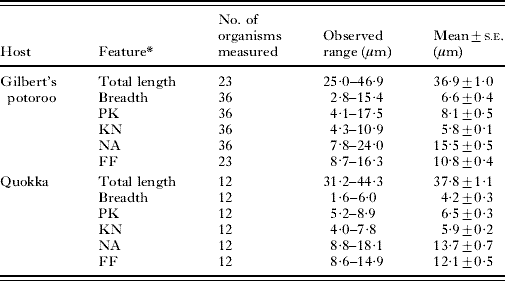
* L, Length of body measured along the mid-line including free flagellum (total length). B, Maximum breadth measured at the level of the nucleus (including undulating membrane). PK, Distance between the posterior end and the kinetoplast. KN, Distance between the kinetoplast and posterior edge of the nucleus. NA, Distance between the anterior edge of the nucleus and the anterior end of the body. FF, Length of the free flagellum.
The mean measurement of each feature was calculated and the statistical significance of any difference between trypanosomes from different host species was determined using a one-way analysis of variance (ANOVA) and the Tukey's Honestly Significant Difference test at a 95% confidence limit.
In vitro culture
In vitro cultures were established by adding 20 μl of fresh blood from each Gilbert's potoroo into 1·8 ml cryopreservation vials containing 1 ml of Modified Sloppy Evans Medium (MSEM) (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999). Cultures were incubated in the dark at room temperature for approximately 10–14 days. Microscopic examination of wet-smear preparations of the medium from each culture was performed weekly after the initial 10 to 14-day incubation to detect motile trypanosomes at 200× and 400× magnification. An aliquot of medium was removed every 10 days and placed into a new culture vial containing fresh MSEM. Once trypanosomes were detected, Giemsa-stained thin smears were prepared for further microscopic examination. Parasites that were detected in MSEM were subcultured into Cunningham's liquid medium (CM) and incubated in the dark at room temperature (approximately 25–27°C) (Cunningham, Reference Cunningham1977; Hamilton et al. Reference Hamilton, Stevens, Gidley, Holz and Gibson2005).
DNA extraction
Whole genomic DNA was extracted from fresh blood samples using the QIAamp® DNA Blood Mini Kit (QIAGEN, Germany) as per the manufacturer's instructions and the DNA stored at 4°C until required.
DNA amplification and sequencing
A 1022 bp fragment of the Trypanosoma sp. 18S rRNA gene was amplified using a nested PCR. The external reverse primer S-762 and the internal forward primer S-825 were previously described by Maslov et al. (Reference Maslov, Lukes, Jirku and Simpson1996). Additional primers, SLF and SLIR, were designed to regions of the 18S rRNA gene (see Fig. 4 for Accession numbers of sequences used). External forward primer, SLF, 5′-GCT TGT TTC AAG GAC TTA GC-3′ and internal reverse primer, SLIR, 5′-ACA TTG TAG TGC GCG TGT C-3′, should amplify a partial 18S fragment from most members of the genus Trypanosoma and some closely related genera such as Crithidia fasciculatea, and Leishmania infantum as assessed by BLASTN GenBankTM searches. The primers amplified a region homologous to that between bases 919 and 1926 in the Trypanosoma sp. wombat H26 (AJ009169). PCR amplification was performed in a 25 μl volume with the final mix containing 10–50 ng of Trypanosoma DNA, 12·5 pmol of primer (external primers SLF forward and S-762 reverse were used in the first PCR round and internal primers S-825 forward and SLIR reverse were used in the second PCR round), 1 unit TAQ DNA polymerase (BIOTECH), 250 mm of each dNTP, 1·5 mm MgCl2, 2·5 units of 10× reaction buffer (BIOTECH) and Baxter Ultra-Pure H2O. The initial denaturing temperature was set at 94°C for 5 min with additional denaturing steps of 50°C for 2 min and 72°C for 4 min followed by 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 60 s. The PCR thermocycler program was completed with a final extension of 7 min at 72°C. A sample of 1 μl of the PCR mixture from the first PCR reaction was used as the template for the second PCR reaction.
