INTRODUCTION
At the species level debate over precise boundaries is critical, not least for practical reasons (Sites and Marshall, 2004). This is particularly so where an organism has become an established and important model for phenomena of more general medical and veterinary importance. Heligmosomoides polygyrus, a paradigm for chronic gastro-intestinal (GI) nematode infections, has been the subject of controversy over its specific identity since first isolated by Ehrenford in the 1950s. The parasite in culture originated from an abnormal infection of Peromyscus maniculatus (deer mouse) in North America (see Forrester and Nielson, 1973). The parasite was then established in laboratory mice, and disseminated throughout the world as a model system for teaching and research (Behnke et al. 1991). This system, based on a single isolation, has been widely used for screening potential anthelminthics (Burg and Stapley, 1989), and as a convenient, manipulable model for investigating the immunology of chronic intestinal nematode infections, such as those caused by hookworms in Man and trichostrongyloid nematodes in domestic livestock (Keymer and Slater, 1990; Scott and Tanguay, 1994). For more than 4 decades, it has been the paradigm for immunosuppression by GI nematodes as a strategy facilitating chronic infections in mammalian hosts (e.g. Behnke, 1987; Monroy and Enriquez, 1992). Parasite-induced modification of host behaviour has provided an additional fertile field of investigation (Behnke, 1987; Barnard et al. 1998; Kavaliers et al. 2003).
The worm from Peromyscus was virtually identical to an Old World nematode, Heligmosomoides polygyrus, a dominant helminth in the Eurasian field or wood mouse Apodemus sylvaticus (see Behnke et al. 1991 for a review of the early taxonomic history of this worm). The life-history traits and ecology of this Operational Taxonomic Unit (OTU) have been explored extensively (Gregory et al. 1992; Quinnell, 1992; Abu-Madi et al. 1998, 2000a; Behnke et al. 1999). Durette-Desset et al. (1972) considered the American worms (H. p. bakeri) to be a sister subspecies to the European OTU (H. p. polygyrus), a relationship which implies a potential for genetic interchange and continuity of biology between the two taxa. This view was upheld by Behnke et al. (1991) and the subspecies have since been elevated to species (Tenora and Barus, 2001; Tenora et al. 2003; evidence from the latter appeared to be largely derived from a personal communication from Behnke, summarizing the observations presented in the current paper, indicating that molecular evidence supported separate species status for these taxa). However, the most important question remains unanswered: Is the OTU H. polygyrus (polygyrus), the dominant endemic GI nematode from the European field mouse, conspecific with the OTU H. polygyrus (bakeri), the widely used laboratory model which was isolated on a single occasion from an American deer mouse? Durette-Desset et al. (1972) supported this notion, and proposed 2 testable hypotheses: (1) that H. p. bakeri and H. p. polygyrus are sister terminal clades and therefore each other's closest relative; and (2) that the two taxa have diverged in recent historical times since the imposition of allopatry when H. p. bakeri was introduced, in house mice, to the New World as a result of human activity during, or shortly after, the conquest some 500 years before present (BP). According to this model the morphological and biological differences between H. p. bakeri and H. p. polygyrus relate to the genetic bottlenecking experienced on introduction to the New World and subsequently when taken into culture. The implications of these hypotheses are considerable in that, if supported, the OTUs would be sufficiently closely related (subspecies or closer) that findings from one could be extrapolated to the other, allowing a synthesis of physiological, immunological and ecological observations of GI nematode biology. However, testing these hypotheses is not straightforward. Heligmosomatids lack informative morphological characters and identification relies heavily on host identity (Durette-Desset, 1971; Asakawa, 1988). Morphological distinction depends on the precise arrangement and number of cuticular ridges (arêtes), which comprise the synlophe (Durette-Desset, 1971; Durette-Desset et al. 1972) and asymmetry of the caudal bursa and arrangement of rays, especially the dorsal rays, complement the identification (Genov and Yanchev, 1981). The synlophe characters in particular are correlated with worm body size and possibly host identity (Durette-Desset et al. 1972) and the range of variation within, and between the taxa is impossible to evaluate, as all cultures of the laboratory model are derived from a single isolate.
