Introduction
Trematode species diversity is underestimated, and one reason for this may be unrecognized cryptic species (Pérez-Ponce de León and Poulin, Reference Pérez-Ponce de León and Poulin2017). Species are deemed cryptic when there are no perceivable diagnostic morphological traits, and genetic analyses reveal more than one distinct lineage (Pérez-Ponce de León and Nadler, Reference Pérez-Ponce de León and Nadler2010). While many cryptic species complexes have been reported, relatively few studies have investigated the extent of morphological similarity among cryptic species complexes. For instance, there is a paucity of investigations that have linked the genetic differences among cryptic lineages with detailed analyses of the morphology and life history of the cryptic lineages (Blasco-Costa et al., Reference Blasco-Costa, Balbuena, Raga, Kostadinova and Olson2010). This lack of integrative analysis has led to poor species-level taxonomic resolution, which ultimately biases estimates of parasite species diversity, and can mask differences in the ecology and life history of cryptic species (Poulin and Leung, Reference Poulin and Leung2010).
Quinqueserialis Harwood, Reference Harwood1939 (Digenea: Notocotylidae) is typical of other trematodes in that the number of species recognized within this genus has fluctuated over time. Up to four nominal species have been recognized in North America, Quinqueserialis quinqueserialis Barker and Laughlin, Reference Barker and Laughlin1911, Quinqueserialis hassalli McIntosh and McIntosh, Reference McIntosh and McIntosh1934, Quinqueserialis floridensis Rausch 1952 and Quinqueserialis zibethicai Gupta 1962. Two of the species were originally described as Notocotylus Diesing 1839 species but were reassigned to Quinqueserialis due to the presence of five rows of ventral papillae, as opposed to three rows among Notocotylus species, and a cirrus armed with heavy spines (Harwood, Reference Harwood1939). Currently, only two of the four taxa are considered nominal species: Quinqueserialis floridensis and Quinqueserialis quinqueserialis (Barker and Laughlin, Reference Barker and Laughlin1911; Rausch, Reference Rausch1952a; Kinsella, Reference Kinsella1971). These two species can be distinguished morphologically based on the placement of the vitelline follicles and overall size, with Q. floridensis specimens being smaller overall and having vitelline follicles posterior to the uterine coils (Rausch, Reference Rausch1952a; Kinsella, Reference Kinsella1969). The two Quinqueserialis species infect rodent hosts such as the round-tailed muskrat (Neofiber alleni True 1884) for Q. floridensis and voles (Microtus pennsylvanicus Ord 1815) and muskrats (Ondatra zibethicus Linnaeus 1766) for Q. quinqueserialis (Barker and Laughlin, Reference Barker and Laughlin1911; Rausch, Reference Rausch1952a, Reference Rausch1952b). The two Quinqueserialis species do not overlap in geographic range, with Q. quinqueserialis distributed from the North American Arctic to parts of the southern United States and Q. floridensis restricted to Florida where Q. quinqueserialis has not been reported (Rausch, Reference Rausch1952a). Thus, there is evidence to support that Q. floridensis and Q. quinqueserialis are distinct species. However, there is still ambiguity within this genus due to similarities in morphology and host use.
A comparative morphological study suggested that two formerly recognized species, Quinqueserialis hassalli and Quinqueserialis zibethicai, are synonymous to Q. quinqueserialis (Kinsella, Reference Kinsella1971). Further evidence for synonymy among these species was overlap in definitive host use (the former infects meadow voles and the latter infects muskrats). However, some of the perceived morphological differences described in Q. hassalli are attributed to host-induced phenotypic plasticity. Adult Q. quinqueserialis parasites in voles tend to be larger than adult stages from the muskrats (Kinsella, Reference Kinsella1971). In the case of Q. zibethicai, the difference in microhabitat (duodenum vs caecum for Q. quinqueserialis) within the definitive host was used to delineate this species (Kinsella, Reference Kinsella1969). However, there are no voucher specimens of Q. zibethicai; therefore, morphological and genetic characteristics of this taxon cannot be evaluated (Kinsella, Reference Kinsella1969).