PCR products were electrophoresed on a 1% agarose gel and visualized using ethidium bromide and UV illumination. Amplified products were purified using QIAquick® PCR Purification kit (QIAGEN) and cloned into a plasmid vector (pGEM®-T, Promega). Purified plasmid DNA was sequenced using SP6 forward promoter primer (Promega) and T7 reverse promoter primer (Promega). Sequencing reactions were performed using an ABI Prism DyeTerminator Cycle Sequencing Core kit (Applied Biosystems, USA) and sequence data was analysed using 4 peaks v 1.7.1 (A. Griekspoor and Tom Groothuis, mekentosj.com).
Genetic characterization and phylogenetic analysis
The partial 18S rRNA gene sequences amplified from the Gilbert's potoroo and quokka were aligned with sequences from 32 other Trypanosoma species/genotypes obtained from the GenBankTM database that represented each of the major clades described previously by Hamilton et al. (Reference Hamilton, Stevens and Gibson2007) (refer to Figs 4 and 5 for GenBank Accession numbers). Alignments were conducted using CLUSTALW (Thompson et al. Reference Thompson, Higgins and Gibson1994). The final alignment consisted of 1165 characters, of which 527 were variable and 395 were parsimony informative, and was used for subsequent phylogenetic analysis and were further aligned manually by eye. jModeltest 0.1.1 (David Posata – http://darwin.uvigo.es/) was used to select an appropriate evolutionary model. A distance similarity matrix was constructed for all aligned Trypanosoma sequences using MEGA 4 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007) based on the maximum composite likelihood algorithm.
Phylogenetic relationships were determined using distance, maximum likelihood and parsimony based methods. Distance (Neighbor-joining and Maximum Composite Likelihood algorithms) and maximum parsimony (Close-Neighbor-Interchange algorithm) analyses were conducted using MEGA 4 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). Statistical support was provided by use of 1000 bootstrap replicates. Maximum likelihood analysis was conducted using PhyML (Guindon and Gascuel, Reference Guindon and Gascuel2003), based on the HKY85 nucleotide substitution model and using 500 bootstrap replicates. All trees included Phytomonas serpens (AF016323), Leptomonas sp. (EF546786) and Leishmania tarentolae (X53916) as outgroup species.
RESULTS
Microscopy
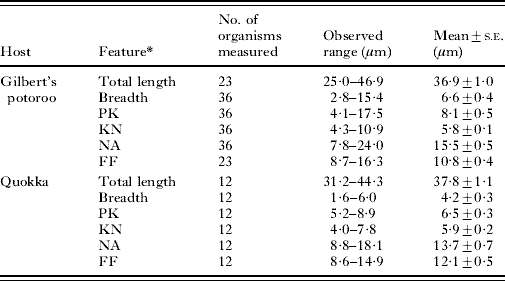
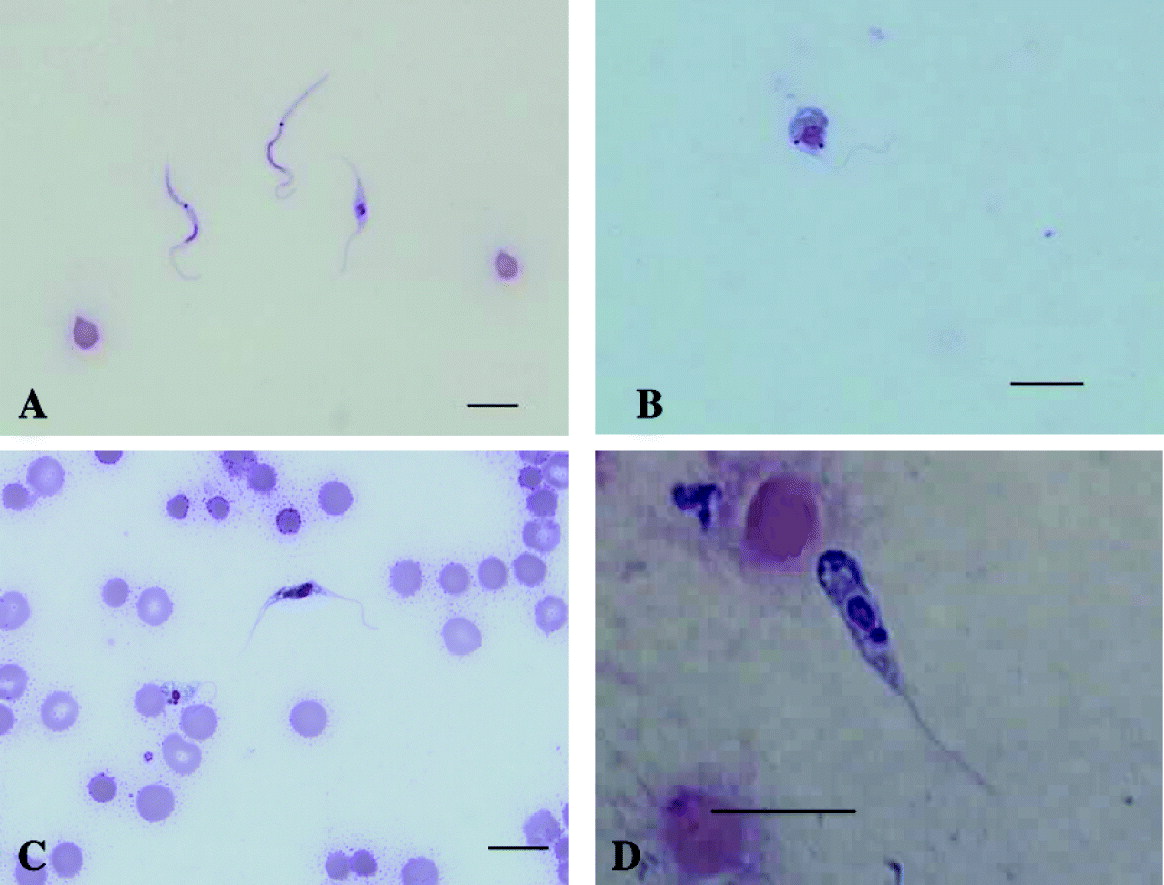