In this study, we used a molecular approach to examine the relationship between the two OTUs of H. polygyrus, by analysing the internal transcribed spacers (ITS) of the ribosomal DNA (rDNA) and the mitochondrial cytochrome c oxidase I (COI) gene of H. polygyrus from the wood mouse A. sylvaticus and comparing it with a laboratory strain of this worm maintained in laboratory mice. Additional material of related Heligmosomoides and Heligmosomum species was also included. This dual molecular approach combines the advantages, and overcomes some of the disadvantages, of focussing on just nuclear or mitochondrial DNA (see Hillis and Dixon, 1991; Mayer and Soltis, 1999; Avise, 2000). It does not, however, clarify precise species boundaries without a statistically defined framework (Sites and Marshall, 2004). We have therefore assessed pairwise differences between populations and used analysis of molecular variance (AMOVA, Excoffier et al. 1992), to test the hypotheses formulated by Durette-Desset et al. (1972) concerning the origin of H. polygyrus bakeri for congruence with variation in the molecular data.
MATERIALS AND METHODS
Parasite collection and fixation
Specimens of Heligmosomoides polygyrus maintained in the laboratory mouse, Mus musculus, in Nottingham since 1976 and isolates from the wood mouse Apodemus sylvaticus from Britain (6 sites), the Channel Isles and Portugal were compared with samples of H. glareoli and Heligmosomum mixtum from Poland (see Table 1). Rodents caught in Sherman traps were killed with an overdose of ether. The gut from each host was removed onto a fine mesh gauze suspended in Hanks' balanced salt solution and placed at 37 °C for up to 1 h to allow worms to migrate into the saline. Individual nematodes were collected from the saline or in some cases removed directly from the intestinal mucosa and preserved in 90% ethanol. Nematodes were stored at −20 °C prior to DNA extraction and sequencing.

Sequencing of the ITS and COI
DNA was isolated from individual worms using a QIAamp® DNA Minikit (Qiagen) and eluted into 200 μl of AE buffer provided with the kit. Initially, for rDNA amplification, primers NCOF (5′ TTGAACCGGGTAAAGTCGT 3′; 3′ end of 18S) from Anderson (1995), and NC1F (5′ ACGTCTGGTCAGGGTTGTT 3′; 3′ end of 5·8S) and NC2R (5′ TTAGTTTCTTTTCCTCCGCT 3′; 5′ end of 28S) from Chilton et al. (1997) were used in 10 μl PCRs. These contained ca. 10 ng DNA, 50 mM KCl, 10 mM Tris-HCl, 1·5 mM MgCl2, 0·2 mM of each dNTP, 0·3 mM of each primer and 1 U Taq DNA polymerase (Invitrogen). The standard thermal profiles and cycle times used were 94 °C for 3 min followed by 94 °C for 30 s, 50 °C for 45 s, 72 °C for 1 min (35 cycles), and 72 °C for 10 min in a PE Applied Biosystems Gene Amp PCR System 9700 thermal cycler. From partial sequences of H. p. bakeri, new primers were designed from the 5′ end of the 5·8S gene, NC1Fb (5′ ACGTCTGGTTCAGGGTTGT 3′) and NC1R (5′ ACAACCCTGAACCAGACGTG 3′). The annealing temperature of the PCRs utilizing these new primers was increased to 60–67 °C (depending on taxa).
The original primers for amplifying the mtDNA cytochrome c oxidase subunit I gene (COI) were designed for Gyrodactylus turnbulli (Cable and Harris, unpublished observations); P9 (5′ (TG)(AT)T(GA) AT(TC)GG(TG)GGTTTTGGTAA 3′) and P10 (5′ TCATACCAAAAGCAGGTA 3′). PCRs commenced at 94 °C for 3 min, followed by 94 °C for 30 s, 50 °C for 30 s, 72 °C for 2 min (35 cycles), and 72 °C for 10 min in a Stratagene® Robocycler Gradient 96. Subsequently, primers LCO (5′ GGTCAACAAATCATAAAGATATTGG 3′) and HCO (5′ TAAACTTCAGGGTGACCAAAAAATCA 3′) (Folmer et al. 1994) were shown to yield more consistent PCR products in 25 μl PCRs with 0·1 mM of each primer, run at 95 °C for 1 min, followed by 95 °C for 1 min, 50 °C for 1 min, 72 °C for 1·5 min (35 cycles), and 72 °C for 7 min in a PE 9700. A negative control sample (without DNA) was included with each ITS and COI amplification.