Relative to the other species in this genus, Q. quinqueserialis has a broad geographic distribution, ranging from the North American Arctic to southern United States (Rausch, Reference Rausch1952b; Detwiler et al., Reference Detwiler, Zajac, Minchella and Belden2012). Across this broad geographic range, differences in host use and spatial distance among populations could potentially result in isolation, leading to diverged populations (Lively, Reference Lively1999). Unlike parasites that use hosts (e.g. birds) that disperse and mix parasites from different populations, Q. quinqueserialis infects a variety of cricetid rodent hosts that generally have limited home ranges, so dispersal by these hosts would be unlikely to promote gene flow of parasites across large distances (Rausch, Reference Rausch1952b; Getz, Reference Getz1961; Marinelli and Messier, Reference Marinelli and Messier1993). The broad distribution and host use of Q. quinqueserialis evokes the question of whether this species comprises a complex of cryptic species.
The uncertainty surrounding species diversity of Quinqueserialis in North America revolves around morphological descriptions from few specimens and limited geographic sampling. For instance, the species Q. quinqueserialis was described from a single specimen collected from a muskrat in Nebraska, USA (Barker and Laughlin, Reference Barker and Laughlin1911). Thus, the extent of intraspecific variation among species in this group remains unknown. Further, only one species in this genus, Q. quinqueserialis, has had their life cycle described and several intermediate hosts identified (Herber, Reference Herber1942; Gagnon and Detwiler, Reference Gagnon and Detwiler2019). There are many gaps in the knowledge of the biology and diversity of this genus and most of the research on this group was completed before the advent of modern molecular technology. Here we use adult morphological and adult and larval DNA sequence data of the 28S nuclear and CO1 mitochondrial genes to determine whether cryptic species are present in the genus Quinqueserialis and to elucidate the taxonomic status of Quinqueserialis spp. in North America.
Materials and methods
Specimen collection
Muskrats, and vole definitive hosts (Microtus spp. Schrank 1798), were field collected in six areas throughout five Canadian provinces and US states in North America: Northern Northwest Territories, Canada; Northern Manitoba, Canada; Southern Manitoba, Canada; Minnesota, USA, Virginia, USA; and Alabama, USA (Table 1). Muskrat carcasses were salvaged from licensed trappers at each location, excluding Northern Manitoba, from 2015 to 2018. Voles were either live-trapped or snap-trapped at three locations where muskrats were collected (Table 1; Government of Manitoba Scientific Collection permit No. WB18783 and WB23398, Government of Northwest Territories Wildlife Collection permit No. WL500642, Virginia Game and Inland Fisheries Scientific Collection permit No. 061288). Upon recovery, voles (Microtus spp.) were euthanized in the field via anaesthetic overdose (Animal Use Protocol 2016-0023, Texas A&M University) followed by cervical dislocation (Animal Use Protocol AC11347, University of Manitoba). Voles were sorted into individual bags and transported to the lab for necropsy. The intestinal tract, from the duodenum to colon, of each muskrat and vole host was sectioned, separated in Petri dishes and opened longitudinally. The contents of each section of intestinal tract were searched for Quinqueserialis spp. adults under a stereomicroscope.
Table 1. Field collection locations and definitive hosts sampled

Prevalence of adult Quinqueserialis quinqueserialis parasites reported at each location and prevalence of adult Quinqueserialis kinsellai n. sp. reported from the type locality
a Voles (Microtus spp.) were trapped at this location in two separate years, 2016 and 2019.
b Adult Q. quinqueserialis specimens from muskrats (Ondatra zibethicus) were donated from collections by Dr. R. Sorensen (Minnesota State University-Mankato). No prevalence data available.
Live parasites were heat killed in 70° C distilled water, then transferred to warm ethanol. The parasites were preserved in 80% EtOH and stored at 4° C for molecular and morphological analysis. Adult worms were initially identified as Quinqueserialis spp. by the presence of five rows of ventral papillae (Harwood, Reference Harwood1939). The holotype specimen for Q. hassalli and a paratype of Q. floridensis were borrowed from the U.S. National Museum of Natural History (NMNH) and included in the morphological analyses. As there is neither a holotype nor a paratype of Quinqueserialis quinqueserialis available, 17 additional specimens identified as Quinqueserialis spp. were also loaned from the NMNH to assess intra- and interspecific morphological variation (Supplementary Table 1).
A voucher image was captured of each worm using an Axio Cam ICcI digital camera connected to an Axio Imager M2 compound microscope (Zeiss Canada Ltd., Toronto, Canada). Drawings were made with the aid of a drawing tube on an Olympus MT5310L compound microscope. Tissue samples were collected from gravid worms prior to staining and mounting to create hologenophore-type vouchers (Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008). Once the tissue sample was retrieved, adult worms were stained with acetocarmine, dehydrated in ethanol, cleared in xylene and mounted in Canada Balsam. An adult worm recovered from the same host as molecular voucher worms was also stained and permanently mounted to create paragenophore-type vouchers (Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008). Voucher and holotype parasite materials from this study are deposited in the Smithsonian National Museum of Natural History, Washington, US (Supplementary Table 2). Vouchers of vole hosts from locations where Q. quinqueserialis was detected are deposited in the Canadian Museum of Nature, Ottawa, ON (Supplementary Table 3).
