Published online by Cambridge University Press: 18 November 2004
Two types of tetraphyllidean merocercoids, Phyllobothrium delphini and Monorygma grimaldii, are well known from most cetaceans world-wide. The role of cetaceans in the life-cycle of these merocercoids is unclear because their specific identity is as yet unknown. The problem is compounded by poor descriptions of both merocercoids. We used light and scanning electron microscopy, and histological techniques to provide a thorough description of merocercoids collected from 11 striped dolphins, Stenella coeruleoalba, from the Spanish Mediterranean. We also described, for the first time, specimens of P. delphini with immature proglottides. Our merocercoids were morphologically similar to those described previously, except in the structure of the apical organ. Intra- and inter-sample variability in the morphology of the apical organ suggested that it degenerates during larval development. A subsample of 16 specimens of P. delphini and M. grimaldii was characterized for the D2 variable region of the large subunit ribosomal RNA gene (LSU) and compared with published tetraphyllidean cestode LSU sequences. P. delphini showed 2 unique signatures that differed from one another by a single base, whereas all sequences of M. grimaldii were identical. This suggests that each type may represent a single species, contrary to previous speculations based on morphological data. All merocercoid specimens formed a clade together with Clistobothrium montaukensis. Based on the low degree of divergence, all specimens of this clade are predicted to be congeneric.
Tetraphyllidean merocercoids (terminology of larval cestodes follows Chervy, 2002) have been reported frequently from most cetacean species and some pinnipeds world-wide (Delyamure, 1955; Dailey & Brownell, 1972; Dailey, 1985; Bester, 1989; Raga, 1994, and references therein). Generally, 2 types have been recognized, i.e. Phyllobothrium delphini (Bosc, 1802) van Beneden, 1868, encysted in the subcutaneous blubber, usually in the abdominal area, and Monorygma grimaldii (Moniez, 1889) Baylis, 1919, encysted mainly in the peritoneum of the abdominal cavity. Both types of larvae have a scolex bearing an apical sucker and 4 monolocular bothridia with accessory suckers, but the scolex of P. delphini is large, has folded bothridia and is connected to a bladder through a short, thick filament, whereas the scolex of M. grimaldii is small, has bothridia with simple margins and is connected to the bladder through a very long and thin filament (Southwell & Walker, 1936; Skrjabin, 1970). Occasionally, other larval cestode types with bothridia lacking accessory suckers have been recorded, encysted in the subcutaneous blubber of some cetaceans (Markowski, 1955; Skrjabin, 1964; Siquier & Le Bas, 2003).
Even though P. delphini and M. grimaldii have been reported frequently from marine mammals in the last two centuries, there are surprisingly few accurate descriptions, especially of the scolex. This could be explained, at least in part, by the difficulty to recover the invaginated scolex intact, especially in the case of M. grimaldii, whose small scolex is invaginated at the end of a very thin and fragile filament. To date, only Mendonça (1984) has published histological sections of the scolex of P. delphini in which the gross morphology can be distinguished. Siquier & Le Bas (2003) were apparently the first to use scanning electron microscopy (SEM) to describe the scolex of P. delphini. Neither method has been employed in the case of M. grimaldii.
Most authors believe that P. delphini and M. grimaldii use marine mammals, especially cetaceans, as a means of infecting predatory or scavenging elasmobranchs (e.g. Southwell & Walker, 1936; Johnston & Mawson, 1939; Dollfus, 1964; Testa & Dailey, 1977; Walker, 2001). However, the specific identity of these larvae is not known. In the case of P. delphini, some authors (Guiart, 1935; Delyamure, 1955; Testa & Dailey, 1977) have recognized different morphotypes. Dailey (1985) suggested that these morphotypes may represent distinct species, whereas morphological uniformity in M. grimaldii suggests that these larvae represent a single species. There is, however, the possibility that observed ‘morphotypes’ represent different phases of development of the same species (Siquier & Le Bas, 2003). The use of molecular systematic techniques is necessary to help clarify these taxonomic issues.
During the parasitological examination of striped dolphins, Stenella coeruleoalba (Meyen, 1833), stranded on the Mediterranean coasts of Spain, a number of specimens of P. delphini and M. grimaldii were obtained. In this study we provide detailed morphological and morphometric descriptions of both types of larvae by using light microscopy, SEM, and fine histology. We also found specimens of P. delphini with immature proglottides and provide, for the first time, a description of this material, which may be useful for shedding light on the ontogenetic changes that occur in these larvae. Finally, we provide a molecular characterization of both larval types in order to further elucidate the identities and phylogenetic affinities of P. delphini and M. grimaldii.