PCR products were purified using a GeneClean III kit (Anachem) and sequenced using an ABI PRISM™ Dye Terminator Cycle Sequencing Ready Reaction Kit, following the manufacturer's guidelines, and run on an ABI 377 automatic sequencer. All samples included in the final analysis were sequenced at least twice using both forward and reverse primers. Sequences were aligned using SEQUENCHER V.3.1.2 (Gene Codes Corporation, Inc.) and verified by eye. Sequences representing the 5·8S, 18S and 28S genes were determined by BLAST searches. The alignment of COI sequences remained in frame for amino acid translation when compared to the sequence from Heligmosomoides polygyrus determined by Sukhdeo et al. (1997). All ITS and COI sequences were trimmed leaving the longest common alignment for all specimens examined. Alternative ITS alignments (322 bp, with nematode outgroups, and 262 bp and 265 bp without these outgroups) were also analysed, but as the resulting tree topologies were almost identical, just the data from a 321 bp alignment is referred to below. Nucleotide sequences for ITS and COI were deposited in GenBank (Accession nos. DQ408618 to DQ408626 and DQ408627 to DQ408635).
Phylogenetic analysis
Parsimony, Distance (neighbour-joining, NJ, and minimum evolution, ME) and Maximum Likelihood (ML) analyses were performed in PAUP* 4.0b 10 PPC (Swofford, 2003) separately on the ITS2 (321 bp) and COI (439 bp) data sets. ITS sequences were included from the related nematodes, Ohbayashinema erbaevei (GenBank AY332647 and AY333381, see Audebert et al. 2005) and Trichostrongylus colubriformis (GenBank S69220, see Hoste et al. 1993). The models of DNA evolution that best fitted the data based on log likelihood scores was calculated using MODELTEST 3.06 (Posada and Crandall, 1998), following the Akaike Information Criterion recommended by Posada and Buckley (2004). ML and ME analyses were performed using the parameter estimates under the best-fit models as K81uf+G and GTR+I+G, respectively, for the alignments of ITS2 and COI sequences. For each analysis, 1000 replicates of the random addition heuristic search option were performed with TBR branch swapping.
Analysis of COI genetic diversity and differentiation
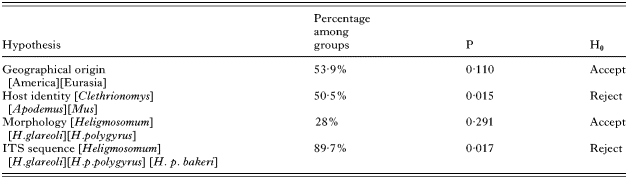
Population genetic structure was determined using minimum spanning network analysis for COI in the program ARLEQUIN 2.000 (Schneider et al. 2000). This program was also employed to calculate pairwise genetic distances for each haplotype, which were then plotted in EXCEL. Finally, ARLEQUIN was used to perform an analysis of molecular variance (AMOVA; Excoffier et al. 1992) to first test whether all clusters were sufficiently distinct to reject the hypothesis that they belong to a single evolutionary group. Subsequent hypotheses, that the individually identified populations are geographically, biologically and ecologically interchangeable, were also tested by AMOVA. Specifically, 4 hypotheses about the grouping of COI haplotypes (Table 2) were tested. (1) There are no significant differences between Eurasian heligmosomatid isolates (H. p. polygyrus, H. mixtum and H. glareoli) and North American isolates (H. p. bakeri). (2) There are no significant differences between heligmosomatids grouped according to host infected (i.e. the vole infecting H. glareoli and H. mixtum vs. the wood mouse infecting H. p. polygyrus vs. the laboratory mouse infecting H. p. bakeri). (3) There are no significant differences between heligmosomatids grouped according to conventionally recognized morphological taxonomy (i.e. H. glareoli vs. H. mixtumvs. H. polygyrus). (4) There are no significant differences between heligmosomatids grouped according to ribosomal DNA identity (i.e. H. glareoli vs. H. mixtumvs. H. p. polygyrus vs. H. p. bakeri). The significance of variation was assessed by 16000 permutation tests (Excoffier et al. 1992).