Using light microscopy, trypanosomes were detected in the blood of Gilbert's potoroos (7/7), and 3 quokkas (3/3). The morphology of the trypanosomes detected in blood smears and buffy coat smears from the Gilbert's potoroo were consistent with trypomastigote life-cycle stages (Hoare, Reference Hoare1972). Three main trypomastigote-like forms were observed, one representing a slender form, a medium form and a broad form. The broad form possessed myonemes which gave this form a striated appearance (Fig. 1C). In all 3 forms the kinetoplast was observed at some distance apart from the nucleus and generally closer situated to the posterior end. The trypomastigote-like stages generally had a pointed posterior end, a well-developed undulating membrane and a free flagellum. Trypomastigotes in various developmental stages were observed in blood smears from the Gilbert's potoroo (Fig. 1A–D), and only 1 trypomastigote-like form was observed in blood smears from the quokka (Fig. 2A). The dimensions of representative trypomastigotes are presented in Table 1. Piroplasmida sp. and Microfilariae (Fig. 2B) were also observed in the potoroo blood smears (7/7 and 2/7 respectively).

Fig. 1. Light photomicrographs of Trypanosoma copemani n. sp. isolated from the blood of a Gilbert's potoroo. (A) Slender trypomastigote form in a Modified Wright's stained blood film from a Gilbert's potoroo. (B) Medium trypomastigote form in a Modified Wright's stained blood film from a Gilbert's potoroo. (C) Broad trypomastigote form in a Modified Wright's stained blood film from a Gilbert's potoroo. (D) Dividing trypanosome form in a Modified Wright's stained blood film from a Gilbert's potoroo (K, kinetoplast; N, nucleus).

Fig. 2. Light photomicrographs of Trypanosoma copemani n. sp. isolated from the blood of a quokka and haemoparasites isolated from the Gilbert's potoroo. (A) Trypomastigote form in a Modified Wright's stained blood film from a quokka. (B) Microfilaria sp. in a Modified Wright's stained blood film from a Gilbert's potoroo.
The fleas collected from the Gilbert's potoroo were identified as Stephanocircus dasyuii of the family Stephanocircidae and the ticks identified as Ixodes australiensis family Ixodidae.
In vitro culture
Trypanosomes from all 7 Gilbert's potoroos were successfully cultured in vitro in both MSEM and Cunningham's media. Examples of the different morphological forms observed in in vitro culture are shown in Fig. 3.