Eleven striped dolphins, Stenella coeruleoalba, stranded during 1998–2001 along the Western Mediterranean coast of Spain (between 40°13′N, 0°17′E and 37°52′N, 0°45′W) were necropsied for parasites. The sample consisted of 3 males (range of total length 155–193 cm) and 8 females (range of total length 154–211 cm). The subcutaneous blubber, peritoneum and mesenteries of the abdominal cavity were inspected immediately and a sample of the cestodes encysted removed while living. All striped dolphins were infected with both larval types. About 25 specimens of each type were collected from each dolphin (the total number of merocercoids per dolphin was not counted). All merocercoids were washed in saline (9‰), examined in a Petri dish with saline and described under a stereomicroscope. The entire filament and scolex were subsequently removed from the cyst. The filament was cut near to the scolex and left in saline until the scolex was fully evaginated.
Three samples of merocercoids were used. The first sample was used to make observations under compound and stereomicroscopes, and to take morphometric measurements. The bladder and filament were drawn in 20 specimens of each morphotype. The bladder was drawn from live specimens that had been in the refrigerator for at least 1 h, allowing the worms to relax. The bladder of relaxed specimens acquired an oblong, flattened shape in all specimens, thus minimizing the potential deformation of the bladder in active animals. The filament and the width of the bladder wall were drawn in 70% (v/v) ethanol-fixed material. The scoleces of 20 live specimens of each morphotype from each of 2 dolphins were fixed in hot 70% (v/v) ethanol by shaking them vigorously in a circular movement. They were then stained with eosin, washed in tap water, dehydrated in 70% (v/v) ethanol and cleared with lactophenol. Temporary mounts were made on cavity slides to avoid deforming the scoleces. Drawings were made with the aid of a drawing tube connected to a compound or stereo light microscope. The internal diameter of the apical organ was visible only in 13 individuals of P. delphini. We measured 16 homologous metrics (Fig. 1) from drawings of each selected specimen. Three specimens of P. delphini showing initial proglottization were fixed in 70% (v/v) ethanol and drawn. Characters described in Fig. 1 were measured from drawings of proglottized specimens, except for the internal diameter of the apical organ, which was not visible, and the bladder size because the bladder of these specimens was not collected intact. Seven proglottides of P. delphini were fixed in 70% (v/v) ethanol, stained with haematoxylin, dehydrated in an ethanol series, cleared in xylene and mounted in Canada balsam. Three additional proglottides were used to obtain transverse sections. Proglottides were processed as indicated above, and thick transverse sections were obtained by cutting the proglottides with a razor blade after they were hardened in xylene. All measurements are in micrometres unless otherwise stated.

Fig. 1. Schematic drawings of a tetraphyllidean merocercoid showing the measurements taken in this study. (A) Specimen in toto. (B) Scolex (bothridium on the left – Phyllobothrium delphini; bothridium on the right – Monorygma grimaldii). For each specimen, measurements of bothridial structures were obtained from 2 bothridia and averaged. BLL, bladder length; BLW, bladder width; BLWT, bladder wall thickness; FL, filament length; FW, filament width; SL, scolex length; MSW, maximum scolex width; SPW, scolex proper width (measured at the level of the scolex mid-length); AMSPL, apical modification of scolex proper length; AMSPW, apical modification of scolex proper width; EDAPO; external diameter of the apical organ; IDAPO, internal diameter of the apical organ; BL, bothridium length; BW, bothridium width (equivalent to bothridium loculus width); ACSL, bothridial accessory sucker length; ACSW, bothridial accessory sucker width.
A second sample of 5 specimens of P. delphini and 5 specimens of M. grimaldii was processed for observation with a vacuum scanning electron microscope (SEM) or an environmental scanning electron microscope (ESEM). Specimens were killed and fixed in a hot solution of 10% (v/v) formalin in Sörensen's phosphate buffer (0·1 M, pH 7·2). For SEM, merocercoids were dehydrated in an ethanol series, critical-point dried in liquid CO2, mounted on specimen stubs using conductive carbon paint, sputter coated with gold-palladium to a thickness of 25–30 nm in a Bio-Rad Sc 500 coating unit and examined in a S-4100 SEM at 5 kV. Specimens for ESEM were washed in saline solution and observed directly with a Philips ESEM XL-30 with a gaseous secondary electron detector.