Table 2. Analysis of variance among heligmosomatid populations based on 439 bp of the mitochondrial cytochrome c oxidase subunit I gene (The percentage of total variance (derived from ΦCT) that is explained by the grouping is indicated as well as the significance levels associated with rejection of H0.)
RESULTS
The amplified rDNA fragment (3′end of 18S, ITS1, 5·8S, ITS2 and 5′end of 28S) varied from 987 bp for Heligmosomum mixtum to 1040 bp for the laboratory isolate of Heligmosomoides polygyrus. Direct sequencing of ITS was problematic with only partial sequences obtained for some samples (Table 1), perhaps reflecting high levels of intra-individual variation in isolates from wood mice. Individual worms contained polymorphisms at 7 positions in the 308 bp ITS2 sequence, but there was little consistent variation between sites, and for example only a single base distinguished the individual Aberdeen worm sequenced from all other worms from wood mice. The Nottingham laboratory strain was compared with the French INRA-Tours strain (Audebert et al. 2005) and the ITS2 of the two strains was identical. Overall, the laboratory strains differed from a consensus sequence for the wood mouse strain by 3·3%.
Part of the variation noted within H. p. polygyrus was due to length variation in a short microsatellite (GT3–4) sequence within ITS2. Most worms exhibited a (GT)3 repeat, but the Aberdeen worm, and 3 of 5 worms from Skegness sequenced contained a (GT)4 unit. Intra-individual ITS variation was also noted within H. glareoli (3/559 bp) but not amongst H. mixtum individuals. The degree of intra-specific variation in the ITS was not explored further as the level of discrimination revealed by mtDNA COI sequencing (see below) was more informative in addressing the aims of the current study.
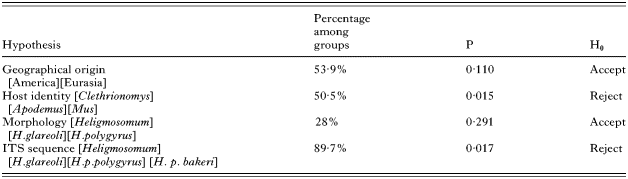
Almost all indels within the ITS region were due to the inclusion of H. mixtum and the outgroups within the data set. Comparison of 5 samples for which there were complete ITS1 and ITS2 sequences revealed greatest variation in ITS2 (22·9% including indels, 15·4% excluding indels), while for ITS1 the corresponding figures were 18·2% and 7·8%. The 5·8S sequence (152 bp) was identical for all worms and was therefore excluded from the analysis, as was the microsatellite repeat unit in the ITS2. Distance and parsimony trees based on ITS2 show complete congruence with all likelihood trees (Fig. 1), with H. p. polygyrus being more similar to H. p. bakeri than to H. glareoli. Support for the terminal clades H. p. polygyrus and H. p. bakeri was strong (over 80% in all trees). Although in a separate genus, Ohbayashinema erbaevei appears to cluster with Heligmosomoides glareoli (another vole-infecting species), suggesting that it is not sufficiently distantly related to be an effective outgroup. For this reason, Trichostrongylus colubriformis, belonging to a different family of nematodes, proved the most suitable outgroup.

Fig. 1. Maximum Likelihood analysis of heligmosomids based on Internal Transcribed Spacer 2 sequence data using likelihood parameters base frequencies=0·2609, 0·1783, 0·2216, 0·3392; rate matrix (1·0000, 8·8865, 2·9368, 2·9368, 8·8865, 1·0000); gamma distribution shape=1·1062; proportion of variable sites=0·4267.