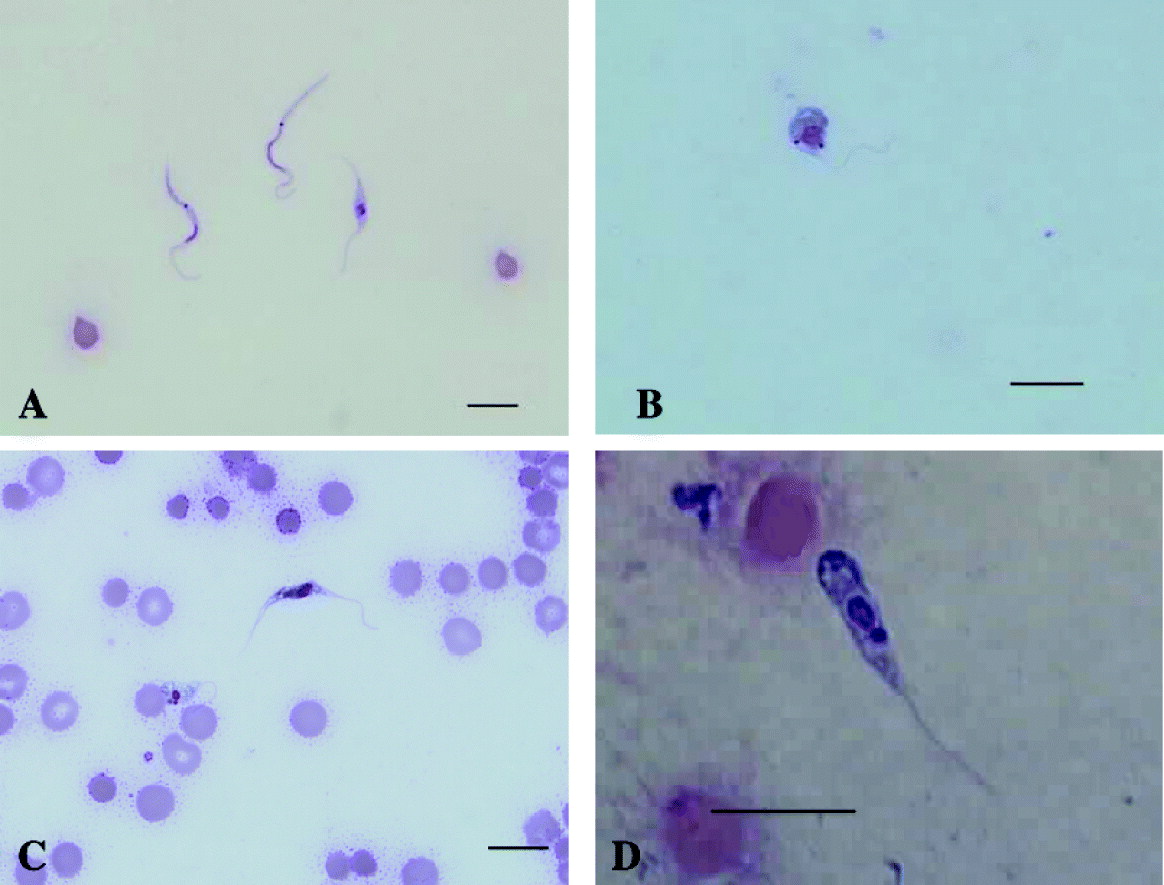
Fig. 3. Light photomicrographs of Trypanosoma copemani n. sp. from in vitro cultures of blood from a Gilbert's potoroo. (A) Trypomastigote and epimastigote forms in a Modified Wright's stained film from an in vitro culture of blood from a Gilbert's potoroo. (B) A dividing sphaeromastigote form in a Modified Wright's stained film from an in vitro culture of blood from a Gilbert's potoroo. (C) Dividing epimastigote form with 2 nuclei and 2 kinetoplasts and sphaeromastigote form in a Modified Wright's stained film from an in vitro culture of blood from a Gilbert's potoroo. (D) Promastigote form in a Modified Wright's stained blood film from an in vitro culture of blood from a Gilbert's potoroo.