Multivariate analysis
Thirteen morphological measurements were analysed for a total of 99 specimens identified as Quinqueserialis spp.: 27 museum specimens (7 museum lots had multiple individuals measured), 15 field-collected paragenophores and 57 field-collected hologenophores. Two museum specimens were excluded from the multivariate analyses because accurate measurements could not be obtained. All morphological features were normally distributed with the exception of the width of uterine coils and width of the left testes, which were log-transformed to meet assumptions of normality prior to analysis. Principal component analysis (PCA) was performed to determine if specimens clustered according to nominal species. This analysis included specimens that were field collected and genetically identified, as well as museum specimens that were morphologically identified by other researchers. The resulting clusters from the PCA were used to assign specimens to three a priori groups that were then analysed in a linear discriminant analysis (LDA). The LDA was applied to 99 specimens to evaluate the morphological differences between them and to identify the morphological features that yield optimal group separation. A random 75% subset of the adult parasite morphological measurements were used to train a parasite species assignment model, which was then used to predict the parasite species identity of the remaining 25% of the data. This process was repeated 1000 times; each with a randomly selected set of training data, and mean prediction success was calculated. The PCA was conducted with the package ‘vegan’ and the LDA was conducted with the package ‘MASS’ in Rstudio 1.1.463 (Venables and Ripley, Reference Venables and Ripley2002; Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019).
Molecular data
To have both a molecular and stained morphological voucher for a single specimen, the anterior third of the worm was removed and used for DNA extraction. Tissue samples were soaked in MilliQ water to remove the EtOH prior to being incubated in 200 μL 5% Chelex solution with 0.2 mg/mL proteinase K at 56 C for 2 hr. DNA samples were then vortexed, boiled at 100 C for 8 min and vortexed again after cooling. Extracted DNA samples were stored at −20° C until polymerase chain reaction (PCR) could be performed. If amplification was unsuccessful with chelex-extracted samples, an adult worm from the same host was used for whole-worm extraction using the Qiagen DNeasy Blood & Tissue kit following a modified manufacturer protocol. These samples were eluted in a total volume of 30 μL of millipore water.
Partial (~1000 base pairs (bp)) 28S rDNA sequences were amplified and sequenced using forward primers LSU-5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′) or 300 F (5′-CAA GTA CCG TGA GGG AAA GTT G-3′) when LSU-5 failed to amplify and 1500 R (5′-GCT AGG GAA ACT TCG-3′) reverse primer (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003). The 28S RNA gene region was targeted because it is commonly utilized to investigate interspecific variation among trematodes and sequences are available for a larger number of trematode species on public databases, including Quinqueserialis quinqueserialis (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003; Detwiler et al., Reference Detwiler, Zajac, Minchella and Belden2012; Blasco-Costa et al., Reference Blasco-Costa, Cutmore, Miller and Nolan2016, Pérez-Ponce de León and Hernández-Mena, Reference Pérez-Ponce de León and Hernández-Mena2019). We amplified ~500 bp of the cytochrome c oxidase subunit I (CO1) mtDNA gene with primers DICE 1F (5′-TTW CNT TRG ATC ATA AG-3′; Moszczynska et al., Reference Moszczynska, Locke, McLaughlin, Marcogliese and Crease2009) and DICE 11R (5′- GCW GWA CHA AAT TTH CGA TC −3′; Van Steenkiste et al., Reference Van Steenkiste, Locke, Castelin, Marcogliese and Abbott2015). The 5′ end of the CO1 mitochondrial gene region is commonly used in DNA barcoding of trematodes (Moszczynska et al., Reference Moszczynska, Locke, McLaughlin, Marcogliese and Crease2009). Mitochondrial DNA accumulates substitutions at a higher rate than nuclear DNA and can therefore distinguish congeneric trematodes more clearly (Vilas et al., Reference Vilas, Criscione and Blouin2005). The 28S amplifications were carried out in 25 μL reactions containing 2 μL of extracted DNA, 2.5 μ m of 10 × buffer, 1.5 μ m of MgCl2, 0.5 μ m of each primer, 0.5 μ m of dNTP and 0.05 μL−1 units Taq polymerase. The CO1 amplifications were carried out in 25 μL reactions containing 5 μL of extracted DNA, 2.5 μ m of 10 × buffer, 3.5 μ m of MgCl2, 0.5 μ m of each primer, 0.5 μ m of dNTP and 0.5 μL−1 units Taq polymerase (Omega Bio-Tek, Georgia, USA). The following thermocycling conditions were used for 28S rDNA amplification with primers LSU-5 and 1500R: 94° C for 3 min, once; 94° C for 1 min, 56° C for 45 s, 72° C for 2 min, 35 times; 72° C for 7 min, once. Amplification of the 28S gene region using primers 300F and 1500R consisted of 95° C for 3 min, once; 95° C for 45 s, 56° C for 30 s, 72° C for 2 min, 40 times; 72° C for 7 min, once. Amplification of CO1 mtDNA was 95° C for 2 min, once; 95° C for 30 s, 50° C for 30 s, 72° C for 1 min, 35 times; 72° C for 10 min, once. PCR products were purified with a MO Bio Laboratories Inc. PCR clean up kit and sequenced in both directions at the Hospital for Sick Children, Toronto, ON.