Sequence data for 532–618 bp for the COI fragment were obtained, with product length being dependent upon the primer set used. Comparison of 599 bp of COI sequence between the Nottingham laboratory isolate of H. polygyrus bakeri (USA) and that maintained in Montreal by Sukhdeo et al. (1997; Accession no. U57034), revealed a single, synonymous base-pair substitution (T to C); a 99·8% similarity between the two laboratory isolates. However, a 439 bp fragment common to all isolates (from laboratory mice and Eurasian wood mice) contained 83 variable and 51 informative sites, with distinct haplotypes from each wood mouse population. Overall, the laboratory isolates differed by 8% (35/439 bp) from the 5 UK wood mouse isolates of H. polygyrus. Variation among these mouse isolates (excluding Guernsey) was less than 1·6%. This variation included single transitions between the Skegness, Aberdeen and Belfast isolates, and 2 transitions between the Staffordshire and Anglesey isolates. MtCOI polymorphisms were not shared between sites, and within sites the variation was consistent between individuals, except at Skegness, where 3 worms exhibited an A/G polymorphism within the population sample. The Guernsey isolate varied by more than 8% from both other wood mouse isolates and from the laboratory strains of Heligmosomoides.
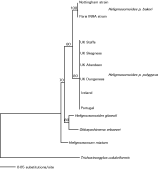
Phylogenetic trees constructed using COI parsimony and distance based trees gave an identical topology, with UK wood mouse isolates forming a single clade with H. glareoli, separated from the two H. p. bakeri isolates. Using Maximum Likelihood, the H. p. bakeri clade was not distinguishable from the H. p. polygyrus/H. glareoli clade, but bootstrap support for all branches was low. In all these analyses, the Guernsey isolate of H. p. polygyrus and H. mixtum provided sister groups for the H. p. polygyrus/H. glareoli/H. p. bakeri clade (data not shown). A minimum spanning network, however, revealed 4 distinct clusters of COI haplotypes, separated from each other by 39 substitutions (Fig. 2). The UK mainland wood mouse isolates of H. p. polygyrus formed a central cluster, closely associated with the Polish isolate of H. glareoli (separated by 9 substitutions). The Polish H. mixtum, the Guernsey wood mouse isolate and the laboratory cultures of H. p. polygyrus then formed equidistantly spaced outliers (Fig. 2). All analyses indicate that the variation in mtCOI sequence is explained by morphology (Heligmosomum vs. Heligmosomoides) and host genus (Clethrionomys vs. Apodemus), or with geographical origin (North America or Guernsey).

Fig. 2. Minimum spanning network for mitochondrial cytochrome c oxidase subunit I haplotypes of Heligmosomoides and Heligmosomum taxa. Heligmosomum mixtum, Heligmosomoides polygyrus bakeri and the Guernsey isolate of H. p. polygyrus are separated by 39 substitutions from the central cluster of mainland UK, H. p. polygyrus haplotypes (Staffordshire, Anglesey, Aberdeen, Belfast and 2 individuals from Skegness, A and B) and H. glareoli. Elsewhere base pair substitutions are represented by short black lines at right angles to the lines connecting haplotypes, see text for details.
Population variability: partitioning according to hypotheses of species relationships
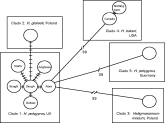
The structuring in COI haplotypes relative to conventional taxonomic groupings is shown in Fig. 3, based on pairwise genetic distances between each pair of haplotypes. Intra-OTU comparisons (H. p. bakeri or H. p. polygyrus) cluster with a pairwise genetic distance of 1–6, whereas inter-OTU genetic distances are generally greater than this. The smallest inter-OTU distances are those between H. p. polygyrus and H. glareoli, which cluster at 9 and 10 (see Fig. 3). Genetic distances between H. p. bakeri and the H. p. polygyrus isolates, and between all OTUs and H. mixtum, fall in the range 39–55. The only exceptions to this pattern are pairwise distances involving H. p. polygyrus from Guernsey, which cluster at a distance of ca. 40 units from other H. p. polygyrus isolates (Fig. 3).

Fig. 3. Pairwise genetic differences of mitochondrial cytochrome c oxidase subunit I sequences among heligmosomid Operational Taxonomic Units (see Table 1 for details of specimens). Pairwise intra-specific comparisons (among both UK mainland isolates of Heligmosomoides polygyrus polygyrus and among the 2 laboratory isolates of H. p. bakeri) indicated with closed bars (with the exception of those involving H. p. polygyrus samples from Guernsey being represented by shaded bars), and inter-specific comparisons (H. p. polygyrus vs. H. glareoli vs. H. p. bakeri) with open bars.