Trypanosomes cultured in MSEM were highly polymorphic with sphaeromastigote (Fig. 3B), promastigote (Fig. 3D), epimastigote (Fig. 3A and C) and trypomastigote-like forms observed (Fig. 3A). The trypomastigotes were extremely slender in form and observed to have an absent or rudimentary undulating membrane. A nucleus and a prominent dark rounded kinetoplast were identifiable in each cell. Active cell division was observed in the epimastigote (Fig. 3C) and sphaeromastigote-like stages. The position and size of the nucleus and kinetoplast in relation to each other varied considerably in the dividing stages. A free flagellum that varied in length was detected in the cultivated trypanosomes. The flagellum arose from near the kinetoplast, ran along the length of the body and emerged from the anterior end.
Trypanosomes cultured in Cunningham's medium were observed as sphaeromastigote and epimastigote-like forms with multiplication observed in both trypanosome stages. The trypanosomes showed similar levels of polymorphism and evidence of multiplication as trypanosomes cultured in MSEM. The epimastigote form was the most abundant life-cycle stage detected when CM cultures were initiated.
Statistical analysis of morphological measurements
The mean of the measurements for each morphological parameter (length, breadth, PK, KN, NA and FF) for 36 trypanosomes isolated from the Gilbert's potoroo, and 12 trypanosomes isolated from the quokka are presented in Table 1. The mean breadth of the trypanosomes isolated from the Gilbert's potoroo was significantly different from the trypanosomes isolated from the quokka (P<0·05).
Genetic characterization of the Trypanosoma spp. isolated from the Gilbert's potoroo and quokka
A 1022 bp region of the 18S rRNA gene was amplified for all sampled Gilbert's potoroo (7/7) and quokkas (3/3). PCR products for each sampled host were plasmid transformed and 3 clones for each transformation were sequenced. Two distinct genotypes were revealed among all sequences, differentiated by 12 nucleotide substitutions among the 1022 bp of sequence. One of the genotypes (referred to as ‘T. copemani’ genotype A) was sequenced from 5/7 Gilbert's potoroos and 2/3 quokkas, while the second genotype (‘T. copemani’ genotype B) was also sequenced from 5/7 Gilbert's potoroos and 2/3 quokkas. Both genotypes were sequenced for 3 Gilbert's potoroos and 1 quokka, representing mixed infections in these hosts. Sequences were deposited to the GenBank database under Accession numbers EU571231-34.
A distance similarity matrix (data not shown) revealed that ‘T. copemani’ genotypes A and B were most similar to an unnamed Trypanosoma species from the common wombat (Vombatus ursinus) (0·3% and 0·9% difference respectively) and were 99·8% similar to each other. The lowest level of genetic difference between already established Trypanosoma species using the same partial region of 18S rRNA gene sequences ranged from 0% between Trypanosoma evansi and Trypanosoma brucei, 1% between Trypanosoma lewisi and Trypanosoma microti and 5% between Trypanosoma rangeli and Trypanosoma conorhini.
Phylogenetic relationships of the Trypanosoma spp. from Gilbert's potoroo and quokka
Phylogenetic analysis of the partial 18S rRNA gene sequence placed ‘T. copemani’ genotype A, genotype B, and the Trypanosoma sp. (genotype C) from the wombat clustered together in a clade with T. pestanai and an unnamed Trypanosoma species from the tick Haemaphysalis hystericus with strong bootstrap support using distance (99%, Fig. 4), parsimony (84%, Fig. 4) and maximum likelihood analysis (99%, data not shown). Further confirmation of the relationships between members within this clade was provided by analysis of a reduced number of Trypanosoma species including the woylie Trypanosoma species (Fig. 5). Six other clades of Trypanosoma species previously described by Hamilton et al. (Reference Hamilton, Stevens and Gibson2007) were also observed with significant support produced for the ‘T. brucei’, ‘Lizard’, ‘T. theileri’ and ‘T. lewisi’ clades (Fig. 4). The relationship of the ‘T. copemani’ clade to the other Trypanosoma species, however, could not be accurately determined due to limited bootstrap support being observed between all major clades.