A third set of specimens was processed for histology. Seven scoleces of M. grimaldii and 10 of P. delphini were fixed in hot buffered 10% (v/v) formalin, dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin. Sections (7–10 μm) were stained with haematoxylin and eosin, mounted in Entellan (Merck) and observed under a light microscope. Two proglottides of P. delphini fixed in 70% (v/v) ethanol were similarly processed.
Voucher specimens have been deposited in the helminth collection of the Natural History Museum, London (NHM; accession nos.: Phyllobothrium delphini, BMNH 2003.10.29.1-10; Monorygma grimaldii, BMNH 2003.10.29.11-20).
In addition to the collections described above, 8 specimens of P. delphini and 8 specimens of M. grimaldii from 3 host individuals were preserved in 95% ethanol for molecular diagnostic analysis. Specimens of P. delphini showing proglottization (see above) were excluded due to their poor condition of preservation, and scoleces were retained for vouchers prior to genomic DNA (gDNA) extraction and deposited in the helminth collection of the NHM (Accession nos. BMNH 2004.8.18.6-21). gDNA was extracted from the specimens using a Qiagen DNeasy™ tissue kit and used for PCR as described by Olson et al. (2003). A fragment (~1400 bp) of the nuclear large subunit ribosomal RNA gene (LSU; spanning domains D1-D3) was amplified using primers LSU5 (5′-TAGGTCGACCCGCTGAAYTTAAGC-3′) and 1200R (5′-GCATAGTTCACCATCTTTCGG-3′) and the middle portion spanning the variable D2 region (~650 bp) sequenced bidirectionally using internal primers 300F (5′-CAAGTACCGTGAGGGAAAGTT-3′) and ECD2 (5′-CTTGGTCCGTGTTTCAAGACGGG-3′). This region of the LSU has proven informative for both diagnostic and phylogenetic work in tetraphyllidean and related taxa (e.g. Brickle et al. 2001; Reyda & Olson, 2003). Contiguous sequences were assembled and edited using Sequencher™ (GeneCodes Corp., ver. 4), and leading and trailing regions of the sequences without overlap were removed prior to analysis. Sequences are available from GenBank under Accession nos. AY741591-1606.
Sequences were screened using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) to confirm their orthology with the LSU genes of cestodes, and aligned by eye using MacClade ver. 4.06 (Maddison & Maddison, 2000) together with available tetraphyllidean LSU sequences (21 taxa). Phylogenetic affinities of the merocercoid sequences with previously characterized adult tetraphyllidean taxa (n=19) were estimated by Bayesian analysis using MrBayes ver. 3.b4 (Huelsenbeck & Ronquist, 2001). Based on the results of MrModeltest ver. 1.1b (Nylander, 2002; a simplified version of ModelTest by Posada & Crandall, 1998), a general time reversible model of nucleotide substitution incorporating among-site rate variation was specified, and the analysis run over 1 million generations, sampling topologies every 100th generation. Other program parameters were as specified in Olson et al. (2003). A consensus tree was constructed using the ‘sumt’ command with a ‘burnin’ value of 250 and the ‘contype=allcompat’ option. Trees were rooted using Echeneibothrium maculatum Woodland, 1927 based on prior analysis of tetraphyllidean and related LSU sequences (see Reyda & Olson, 2003). Comparisons of uncorrected genetic distances (shown parenthetically as the percentage difference; i.e. no. of substitutions/no. of sites compared*100) were calculated using PAUP* ver. 4.0b10 (Swofford, 2001) based on a re-alignment of taxa from the clade (marked with an asterisk in Fig. 5) including only the unique merocercoid sequences together with Clistobothrium montaukensisRuhnke, 1993. The high similarity of these sequences necessitated only a single 1 bp insertion in the alignment.
Some of the morphological terms have been inconsistently and ambiguously used to describe M. grimaldii and P. delphini. For instance, many authors have used the term ‘myzorhynchus’ to refer to the apical region of the scolex of these larvae (Baer, 1932; Southwell & Walker, 1936; Dollfus, 1964; Testa & Dailey, 1977; Mendonça, 1984; Raga, 1985; Balbuena, 1991; Soulier, 1993; Siquier & Le Bas, 2003), but the use of this term has often been questioned (Wardle & McLeod, 1952; Euzet, 1959; Caira, Jensen & Healy, 1999). To avoid further confusion, in this study we have adopted the workable definitions provided by Caira et al. (1999) for some conflicting characters of the scolex. We used ‘apical modification of scolex proper’ for the apical region of P. delphini and M. grimaldii because this region is externally modified as a mobile, dome-shaped structure, and its tissue is internally continuous with that of the scolex proper (see Results). Likewise, we used ‘apical organ’ for the structure we observed on the tip of the scolex because there was a discrete, histological boundary between this structure and the surrounding tissue (Caira et al. 1999).