AMOVA (Excoffier et al. 1992) used to test 4 explicit hypotheses about the structuring of mitochondrial COI haplotypes (Table 2) indicated the following. (1) There was no significant partitioning of the mtCOI variance between Eurasian heligmosomatid isolates (H. p. polygyrus, H. mixtum and H. glareoli) and the North American H. p. bakeri (H0 accepted, P>0·11). (2) There is, however, significant partitioning of haplotype variance between heligmosomatids grouped according to host infected (H0 rejected, P<0·05). (3) There was no significant partitioning in haplotype variance between heligmosomatids grouped according to conventionally recognized morphological groupings, which treat H. p. polygyrus and H. p. bakeri as a single taxon (H0 accepted, P>0·29). (4) There was significant partitioning of haplotype variance between heligmosomatids grouped according to ribosomal DNA identity, which separates H. p. polygyrus and H. p. bakeri (H0 rejected, P<0·05).
Structuring in COI haplotypes was therefore significantly correlated with host identity (voles vs. wood mice vs. house mice) and with nematode OTU as established by ribosomal ITS sequence identity (H. mixtum vs. H. glareolivs. H. polygyrus polygyrus vs. H. polygyrus bakeri). It was not correlated with geographical origin or with conventional taxonomic assignation (H. mixtum vs. H. glareolivs. H. polygyrus).
DISCUSSION
The present findings suggest that Heligmosomoides polygyrus polygyrus and H. p. bakeri are terminal sister clades. Analysis of ribosomal ITS2 sequences showed that these taxa cluster together, distinct from a second grouping including H. glareoli, Heligmosomum mixtum and the vole heligmosomatid Ohbayashinema erbaevei. Tree topology was consistent in linking all isolates of H. p. polygyrus with laboratory isolates of H. p. bakeri, but the relationship between the two OTUs was quite distant. The separate terminal clades have significant bootstrap support, and the ITS2 sequences of the two taxa differed by 3%. With the more rapidly evolving COI sequences, a cluster of terminal clades corresponding to the UK isolates of H. p. polygyrus were clearly separated by 39 mutational steps from the laboratory isolates.
Species status in nematodes is often simply inferred by molecular distance between isolates. Thus, Blouin (2002) stressed the value of mitochondrial loci in highlighting large molecular distances between nematode species. This assumes a greater interspecific genetic distance between taxa than the intraspecific distances, a condition that appears to hold in the present case, in all pairwise comparisons of genetic distance except those including the Guernsey isolate of H. p. polygyrus. The cryptic Haemonchus placei, for example, was differentiated by 15% sequence variation from H. contortus at the mitochondrial ND4 locus (Blouin et al. 1997), while Zhu et al. (2002) recorded differences in ITS sequence of approximately 2% amongst the cryptic nematode species of the Pseudoterranova decipiens complex. In both cases, as in the H. polygyrus complex, species status is based on little more than small morphological differences, differences in host identity and sequence variation. This assumption that molecular differences match differences in specific status also underlies the work of Eyualem and Blaxter (2003), who showed that ribosomal DNA sequences matched closely with species of Panagrolaimus as indicated by cross-breeding experiments but not by morphology. Nevertheless, simple sequence differences are not by themselves sufficient to establish species identity. Adams (1998) in particular criticized the application of typological and biological species concepts to nematodes, recommending instead phylogeny reconstruction based on the Phylogenetic Species Concept. Blouin (2002) reflected this, noting that molecular distances should be only the first step in identifying cryptic nematode species, and that full recognition of their status requires considerable further work. ITS sequences, which change by concerted evolution, are generally homogenous within a species (Gasser et al. 1996), although exceptions within the nematodes are known. Hypodontus macropi, from kangaroos, for example, showed up to 5% intra-specific variation at ITS2, due primarily to slippage at dinucleotide repeats (Gasser et al. 2001), while the plant parasite Meloidogyne had a wide diversity of ITS sequence clusters, related to its evolutionary history of divergence and interspecific hybridization (Hugall et al. 1999). Thus, our analysis, with sequence differences of this magnitude in both ITS and COI between the two OTUs of H. polygyrus, suggests, but does not confirm, their status as independent species.