Fig. 4. Distance-based phylogenetic tree of 32 Trypanosoma species inferred using partial 18S rRNA gene sequences (790 positions). Relationships were determined using Neighbor-joining and Maximum Composite Likelihood methods. Bootstrap values are shown as percentages of 1000 replicates and branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. Values shown in bold represent support for each clade using distance, maximum parsimony and maximum likelihood algorithms respectively. The scale bar represents the proportion of base substitutions per site. Species from Australian marsupials are shown with an asterisk.

Fig. 5. (A) Linearized distance-based phylogenetic tree inferred using partial 18S rRNA gene sequences (503 positions) showing the relationship of the proposed new species T. copemani to other closely related species. (B) Subsection of a distance-based phylogenetic tree inferred using partial 18S rRNA gene sequences (959 positions) revealing the 3 distinct genotypes within the species T. copemani (A, B and C). Relationships were determined using Neighbor-joining and Maximum Composite Likelihood methods. Bootstrap values are shown as percentages of 1000 replicates and units are in number of base substitutions per site. New sequences are shown in bold.
Proposed species description
On the basis of genetic characterization of a partial region of the 18S rRNA gene and phylogenetic analysis a new species of Trypanosoma is proposed.
Trypanosoma copemani n. sp
Description: Trypomastigote form: Trypanosoma copemani n. sp. appeared slightly curved with a pointed posterior end, a large rounded nucleus, prominent black stained oval kinetoplast, a well developed undulating membrane and a long free flagellum. The nucleus was usually located towards the middle of the body within a granular cytoplasm. Three main forms were observed, one representing a slender form, a medium form and a broader form. The broad form displayed myonemes giving it a striated appearance. In all three forms the kinetoplast was observed at some distance apart from the nucleus and generally closer, situated to the posterior end. The total length ranged from 25 to 46 μm (mean 36·9 μm), the breadth including the undulated membrane ranged from 2·8 to 15·4 μm (mean 6·6 μm) the posterior to kinetoplast ranged from 4·1 to 17·5 μm (mean 8·16 μm), the kinetoplast to nucleus ranged from 4·3 to 10·9 μm (mean 5·8 μm) while the nucleus to anterior ranged from 7·8 to 24·0 μm (mean 15·53 μm) and the free flagellum from 8·7 to 16·3 μm (mean 10·8 μm).
Type host. Gilbert's potoroo (Potorous gilbertii)
Other host. Quokka (Setonix brachyurus)
Type locality. Two Peoples Bay Nature Reserve (34°58'S, 118°11'E) near Albany, Western Australia
Location in host. Systemic circulation
Pre-patent period. Unknown
Patent period. Unknown
Etymology. Named after the late Associate Professor Douglas Bruce Copeman for his contribution to Australian parasitology.
DISCUSSION
Only recently has the diverse range of Trypanosoma species of Australian marsupials begun to be morphologically and genetically characterized and placed in an evolutionary context among other Trypanosoma species of the world. The discovery of 2 genetically unique trypanosomes within the Gilbert's potoroo and quokka raises questions regarding the speciation of members of the Trypanosomatidae and the need for more clearly defined parameters regarding the morphological and genetic based classification. This study proposes the name Trypanosoma copemani n. sp. (subcategorized into 3 genotypes) to be used to describe trypanosomes from Gilbert's potoroo, quokka and the common wombat; however, further genetic and biological data are necessary before more conclusive taxonomic categorization can be made.
Infection and pathogenesis
While only 7 Gilbert's potoroos and 3 quokkas were captured and sampled during this study due to population size and the nocturnal and timid nature of native Australian animals, all Gilbert's potoroo and quokka that were screened were found to be infected with a T. copemani n. sp. This is at odds with previous studies that showed that Australian mammals had a low prevalence of infection with trypanosomes that was considered to be a function of low parasitaemia (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999). The observed differences between the findings of this study and previous studies may be because these studies sampled animals housed either permanently, or wild animals held temporarily for treatment, in zoological parks and not in free-living populations (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999; Hamilton et al. 2004). It is possible that captive animals are not exposed to the same degree of challenge from infected vectors and reservoir hosts as are animals that are in their natural ecological niche.