Phyllobothrium delphini (Bosc, 1802) van Beneden, 1868
Description of merocercoid (Table 1, Figs 2 and 3)
Table 1. Measurements of Phyllobothrium delphini from the present study and the literature (Scolex measurements have been taken from evaginated scoleces, except for those of Testa & Dailey (1977). Range (mean±S.D.) [coefficient of variation (%)]. Measurements in micrometres unless otherwise stated.)


Fig. 2. Scanning electron micrographs and histological sections of the scolex of Phyllobothrium delphini collected from Mediterranean striped dolphins. (A) Lateral view of scolex; (B) apical view of scolex; (C) longitudinal section of the scolex; (D) transversal section of the scolex; (E) longitudinal section of the apical modification of scolex proper and the apical organ; (F) longitudinal section of the apical organ protruded; (G) apical organ (SEM); (H) longitudinal section of the apical organ resembling a collapsed cup. AO, apical organ; AMSP, apical modification of scolex proper; AS, accessory sucker; BL, bothridial loculus; BC, basophilic cells; BS, bothridial stalk; MB, muscular bundles; MM, muscular membrane; SP, scolex proper.

Fig. 3. Immature proglottides in a filament of Phyllobothrium delphini. The filament of this specimen is invaginated (i.e. the external tegument is internal), and laterally flattened (osmoregulatory canals appear running longitudinally along its central axis). (A) Transverse thick section of a proglottis; (B) whole mount; (C) transverse histological section of a proglottis. ET, external tegument; DOC, dorsal osmoregulatory canal; GP, approximate location of the genital pore; O, primordium of ovary; VD, primordium of vas deferens; T, testes; VA, primordium of vagina; VOC, ventral osmoregulatory canal.
Bladder sub-spherical to ovoid, with scolex invaginated at end of short thick filament connected to bladder. Filament with 2 osmoregulatory canals running longitudinally along each side, forming zigzag pattern. Scolex with 4 monolocular bothridia with folded margins and round anterior accessory sucker, occupying 14·8–22·4% (18·8±1·8%, n=40) of bothridium length (Fig. 2A and B). Loop of osmoregulatory canal enters each bothridium and runs parallel to bothridium margin, at distance of about quarter of bothridial diameter. Each bothridium connected to scolex proper by short stout stalk (BS in Fig. 2C and D). Posterior half of bothridial loculus free. Anterior part of bothridium with free rims. Margins of bothridial loculus unite with upper part of accessory sucker (Fig. 2A and B).
Apical modification of scolex proper dome-shaped in frontal plane, conical in sagittal plane, and cruciform in apical view, with bothridia located between cross-arms. Apical modification of scolex proper neither invaginable nor retractable, but continuously moving and deforming in live specimens. Histological sections of this region reveal muscular bundles and some basophilic cells (Fig. 2E). No histological differences between apical region and scolex proper (Fig. 2C and E).
Apical organ located at tip of apical modification of scolex proper. Some shape variability was found: in some individuals, apical organ resembled collapsed cup (Fig. 2E, G and H), whereas in other individuals it was protruded and more rounded (Fig. 2F). Internally, apical organ with sac-like structure delimited by thin muscular membrane, containing basophilic cells but not radial muscles (Fig. 2F and H).
Nine specimens of P. delphini collected from 1 striped dolphin showed initial proglottization along filament. In some individuals, only anterior part of filament proglottized, whereas in others the entire filament was proglottized. Proglottized individuals with filaments much longer than non-proglottized individuals (Table 1). Scolex of proglottized worms either evaginated or invaginated within filament, and morphologically very similar to that of non-proglottized individuals from same dolphin. Accessory suckers of bothridia occupying 16·6–19·0% (17·6±1·3%, n=3) of bothridium length. Apical organ dome-shaped in all individuals. Scolex structures larger than in non-proglottized specimens (Table 1). Proglottis length increases further from scolex. Neck measured in 2 specimens 274 and 295 in length. Proglottides with genital primordia of male and female systems in most developed specimen are shown in Fig. 3. Round testes clearly distinguishable in 7 proglottides (Fig. 3A, B and C), numbering 90–134 (107±18) per proglottis and measuring 20–31 (26±3, n=42). Large group of cells (probably primordium of ovary) observed in middle of proglottides (Fig. 3A and B). Thick cord of cells (probably primordium of vagina) arises from ovary and runs transversally to lateral margin (Fig. 3B and C). Thin cord of cells (probably primordium of vas deferens) runs parallel to thick cord (Fig. 3B). According to arrangement of cell cords, genital pores appeared irregularly alternating (Fig. 3B).