Contigs were constructed and sequences were assembled using Sequencher 4.1 (Gene Codes Corp., Ann Arbor, MI, USA) and were aligned in MEGA 7.0 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Genetic distances between specimens were calculated at both gene regions with uncorrected p-distance in MEGA. A genetic benchmark of >1% different at the 28S gene region and >5% different at the CO1 gene region was interpreted as evidence of distinct species (Vilas et al., Reference Vilas, Criscione and Blouin2005). These cut offs were used to assign specimens to species groups, and genetic distances between and among species was calculated with uncorrected p-distance in MEGA.
Results
Specimens identified as Q. quinqueserialis were field collected from at least one host within the six sampling areas (Table 1). Prevalence of Q. quinqueserialis infection was 83% (114/123) in muskrats and 27% (35/128) in voles (Microtus pennsylvanicus, M. oeconomus Pallas 1776 and Myodes spp. Pallas 1811) and jumping mice (Zapus sp. Coues 1875). Notably, only meadow voles (M. pennsylvanicus) and a single tundra vole (M. oeconomus) were infected with Q. quinqueserialis.
Morphological data
Thirteen morphological characters were measured and analysed for the field-collected and museum Quinqueserialis sp. specimens (Table 2). The first and second principal components accounted for 82% of the total observed morphological variation. The first principal component (PC1) accounted for 71% of the total variation (eigenvalue = 9.2). PC1 was interpreted as describing overall body size, meaning all morphological variables contributed significantly (cut-off value of factor scores was 0.8, determined following Abdi and Williams, Reference Abdi and Williams2010). The second principal component (PC2) accounted for 12% of the total variation (eigenvalue = 1.5). PC2 was interpreted as describing the width and length of the oral sucker, which both contributed positively, and the width of the uterine coils, which contributed negatively.
Table 2. Comparative morphological data for three Quinqueserialis species

Measurements (μm) of 13 morphological variables of three species of Quinqueserialis found in North America: Quinqueserialis quinqueserialis, Quinqueserialis floridensis, Quinqueserialis kinsellai n. sp.
Among the specimens identified as Q. quinqueserialis, those from voles were generally larger than those from muskrats (Fig. 1). The museum specimens identified as Q. floridensis formed a separate cluster along PC2 from those identified as Q. quinqueserialis. The Q. floridensis specimens were smaller than the specimens in the other clusters and had wider uterine coils (Fig. 1). There was a third cluster consisting of museum specimens identified as Q. quinqueserialis from voles and specimens from field-collected voles. This cluster separated from the Q. floridensis specimens along PC1 and from the Q. quinqueserialis specimens along PC2 (Fig. 1). Specimens within this cluster were larger than Q. quinqueserialis specimens, had wider uterine coils, but had relatively smaller oral suckers. The three clusters formed by the PCA were used to assign specimens to groups for the LDA. These groups are subsequently referred to as: Q. quinqueserialis, Q. floridensis and Q. quinqueserialis morphotype 2.

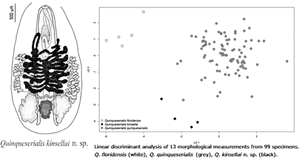
Fig. 1. PCA of Quinqueserialis spp specimens. PCA of 13 morphological measurements of 99 Quinqueserialis spp. specimens. Three species of Quinqueserialis demarcated by colour: Q. quinqueserialis (grey), Q. floridensis (white) and Q. kinsellai n. sp. (black), first referred to as Q. quinqueserialis morphotype 2 in text and described as a novel species herein. Quinqueserialis spp. specimens demarcated by shape according to host species.