The AMOVA (Excoffier et al. 1992) more rigorously establishes whether variation in molecular sequences is partitioned along discontinuities observed with other approaches, reflecting differences between valid species following independent evolutionary trajectories. In the present case, it is clear from ITS2 and COI datasets that H. p. polygyrus and H. p. bakeri represent significantly different, if closely related, clades. For these clades to be elevated to species status, there should be no evidence of recent genetic interchange between them. If a H. p. bakeri COI haplotype or ITS sequence was recovered from a nematode from a European wood mouse, for example, support for the specific status of the two forms would be greatly weakened. In fact, there is congruence between molecular sequences and the biological taxa they represent; the group of COI haplotypes collected from European wood mouse parasites clustered closely together, separated by 39 mutational steps from the two COI isolates from the laboratory isolates. There was no significant association between different haplotypes and geographical origin, but there was a significant relationship between haplotypes and host identity (wood mouse vs. laboratory mouse vs. voles), and between haplotypes and the OTUs recognized on the basis of rDNA sequences (Heligmosomum mixtum vs. Heligmosomoides glareolivs. H. polygyrus polygyrus vs. H. p. bakeri). These associations suggest strongly that H. p. bakeri and H. p. polygyrus, with their differences in ITS and COI sequences, host identity, ecological preference and geographical origin do indeed represent distinct species.
Previous workers had suspected that the difference between laboratory isolates of H. polygyrus and those from wood mice might be considerable (Durette-Desset et al. 1972; Durette-Desset, 1985; Asakawa, 1988). In particular, Quinnell et al. (1991) reported that H. p. polygyrus from wild wood mice was unable to mature in laboratory mice, since very few larvae survived the tissue phase of infection. Some H. p. bakeri completed their development to the adult stage in wood mice, but most were rejected within 2–3 weeks unless the animals were treated with the immunosuppressive drug cortisone. Also, Abu-Madi et al. (1994) found differences between the two OTUs in protein synthesis and Abu-Madi et al. (2000b) showed, using RFLP, that the taxa could be distinguished by 8 endonuclease digestions of the ITS region, a finding consistent with the 3% sequence divergence at this locus.
The question remains as to whether the two taxa could be recently diverged sister species. Peromyscus maniculatus is not thought to be the natural host for the laboratory form (Forrester and Nielson, 1973), and the earliest records of the worm in America were from the mouse Mus musculus (e.g., Spurlock, 1943). In Europe an additional subspecies, H. p. corsicus was identified from the house mouse in Corsica (Durette-Desset et al. 1972), raising the possibility that H. polygyrus is a Holarctic taxon, spread to the New World with commensal house mice, from which it has spread to infect Nearctic rodents, undergoing subspeciation as a result (Durette-Desset, 1985). According to this hypothesis, H. polygyrus spread first into Mus and Reithrodonontomys, then into Phenacomys, both subspecific radiations occurring in the 300 years since mice were first introduced into the New World (Durette-Desset, 1985). However, it would be most surprising if H. p. bakeri had undergone sequence divergence of 3% in the ITS and 8% in the COI since putative introduction to the New World via trans-Atlantic shipping. Nieberding et al. (2004a) estimated a divergence rate at the mitochondrial cyt b gene of approximately 2·5% per million years, suggesting that the split between H. p. polygyrus and H. p. bakeri is at least 3 million years old. Based on our present findings, the hypothesis of Durette-Desset (1985) that H. p. bakeri reached the Nearctic with imported house mice from Europe cannot be rejected. However, if this did occur, the extent of sequence divergence indicates that this parasite was already distinct from H. p. polygyrus before introduction. Alternatively, Asakawa (1988) has suggested that the polygyrus strain spread to North America from the Palaearctic via Asia in murid hosts, subspeciating in the process. Based on morphology, H. polygyrus bakeri has been recorded in Japanese Mus musculus while an apparently closely related species, H. neopolygyrus, parasitizes Apodemus peninsulae (see Asakawa, 1991; Asakawa and Ohbayashi, 1986). Since other species of the genus Heligmosomoides probably spread to North America via the Bering Strait in microtids, some of which may have secondarily adapted to non-microtid hosts (Asakawa, 1988), H. p. bakeri is more likely to be a member of these lineages, rather than a close relative of the other European H. polygyrus subspecies. Asakawa's (1988) hypothesis is also more consistent with the degree of divergence in ITS and COI sequences between the two nematodes, and suggests that the sister taxa of H. p. bakeri are probably to be found in North American or Eastern Eurasian rodents.