Site: encysted in the subcutaneous blubber, especially in the posterior body half of striped dolphins.
Monorygma grimaldii (Moniez, 1889) Baylis, 1919
Description of merocercoid (Table 2, Fig. 4)

Fig. 4. Scanning electron micrographs and histological sections of the scolex of Monorygma grimaldii collected from Mediterranean striped dolphins. (A) Lateral view of scolex; (B) apical view of scolex; (C) longitudinal section of the apical modification of scolex proper and the apical organ; (D) longitudinal section of the scolex; (E) non-everted apical organ (SEM); (F) longitudinal section of a cup-shaped apical organ; (G) everted apical organ to form a knob-like structure (SEM); (H) longitudinal section of an everted apical organ. AO, apical organ; AMSP, apical modification of scolex proper; AS, accessory sucker; BL, bothridial loculus; BC, basophilic cells; MB, muscular bundles; MM, muscular membrane; SP, scolex proper.
Table 2. Measurements of Monorygma grimaldii from the present study and the literature (Scolex measurements have been taken from invaginated scoleces, except for data of the present study and those of Skrjabin (1970). Range (mean±S.D.) [coefficient of variation (%)]. Measurements in micrometres unless otherwise stated.)

Bladder ovoid to almond-shaped, connected to internal long slender filament ending with invaginated scolex. In live specimens, filament tangled in fluid filled bladder; when larva fixed in 70% (v/v) ethanol, fluid precipitates around filament, such that it appears to be surrounded by amorphous porous parenchyma. Filament bears 2 osmoregulatory canals running longitudinally along each edge, tracing close zigzag pattern. Scolex bears 4 sessile monolocular bothridia with simple edges and anterior accessory sucker; accessory sucker occupies 25·6–41·1% (31·7±3·3%, n=40) of bothridial length (Fig. 4A and B). Bothridia slightly tapered anteriorly, attached to scolex proper only by anterior part of bothridial loculus. Margins of bothridial loculus unite laterally with accessory sucker (Fig. 4A).
Apical modification of scolex proper large, sub-spherical, dome-shaped, neither invaginable nor retractable, but continuously moving and deforming in live specimens. Histological sections of this region showed muscular bundles and basophilic cells (Fig. 4C and D). No histological differences between apical region and scolex proper (Fig. 4C and D).
Apical organ located at tip of apical modification of scolex proper, cup-shaped in some individuals (Fig. 4E and F) and conical or knob-shaped in others (Fig. 4G and H). Histological sections revealed that apical organ has sac-like structure, is slightly muscular and with some basophilic cells but lacks radial muscle fibres, and is delimited from surrounding tissue by thin muscular membrane (Fig. 4F and H).
Site: Most worms encysted in the peritoneum of the abdominal cavity of striped dolphins; some of them also in the peritoneum of testes and the mesentery of the rectum and uterus.
A total of 586 characters was included in the analysis of the D2 variable region of the LSU gene, of which 250 were parsimony informative. Three unique LSU signatures were present among the merocercoid sequences, and a consensus tree resulting from Bayesian analysis (Fig. 5) showed that these formed a clade together with C. montaukensis and a previously published sequence of a metacestode collected from squid (Loligo gahi; see Brickle et al. 2001). Sequences of P. delphini were identical except for 1 which differed by a single G/A transition (0·16%), whereas all sequences of M. grimaldii were identical and differed from the LSU signature of P. delphini by only 3 C/T transitions (0·48%), the most common substitution class due to the regular formation of G-T, as well as G-C, pair bonds in the secondary structure of rDNA (see comparison of substitution classes in Olson et al. 2001). The genetic distance between the signature of P. delphini with that of C. montaukensis was 1·7% and between M. grimaldii and C. montaukensis was 1·1%, whereas between the metacestode from Loligo gahi and C. montaukensis was 0·32%.