The trained LDA run on 13 morphological characters (Table 2) separated the three species with an average 95.3% accuracy. The first canonical function clearly discriminated Q. floridensis specimens from the other two groups, whereas the second canonical function discriminated Q. quinqueserialis morphotype 2 from Q. quinqueserialis (Fig. 2). The specimens of Q. floridensis and Q. quinqueserialis morphotype 2 were all assigned to their a priori groups, whereas one Q. quinqueserialis specimen was misclassified as Q. quinqueserialis morphotype 2 (4.7% misclassification). These misclassifications could be attributed to the higher dispersion and overlap among specimens of Q. quinqueserialis and Q. quinqueserialis morphotype 2 (Fig. 2). Five variables were associated with the discriminatory power of the model: oral sucker length, oral sucker width, left testes width, left vitellaria length and width of uterine coils (Table 2).

Fig. 2. LDA of three Quinqueserialis species. LDA of 13 morphological measurements of 99 specimens assigned to three taxa: Quinqueserialis quinqueserialis, Quinqueserialis floridensis and Quinqueserialis kinsellai n. sp., first referred to as Q. quinqueserialis morphotype 2 in text and described as a novel species herein.
Molecular analysis
For the mitochondrial gene, we generated 85 CO1 sequences (510–606 bp, MW853836-MW853920) from both larval and adult Quinqueserialis parasites from 61 individual hosts (muskrats, voles and snails), including 56 adult sequences of which 25 are from vole hosts, and 31 are from muskrat hosts. For the nuclear gene region, we generated 91 partial 28S sequences (912–1271 bp, MW934276- MW934366) from both larval and adult Quinqueserialis parasites, including 61 adult sequences of which 19 are from vole hosts and 42 sequences are from muskrat hosts. There were two unique 28S haplotypes.
The mean genetic distance at the CO1 gene region between Q. quinqueserialis and Q. quinqueserialis morphotype 2 was 10% (over 452 bp), which is two times the p-distance difference for species suggested by Vilas et al. (Reference Vilas, Criscione and Blouin2005). The mean genetic distance at the CO1 gene region within Q. quinqueserialis was 0.76% (over 452 bp), while there was no genetic variation at the CO1 gene region within Q. quinqueserialis morphotype 2. The mean genetic distance at the 28S gene region between Q. quinqueserialis and Q. quinqueserialis morphotype 2 was 1.6% (over 810 bp), which is above the 1% p-distance difference for species suggested by Vilas et al. (Reference Vilas, Criscione and Blouin2005). There was no intraspecific genetic variation at the 28S gene region for both Q. quinqueserialis and Q. quinqueserialis morphotype 2 specimens. Genetic comparisons of Q. quinqueserialis and Q. quinqueserialis morphotype 2 to Q. floridensis specimens could not be completed as DNA could not be extracted from the museum specimens.
Examination of morphological data and genetic sequencing of Q. quinqueserialis from the same host species (meadow vole) and locality (Churchill, MB, Canada) confirmed the distinctness of Q. quinqueserialis morphotype 2. Thus, we propose erecting this morphotype as a species and propose the name Quinqueserialis kinsellai n. sp.
Quinqueserialis kinsellai n. sp.
Type-host: Microtus pennsylvanicus, the meadow vole (Cricetidae). Two hosts vouchered at the Texas A&M Biodiversity Research and Teaching Collections, College Station, TX, USA (TCWC 66632 and TCWC 66647).
Type-locality: Churchill Northern Studies Centre, Churchill, Manitoba, Canada (58.739218 N. -93.817874W).
Site: Small intestine and caecum of intestine.
Type-material: Holotype (USNM 1643534) and paratypes (USNM 1643530-1643533) deposited at the Smithsonian National Museum of Natural History, Washington, D.C., United States.
Representative DNA sequences: COX1 (MW853846, MW853847, MW853848, MW853855), 28S (MW934288, MW934289).
Etymology: The species is named for Dr J. Michael Kinsella in recognition of his contributions to the knowledge of Quinqueserialis spp. taxonomy and biology.