Interestingly, piroplasms were observed in the blood of the Gilbert's potoroo, which have recently been described as Theileria gilberti n. sp. (Lee et al. Reference Lee, Ryan, Jefferies, McInnes, Forshaw, Friend and Irwin2009). An earlier study by Cox (Reference Cox1977) showed that piroplasms enhance and prolong trypanosome infections particularly in stercorarian-transmitted trypanosomes and generally occur together in the wild. This may account for the relatively high prevalence of trypanosome infection seen in the Gilbert's potoroo. Microfilaria were also detected in 2 of the Gilbert's potoroo sampled and, to the authors' knowledge, this is the first time that microfilaria have been reported in the Gilbert's potoroo (O'Donoghue and Adlard, Reference O'Donoghue and Adlard2000).
Despite the presence of these various parasite species, no obvious clinical signs were exhibited by either Gilbert's potoroo or quokka. Parasitic infections have most likely co-evolved with their hosts and therefore probably prove little or no health risk to the endangered Gilbert's potoroo and quokka. However, if any changes occur in the ecology of infection within these animals then overt disease may occur. Constant wildlife monitoring and management of the native fauna within Two Peoples Bay, Albany, Western Australia is advisable to prevent the introduction and potential spread of new diseases that may pose as a risk to the natural ecology of these animals.
Morphology
Morphological characterization showed that T. copemani n. sp. isolated from Gilbert's potoroo and quokka was highly polymorphic, with various life-cycle stages detected in both blood films and in vitro cultures.
The morphological characteristics observed in blood smears and in smears from in vitro cultures were typical of Trypanosoma trypomastigotes, epimastigotes, promastigotes and sphaeromastigote-like forms. The epimastigote, and trypomastigote were the most principal life-cycle stages observed, with the former being the most abundant. These findings are similar to observations by Noyes et al. (Reference Noyes, Stevens, Teixeira, Phelan and Holz1999) who successfully cultured novel native Australian Trypanosoma spp. isolated from a common wombat (Vombatus ursinus) and an eastern grey kangaroo (Macropus giganteus giganteus) and observed that epimastigotes and sphaeromastigotes were the most abundant life-cycle stages in the wombat cultures while promastigotes were the most abundant form observed in the cultures from the eastern grey kangaroo. The promastigote life-cycle stage from the Gilbert's potoroo compared to promastigotes studied by Noyes et al. (Reference Noyes, Stevens, Teixeira, Phelan and Holz1999) differed in both their abundance and their morphological characteristics. Promastigotes isolated from the cultures of kangaroo blood showed a swollen anterior end that lacked an emergent flagellum whereas the promastigote like-stages from the Gilbert's potoroos were less abundant and contained an emerging anterior free flagellum.
The 3 different morphological trypomastigote blood stream stages observed in the Gilbert's potoroo may represent different multiplication stages. The large stout trypanosome appears to represent a pre-division stage as seen in T. lewisi (Herpetosoma), which is known to be accompanied by growth of the body and forward migration of the kinetoplast (Hoare, Reference Hoare1972). The slender stage showed similar morphological characteristics to tick nymph (Ixodes holocyclus) trypanosomes isolated from wild adult bandicoots (Isoodon obesulus) (Mackerras, Reference Mackerras1959) and may represent the vector stage which gives rise to the medium trypomastigotes stage. Definitive morphological characterization of the trypanosomes isolated from the Gilbert's potoroo will, however, require examination of all life-cycle stages that occur in vitro and in vivo to determine the complexity of each stage.
The relative size and the morphological characteristics of the trypanosomes isolated from the Gilbert's potoroo suggest that they may either belong to the Megatrypanum or Herpetosoma subgenus as described by Hoare (Reference Hoare1972). However, there have been concerns raised recently about the validity of the use of this terminology (Stevens et al. Reference Stevens, Teixeira, Bingle and Gibson1999). Therefore, we have adopted the current terminology, which may require review once a new classification system has been defined (Hoare, Reference Hoare1972).