The morphology of the tetraphyllidean merocercoids described in this study agrees well with the available descriptions of P. delphini and M. grimaldii, except for the structure of the apical organ. In some descriptions of both types of larvae, the apical organ was not described (Linton, 1905; Baer, 1932; Guiart, 1935; Garippa, Scala, & Pais, 1991). However, most authors described or illustrated the apical organ as an apical sucker (Southwell & Walker, 1936; Delyamure, 1955; Dollfus, 1964; Skrjabin, 1964; Skrjabin, 1970, 1972; Testa & Dailey, 1977; Mendonça, 1984; Raga, 1985; Balbuena, 1991; Siquier & Le Bas, 2003). In specimens of P. delphini, Johnston & Mawson (1939) described this organ as an apical plug. In our specimens, the apical organ always appeared as a sac-like structure delimited by a thin muscular membrane. However, we found structural variability even in specimens from the same individual dolphin; in some individuals, the apical organ resembled a collapsed cup, whereas in others it was dome-shaped. Although the causes of this variability are unclear, Hamilton & Byram (1974) described the in vitro development of plerocercoids of the onchobothriid Acanthobothrium sp. and found substantial changes in the structure of the apical sucker that are relevant for our case (see also Chambers, Cribb & Jones, 2000): in early development, a typical sucker (i.e. a cup-shaped structure with radial musculature) was observed. Later on, the apical sucker began to degenerate, and subsequently collapsed and lost the radial muscles. Then, the degenerated sucker everted, acquiring a dome-shape appearance. Finally, the sucker detached from the scolex. The entire process was observed to occur at the larval stage since it was not accompanied by proglottization and sexual differentiation of worms (Hamilton & Byram, 1974). Accordingly, there is the possibility that the variability observed in the apical organ of P. delphini and M. grimaldii may result from a similar degenerative process (compare Figs 2E, F, H and 4C, F, H with Figs 8–12 in Hamilton & Byram, 1974). In fact, several authors (Southwell & Walker, 1936; Wardle & McLeod, 1952) suggested that the apical sucker of P. delphini disappears during development. It is therefore possible that the specimens described as having an apical sucker were in an early stage of development, while the specimens we have described were in intermediate phases of development.
Our re-descriptions of P. delphini and M. grimaldii provide the most complete account of morphometric data of these larvae to date and illustrate the range of morphological variation in specimens collected from one host species in a single locality. Minor differences of both P. delphini and M. grimaldii were found with respect to published data, but the ranges of morphometric variables generally overlapped. Moreover, part of the morphometric variability observed could be attributable to differences in the processing methods (see references in Table 1 and 2), or the degree of development of the larvae (Siquier & Le Bas, 2003).
Although diagnosing species via molecular analysis is still at an early stage, it is reasonable to expect that the low variation (<0·5%) observed within the samples of P. delphini may be well within the genetic variation of a single morphological species (see, e.g. Brickle et al. 2001), and may thus represent population-level differences. Based on previous descriptions and their own morphological data, Testa & Dailey (1977) defined 11 morphotypes of P. delphini that were suggested to represent different species (see also Dailey, 1985); up to 6 morphotypes were found in a single host species in the same locality. A cursory examination of samples of P. delphini from Mediterranean striped dolphins suggested the presence of at least 4 of the morphotypes described by Testa & Dailey (1977), yet genetic data point to the existence of only 2 genetic signatures that differ by a single base. It is likely therefore that the morphological variation reported by early authors (Guiart, 1935; Delyamure, 1955) and by Testa & Dailey (1977) may be intraspecific, at least in part, and perhaps associated with ontogenetic changes (see Siquier & Le Bas, 2003).
The names Phyllobothrium delphini and Monorygma grimaldii have been used historically as convenient labels to recognize the 2 types of merocercoids commonly found in marine mammals; however, the taxonomic validity of these names is questionable. Apparently, neither P. delphini nor M. grimaldii fulfill the current diagnosis of their putative genera (see Ruhnke, 1996 and Euzet, 1994, respectively). Obviously, the problem is that the generic concepts of tetraphyllideans are based on adults, so the use of diagnostic criteria for P. delphini and M. grimaldii can be justified only if their scolex is not modified through development to the adult stage. At present, we can state only that the scolex morphology of proglottized specimens of P. delphini does not differ substantially compared to that of non-proglottized specimens. Therefore, a molecular analysis is fundamental to positively match these larval forms with adult specimens of ‘P. delphini’ and ‘M. grimaldii’.