Description × Fig. 3A–D

Fig. 3. Quinqueserialis kinsellai n. sp., ventral views. (A) Ventral view of the holotype (USNM 1643534) showing distribution of glands. Scale = 500 μm. (B) Ventral view of the holotype showing internal features, ventral glands omitted, lacking oral sucker. Scale = 500 μm. (C) Ventral view of paratype (USNM 1643530) showing oral sucker. Oral sucker folded over genital pore and specimen torn at posterior margin of the cirrus. Scale = 500 μm. (D) Ventral view of paratype (USNM 1643532) showing terminal portion of genital ducts. Scale = 500 μm.
The morphological description is based on four partially mounted adult specimens and one whole-mounted adult specimen. Measurements are presented in μm (mean ± s.d.). Body oblong, and slightly attenuated anteriorly, with concave ventral surface, 3535–4385 long (4051 ± 362) by 1437–2006 (1716 ± 232) in greatest width (Fig. 3). Ventral surface with five rows of glands with an average of 13 papillae in the lateral rows, 15 papillae in the proximal rows and 15 papillae in the medial row (Fig. 3A). Oral sucker is 319–441 (358 ± 57) in diameter (Fig. 3C). The oesophagus is short, and typical for genus. Intestinal caeca pass medial to vitellaria and testes and end blindly posterior to the posterior margins of the testes. Excretory pore median and situated at level just posterior to ends of intestinal caeca. Testes 613–857 (719 ± 77) long by 384–608 (482 ± 78) wide, lobed and immediately posterior to vitellaria. Cirrus armed with conical spines is 496 long with a cirrus sac that is 665–838 (763 ± 89) long by 104–224 (155 ± 62) wide (Fig. 3D). Ovary is lobed, intercaecal and situated at the same level as testes. Ovary is 362–635 (460 ± 120) long by 241–355 (333 ± 63) wide. Mehlis gland is anterior to the ovary. Uterus with >10 convoluted transverse loops, which extend laterally beyond margins of intestinal caeca. Uterine coils 1073–1414 (1297 ± 160) in maximum width. Metraterm is strongly developed, 416–718 (585 ± 118) long by 180–247 (207 ± 24) wide. Vitellarium consists of two lateral groups of numerous follicles arranged in clusters that extend from the anterior margin of the testes to the base of the metraterm, 925–1415 (1200 ± 152) in maximum length (Fig. 3B, C). Eggs are ovoid with polar filaments 17 (17.4 ± 0.2) long by 11 (11.5 ± 0.7) wide.
Remarks
This material exhibits some diagnostic characteristics of Q. quinqueserialis, i.e. five rows of ventral papillae, armed cirrus, lateral groups of vitellaria arranged in clusters (Harwood, Reference Harwood1939; Kinsella, Reference Kinsella1971). However, Q. kinsellai n. sp. is characterized by the convoluted transverse loops of the uterus that extend beyond the margins of the intestinal caeca while the loops of the uterus are not convoluted and only extend past the intestinal caeca in certain hosts among Q. quinqueserialis specimens. On average, the testes and uterus of Q. kinsellai n. sp. are larger than those of Q. quinqueserialis, however the upper limits of the ranges of these features in Q. quinqueserialis specimens overlap with the ranges of these features in Q. kinsellai n. sp. Despite overlap in the size range of many morphological features, both multivariate approaches (PCA and LDA) clearly indicate that each species is a distinct morphological cluster. Further, Q. kinsellai n. sp. has a larger body size than Q. floridensis and does not exhibit the diagnostic feature of vitelline clusters restricted to a position posterior to the uterine coils as Q. floridensis exhibits. However, as multivariate analyses are required to differentiate Q. kinsellai n. sp. from Q. quinqueserialis and Q. floridensis, we consider this species functionally cryptic. A larger sample size of Q. kinsellai n. sp. is required to determine whether this species has diagnostic morphological traits. The observed genetic divergence support the distinct species status of Q. kinsellai n. sp.