The presence of a long free flagellum, prominent rod-shaped kinetoplast and the relative large distance between the kinetoplast and the nucleus in the trypomastigote blood stream form suggest that the trypanosomes from the Gilbert's potoroo are more likely to represent trypanosomes from the Herpetosoma subgenus as Megatrypanum trypanosomes are characterized as having kinetoplasts situated close to the nucleus a short to absent free flagellum, are large in length and have a small kinetoplast (Hoare, Reference Hoare1972). The total length of T. copemani n. sp. isolates ranges between 25·0 μm and 46·9 μm which falls within the known range for classification into the Herpetosoma subgenus. The length of existing members of the Herpetosoma ranges from 16·8 μm (T. sigmodoni) to 47 μm (T. myceta) (Hoare, Reference Hoare1972).
Evolutionary and taxonomic relationships
The 18S rRNA gene was chosen as the basis of molecular characterization in this study because it is conserved throughout the eukaryotes (Stevens et al. Reference Stevens, Noyes and Gibson1998) and it is regarded as the gene of choice for phylogenetic analysis of kinetoplasts (Haag et al. Reference Haag, O'Huigin and Overath1998; Stevens et al. Reference Stevens, Noyes and Gibson1998; Hamilton et al. Reference Hamilton, Stevens, Gidley, Holz and Gibson2004). Nucleotide variation within the 18S rRNA gene has been suggested as a means of species differentiation, although no universal number of mutational changes has been postulated for species level classification. Within this study, the level of genetic variation within the partial 18S sequence between established species ranged from 0 to 5%, making it difficult to assess accurately whether novel genotypes represented new species.
Two distinct genotypes of Trypanosoma from the same geographical location were found to infect both the Gilbert's potoroo and the quokka representing a new species with 2 genotypes. Trypanosoma copemani n. sp. genotype A and genotype B were found to be genetically similar to a Trypanosoma sp. (genotype C) isolated from the common wombat (Hamilton et al. Reference Hamilton, Stevens, Gidley, Holz and Gibson2005). These novel species were shown to be phylogenetically similar, forming a distinct clade with high statistical support. It is likely that the wombat isolates belong to T. copemani n. sp.; however, further data would be required to prove this. The evolutionary significance and resolution of the relationship of the T. copemani n. sp. clade to the other Trypanosoma clades is difficult to determine due to alignment problems involving the 18S rRNA gene with its regions of high nucleotide variation and large insertions and deletions. Further phylogenetic analyses using the entire open reading frame of the 18S rRNA gene, and other loci such as the glycosomal glyceraldehyde phosphate dehydrogenase (gGAPDH) and multiple gene analysis are therefore needed (Hannaert et al. Reference Hannaert, Opperdoes and Michels1998; Hamilton et al. Reference Hamilton, Stevens, Gidley, Holz and Gibson2004, Reference Hamilton, Stevens and Gibson2007).
Genotypes A, B and C of T. copemani n. sp. formed a clade with the KG1 Trypanosoma tick (Haemaphysalis hystricis) isolate (Thekisoe et al. Reference Thekisoe, Honda, Fujita, Battsetseg, Hatta, Fujisaki, Sugimoto and Inoue2007), the woylie Trypanosoma species (Smith et al. Reference Smith, Clark, Averis, Lymbery, Wayne, Morris and Thompson2008) and T. pestanai, which was isolated from a badger in Portugal. The genetic distances between T. copmani and the KG1 Trypanosoma tick isolate and the woylie Trypanosoma species, however, are large (13·4–34·8% respectively). The tick species, Ixodes australiensis, was collected from the Gilbert's potoroo and may play a role in both the transmission of trypanosomes and piroplasms in the south-west of Western Australia. Indeed, the slender form of trypanosomes cultivated from the potoroo had similar morphological characteristics to the trypanosomes isolated from wild bandicoot tick nymphs (Ixodes holocyclus) (Mackerras, Reference Mackerras1959). Further investigation into the role of ticks and fleas as vectors for all members of this newly described clade is warranted.
We would like to thank staff from the Department of Environment and Conservation, Albany, Western Australia, for support in the field aspects of this study. The trypanosome was originally noted by Dr Mary McConnell, VetPath, Ascot, W.A. during haematological analysis of potoroo blood in 2001. We are grateful to Dr Patrick Hamilton from Bristol University, UK, for his advice and for providing reference DNA from Australian Trypanosoma sp., Dr Clare Constantine, Dr William Ditcham, Dr Scott Edwards, Gordon Thomson, Yazid Abdad, and Linda McInnes for various assistance with this paper.