Bayesian analysis indicated that both types of merocercoids cluster together with adult specimens of C. montaukensis collected from sharks off the Western Atlantic coast of Montauk, New York, and with some metacestodes collected from squid in the Falkland Islands (Brickle et al. 2001); all these taxa are separated by slight genetic distances. Bayesian analysis also indicated that P. delphini is far separated from Phyllobothrium lactuca van Beneden, 1849, the type species of the genus Phyllobothrium van Beneden, 1849. These results have 2 interesting taxonomic implications. First, the analysis suggests that P. delphini should be removed from the genus Phyllobothrium. Second, it is premature to determine whether the low degree of genetic divergence observed in the clade containing P. delphini, M. grimaldii, C. montaukensis and the squid metacestode represent population, strain or species-level differences but, based on the levels of inter-generic genetic distances as evident in Fig. 5, it might be reasonable to assume that all of these taxa are congeneric. In the case of P. delphini, this suggestion seems to be supported by morphological data. If we assume that the structure of the scolex of proglottized specimens of P. delphini does not change substantially in the definitive host (see Freeman, 1973), the morphology of the scolex would fit with the description given by Ruhnke (1993) in the generic diagnosis of ClistobothriumDailey & Vogelbein, 1990: ‘scolex with 2 dorsal and 2 ventral pedunculate bothridia and dome-shaped or cruciform apical region. Myzorhynchus absent. Each bothridium with single apical, muscular, round sucker. Posterior loculus foliose or folding flap of tissue’. Thus, the only difference between P. delphini and the adult of species of Clistobothrium is that the latter lacks an apical organ. However, we have suggested above that the apical organ degenerates during the larval development and may disappear at the adult stage. Our taxonomic suggestion agrees with the opinions of several authors (Southwell & Walker, 1936; Johnston & Mawson, 1939; Wardle & Mc Leod, 1952), who speculated that P. delphini might actually be Clistobothrium tumidum (Linton, 1922) Ruhnke, 1993 (syn. Phyllobothrium tumidum) based on the morphological resemblance of the scolex. Our taxonomic suggestion is also compatible with ecological data. The 3 known species of Clistobothrium are restricted to large pelagic sharks of the family Lamnidae (mackerel sharks) that are known to feed on cetaceans and pinnipeds, as well as on cephalopods (Linton, 1922; Dailey & Vogelbein, 1990; Ruhnke, 1993). In addition, species of Clistobothrium have been reported in localities of the Pacific, the Atlantic and the Mediterranean basins (Linton, 1922; Euzet, 1959; Dailey & Vogelbein, 1990; Ruhnke, 1993), where P. delphini and M. grimaldii are also known to occur (see Raga, 1994, and references therein).

Fig. 5. Results of Bayesian analysis with nodal support shown as percentage posterior probabilities; N.B. branch lengths are proportional to observed character change, not as estimated by Bayesian analysis. Re-alignment and direct comparison of genetic distances were compared for the taxa inclusive of the clade indicated by an asterisk (see text). GenBank sequence Accession numbers shown parenthetically.
The generic assignation of M. grimaldii is much more problematic. The scolex morphology of these merocercoids is very different from that of P. delphini and that of Clistobothrium (the scolex morphology of squid metacestodes from Falkland Island has not been described, see Brickle et al. (2001)). We were unable to find proglottized specimens of M. grimaldii, so we ignore whether there might be substantial changes in scolex morphology when merocercoids begin proglottization. However, according to the slight differences observed between non-proglottized and proglottized specimens of P. delphini, we should not expect deep transformations in proglottized M. grimaldii.
In summary, our study provides, for the first time, a detailed morphological analysis of P. delphini and M. grimaldii, and a description of proglottized specimens of P. delphini. We also provide the first molecular analysis of the 2 larval types, which reveals that little variability exists within each type, and that both types might be congeneric with C. montaukensis. This suggestion is supported by morphological and ecological data in the case of P. delphini, but not in the case of M. grimaldii. The lack of congruence between molecular and morphological analyses will be solved definitively when a complete phylogenetic tree of the Tetraphyllidea, as well as molecular data from adult forms of Mediterranean species of tetraphyllideans, are available.