Life cycle investigation
Currently, only the adult stage of this species has been described. Specimens from two individual meadow voles were collected from Churchill, MB, Canada in 2016. In 2019, potential first intermediate host gastropod snails were collected and screened for trematode parasite stages in Churchill, MB, where the infected definitive hosts were collected. In total, 817 snails (Gyraulus spp., Lymnaea spp., Physella spp. Planorbdella trivolvis, Planorbula armigera, Promenetus exacuous) were screened and 36 snails were infected with redia that produced cercariae with the monostome morphotype. Of the infected snails, rediae from 27 snails were sequenced (CO1 MW853894-MW853912, and MW853916-853920; 28S MW934341-MW934355). None of the DNA sequences from rediae were genetically similar to the DNA sequences from the adult specimens of the novel species Q. kinsellai n. sp. (CO1: 7–11% different at 454 bp; 28S: 2% different at 885 bp). Instead, the CO1 sequences from the rediae were similar to adult and larval Q. quinqueserialis (1–3% different at 454 bp) sequences from the field-collected specimens in the other four sampling regions (MW853836-853845, MW853849-853854, MW853856-853893, MW853913-853915). Redia sequences that were not genetically identical to either Q. kinsellai or Q. quinqueserialis were queried using the blast algorithm on GenBank. All sequences with e-value of 0 and percent identity >95% were retained in an alignment in MEGA 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Additionally, the GenBank sequences included within the alignment had query coverage of at least 90%. The alignments were trimmed to compare regions where all sequences were of equal length. The trimmed alignments were used to calculate p-distance using MEGA 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Rediae from five snails (Gyraulus sp. and Physella gyrina) were genetically similar at the CO1 gene region to unidentified Notocotylus sp. sequences (CO1: 2–5% different at 446 bp, MW853916-MW853920). The 28S sequences from the rediae were genetically identical to Q. quinqueserialis adult and larval (0% different at 885 bp) sequences from the field-collected specimens in the other four sampling regions (MW934276-934287, MW934290-934340, MW934356-934365). In addition, a redia from an infected Gyraulus sp. snail was genetically similar to sequences from an unidentified Notocotylus sp. and Pseudocatatropis dvoryadkini (1% different at 785 bp), and were identical to a redia from a Lymnaea elodes snail field collected in Northwest Territories, Canada (MW934366). The snail hosts found to be infected with Q. quinqueserialis were consistent with those identified in other locations throughout Canada (Gagnon and Detwiler, Reference Gagnon and Detwiler2019). Vouchers of snail hosts from locations where Q. quinqueserialis was detected in Manitoba are deposited in the Manitoba Museum, Winnipeg, MB, Canada (Supplementary Table 4). Presently, the intermediate host(s) of Q. kinsellai n. sp. remains to be identified.
Discussion
This study is the first to incorporate genetic and morphological data focused on characterizing species in the genus Quinqueserialis. We hypothesized that cryptic species may be detected, and found a novel species that is functionally cryptic, Q. kinsellai n. sp. However, both PCA and LDA analyses demonstrated the presence of three morphologically distinct Quinqueserialis species. Two of the three species were also genetically distinct, while the remaining species, Q. floridensis, requires additional sampling as all known specimens cannot be sequenced (i.e. permanent slides or stained and stored in ethanol). Our study confirmed that Quinqueserialis spp. diversity was underestimated because a novel species, Q. kinsellai n. sp., was discovered. Importantly, if using morphology alone, this new species could be misclassified as Q. quinqueserialis due to morphological similarity and host-induced phenotypic plasticity in specimens from vole hosts of the latter species. Thus, we demonstrate that by integrating morphology, genetics and host use, we can make sense of inter- and intra-specific variation to clarify taxonomy and host use of groups with a long history of inadequate descriptions, poor specific diagnoses and extensive synonymy (Smith, Reference Smith1954; Kinsella, Reference Kinsella1971).
The multivariate analyses of 13 measurements of seven morphological traits revealed three clusters representing separate Quinqueserialis species. However, overlap in measurements of morphological trait ranges, and overlap among specimens of Q. quinqueserialis and Q. kinsellai n. sp. in the LDA support the conclusion that the two species are functionally cryptic. Cryptic species are hypothesized to occur more frequently among trematodes than any other helminth taxa (Pérez-Ponce de León and Poulin, Reference Pérez-Ponce de León and Poulin2017). However, in our study, the underestimated diversity of trematode parasites may be more attributed to limited geographic sampling and a lack of studies of Quinqueserialis that integrate molecular and morphological analyses. For Quinqueserialis, most studies used either morphology or genetics to characterize parasites (e.g. Kinsella, Reference Kinsella1971; Detwiler et al., Reference Detwiler, Zajac, Minchella and Belden2012). The latter study is typical of the ‘molecular prospecting’ approach and does not include in-depth morphological analyses (Blouin, Reference Blouin2002). In the case of Quinqueserialis, the size of the oral sucker and the width of the uterine coils were among the morphological traits that contributed to the discriminatory power of the LDA between species. However, the width of the uterine coils has been shown to be subject to host-induced phenotypic plasticity among Q. quinqueserialis specimens (Kinsella, Reference Kinsella1971). The intraspecific variation observed in this trait could explain the 4.7% misclassification rate of the LDA. Thus, while the three species form separate clusters they remain functionally cryptic, as 4.7% of Quinqueserialis specimens collected could be misidentified even when analysing morphology with multivariate analyses, leading to erroneous diversity estimates. The possibility of morphological misidentification demonstrates the importance of integrating morphology and genetic data, as gene sequencing informs species boundaries.