We thank our colleagues from the Marine Zoology Unit, Instituto Cavanilles de Biodiversidad y Biología Evolutiva (University of Valencia), for their assistance with the necropsies of animals, to the staff of the Servicio Central de Soporte a la Investigación Experimental of the University of Valencia for their technical assistance with the SEM and ESEM, and to Dr J. Pertusa (University of Valencia) for his assistance with the histological techniques. Thanks are also due to Dr B. B. Georgiev (Bulgarian Academy of Sciences) for his comments and assistance. The comments of two anonymous referees are highly appreciated. Cetaceans were collected thanks to an agreement between the Conselleria de Medio Ambiente (Generalitat Valenciana) and the University of Valencia. This work has been supported by projects BOS2002-00878 and REN2003-01758 from the Spanish Government and by project GV04B-304 from the Valencian Government. P.D.O. and D.T.J.L. were supported by a Wellcome Trust Senior Fellowship to D.T.J.L. (043965/Z/95/Z). F.J.A. benefits from a ‘Ramón y Cajal’ contract from the Ministerio de Ciencia y Tecnología of Spain. The first author holds a doctoral fellowship from the Conselleria de Cultura, Educación y Ciencia of the Generalitat Valenciana.
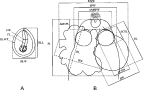
Fig. 1. Schematic drawings of a tetraphyllidean merocercoid showing the measurements taken in this study. (A) Specimen in toto. (B) Scolex (bothridium on the left – Phyllobothrium delphini; bothridium on the right – Monorygma grimaldii). For each specimen, measurements of bothridial structures were obtained from 2 bothridia and averaged. BLL, bladder length; BLW, bladder width; BLWT, bladder wall thickness; FL, filament length; FW, filament width; SL, scolex length; MSW, maximum scolex width; SPW, scolex proper width (measured at the level of the scolex mid-length); AMSPL, apical modification of scolex proper length; AMSPW, apical modification of scolex proper width; EDAPO; external diameter of the apical organ; IDAPO, internal diameter of the apical organ; BL, bothridium length; BW, bothridium width (equivalent to bothridium loculus width); ACSL, bothridial accessory sucker length; ACSW, bothridial accessory sucker width.

Table 1. Measurements of Phyllobothrium delphini from the present study and the literature

Fig. 2. Scanning electron micrographs and histological sections of the scolex of Phyllobothrium delphini collected from Mediterranean striped dolphins. (A) Lateral view of scolex; (B) apical view of scolex; (C) longitudinal section of the scolex; (D) transversal section of the scolex; (E) longitudinal section of the apical modification of scolex proper and the apical organ; (F) longitudinal section of the apical organ protruded; (G) apical organ (SEM); (H) longitudinal section of the apical organ resembling a collapsed cup. AO, apical organ; AMSP, apical modification of scolex proper; AS, accessory sucker; BL, bothridial loculus; BC, basophilic cells; BS, bothridial stalk; MB, muscular bundles; MM, muscular membrane; SP, scolex proper.

Fig. 3. Immature proglottides in a filament of Phyllobothrium delphini. The filament of this specimen is invaginated (i.e. the external tegument is internal), and laterally flattened (osmoregulatory canals appear running longitudinally along its central axis). (A) Transverse thick section of a proglottis; (B) whole mount; (C) transverse histological section of a proglottis. ET, external tegument; DOC, dorsal osmoregulatory canal; GP, approximate location of the genital pore; O, primordium of ovary; VD, primordium of vas deferens; T, testes; VA, primordium of vagina; VOC, ventral osmoregulatory canal.

Fig. 4. Scanning electron micrographs and histological sections of the scolex of Monorygma grimaldii collected from Mediterranean striped dolphins. (A) Lateral view of scolex; (B) apical view of scolex; (C) longitudinal section of the apical modification of scolex proper and the apical organ; (D) longitudinal section of the scolex; (E) non-everted apical organ (SEM); (F) longitudinal section of a cup-shaped apical organ; (G) everted apical organ to form a knob-like structure (SEM); (H) longitudinal section of an everted apical organ. AO, apical organ; AMSP, apical modification of scolex proper; AS, accessory sucker; BL, bothridial loculus; BC, basophilic cells; MB, muscular bundles; MM, muscular membrane; SP, scolex proper.

Table 2. Measurements of Monorygma grimaldii from the present study and the literature

Fig. 5. Results of Bayesian analysis with nodal support shown as percentage posterior probabilities; N.B. branch lengths are proportional to observed character change, not as estimated by Bayesian analysis. Re-alignment and direct comparison of genetic distances were compared for the taxa inclusive of the clade indicated by an asterisk (see text). GenBank sequence Accession numbers shown parenthetically.