Our study increases the number of nominal species in North America from two to three. Before our study, four species of Quinqueserialis were described in North America, though only two were considered valid by Kinsella (Reference Kinsella1971). Our results genetically confirm the presence of one of the nominal species, Q. quinqueserialis, throughout North America, but also reveal a novel species occurring in meadow voles in Churchill, MB, Canada. While this species is genetically different from Q. quinqueserialis at both the nuclear 28S and mitochondrial CO1 gene regions, it is functionally morphologically cryptic. According to the criteria for the genetic species concept, these two parasite groups could be designated as different species as they are two distinct evolutionary units (Baker and Bradley, Reference Baker and Bradley2006). However, in the case of Q. floridensis, we only have evidence to support the morphological species concept because no genetic data could be obtained from the museum specimens. Quinqueserialis floridensis specimens can be distinguished from the other two species by its overall smaller body size, the distribution of the vitellaria and the wide, lateral extent of the uterine coils. Due to its distinguishing morphological characters, and its unique host association with the round-tailed muskrat, this species should remain valid until further sampling and genetic sequencing can be completed.
In conclusion, we found evidence for crypsis among Quinqueserialis species, and that diversity within this genus was underestimated likely due to restricted geographic sampling. A novel species, Q. kinsellai n. sp., genetically distinct from Q. quinqueserialis and morphologically distinct from Q. floridensis, was found at the periphery of the known range for this genus in North America. Our data suggests that delimiting species using solely morphology may be imprecise, as 4.7% of specimens in this genus were misclassified. Thus, this study demonstrates the importance of integrating many sources of evidence, such as morphology, genetics, host use and geographic distribution in determining trematode diversity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000792.
Acknowledgements
Field collections of Quinqueserialis throughout Canada and the US were done with the assistance of many people. We thank fur trappers John Christie, John Nelson, Ryan McLeod and Danny Gordon for donating muskrat carcasses that were fortunately infected with adult worms in Northwest Territories and Manitoba, Canada. We are grateful for the advice and consultation from the Gwich'in Renewable Resources Board and the Inuvik Hunters and Trappers Committee in Inuvik, NT, Canada. Collecting in this area would have been impossible without the guiding expertise of Hank Angasuk, Ryan McLeod and Scott Kasook. Lab space during field collection was provided by Robert Sorensen (Minnesota State University-Mankato), the Aurora Research Institute (Inuvik, NT, Canada) and the Churchill Northern Science Centre (Churchill, MB, Canada). We thank several undergraduate volunteers for their assistance with field collections as well as Asma Sultana, Scott Malotka, Emma Rempel, Katelyn Lasater and Olwyn Friesen for assisting with molecular work, necropsies and reviewing.
Author contribution
DKG and JTD conceived, designed the study and wrote the article. DKG conducted field collections, gathered data and performed statistical analyses. WCP and LKB conducted field collections, and provided critical revisions to the article. ELK created the line drawings for the novel species described herein and provided critical revisions to the article.
Financial support
This research was supported by NSERC (J.T.D., Discovery Grant), the University of Manitoba (J.T.D. Fieldwork Support Program), Macroecology of Infectious Disease Research Coordination Network Travel Grant funded by a joint NSF/NIH/USDA grant (DEB 1316223; W.C.P.), American Society of Parasitology Willis A. Reid Graduate Research Grant (W.C.P.), Texas A&M University Office of Graduate and Professional Studies Research Award (W.C.P.), Oakes-Riewe Environmental Research Award (D.K.G.), University of Manitoba Indigenous Master's Excellence Award (D.K.G.), University of Manitoba Master's Award for Indigenous Students (D.K.G.), Churchill Northern Studies Centre Northern Research Fund (W.C.P.; D.K.G.) and the Northern Scientific Training Program (D.K.G.).
Conflicts of interest
The authors declare there are no conflicts of interest.
Ethical standards
Small mammal trapping was performed to standard of approved Animal Use Protocol AC11347 submitted by DKG and JTD to the Animal Care Committee at the University of Manitoba and approved Animal Use Protocol 2016-0023 submitted by WCP to the Institutional Animal Care and Use Committee at Texas A&M University.