Although AMOVA supports the specific identity of the laboratory isolate, it raises substantial doubts about the status of H. p. polygyrus. The single COI haplotype from H. glareoli is only 9 mutational substitutions from the cluster of H. p. polygyrus haplotypes, while a haplotype from wood mouse parasites from Guernsey is almost 40 substitutions distant from mainland UK forms. Nieberding et al. (2004b) in their survey of mitochondrial cyt b variation in the wood mouse OTU, also recorded distances between haplotype clusters in excess of 30 steps. They interpreted this as evidence of deep and ancient structuring, a consequence of evolution in separate glacial refugia, dated to 1–2 million years ago (MYA) (Nieberding et al. 2004b). The lack of correlation between the loci sequenced (cyt b by Nieberding et al. 2004a,b; COI and ITS in the present study) makes direct comparison difficult. However, Nieberding et al. (2004b) included Irish specimens in their work, and comparison with our Belfast specimens suggests that all UK mainland populations (excluding the Guernsey isolate) would cluster in the Danish/Irish clade 5 of Nieberding et al. (2004b). This perhaps is not surprising, as it is generally accepted (Berry, 1985) that Apodemus sylvaticus reached Ireland by human intervention, probably in the medieval period, either from mainland Britain or from one of the Scandinavian kingdoms. Nieberding et al. (2004b) did not include rDNA sequences for their Heligmosomoides samples, making it difficult to be sure that all of their clades represent an interbreeding, panmictic population. However, we sequenced the ITS from H. polygyrus from Portugal and found it to be identical to that from worms from the UK, which supports the proposal that all H. polygyrus isolates from wood mice are drawn from a single panmictic taxon. We nevertheless suspect that H. p. polygyrus may be paraphyletic or polyphyletic, and may include strains from different hosts and geographical areas which have followed independent evolutionary trajectories in the past but have since coalesced with the amelioration of the periglacial environment following the Last Glacial Maximum. This may be particularly so in southern Europe, where the status of H. p. corsicus from house mice remains unclear. Clearly, the phylogenetic history of H. p. bakeri is distinct from that of H. p. polygyrus, and we therefore suggest that the laboratory OTU of Heligmosomoides is raised to full species rank. However, because it is currently impossible to recreate a single phylogenetic history for H. polygyrus polygyrus in the sense of Adams (1998), the status of this taxon requires further investigation.
The question remains which name to use for the heligmosomatid from laboratory mice. The simplest solution appears to be to raise the current subspecies to specific rank as suggested by Tenora and Barus (2001) and Tenora et al. (2003), based in part on the molecular evidence presented in the current paper. According to this interpretation, the laboratory strain then becomes Heligmosomoides bakeri (Durette-Desset et al. 1972; Tenora and Barus, 2001), while worms from Apodemus sylvaticus remain H. polygyrus (Dujardin, 1845). The type description is then that of Durette Desset et al. (1972), and type material (paratypes) is deposited in the United States National Museum Helminthological Collection (USNM tube No. 72184). We appreciate that this proposal does not favour taxonomic stability, as the majority of published papers concern the laboratory model, now to be called H. bakeri. However, this revision is considered to be justified as a name change drastically alters our perception of the host range and degree of specialization of these nematodes. There is some evidence that H. bakeri produces immunosuppressive molecules that facilitate long-term survival in the laboratory mouse (Pritchard et al. 1994). A better understanding of the taxonomy and evolution of these worms will lead to a more directed hypothesis-driven approach to analysing the role of immunosuppressive molecules in this genus, potentially an important factor in the speciation of heligmosomatid nematodes.
This work was funded by a Natural Environment Research Council (NERC), UK, Advanced Research Fellowship (NER/J/S/2002/00706) to J.C. We are grateful to Dr Les Chappell and Professor Ian Montgomery for the provision of worms from wood mice caught in Aberdeen and Belfast respectively, and to Professor Edward Sinski, Dr Anna Bajer, and the staff of the University of Warsaw field station at Urwitalt for their hospitality. Finally, we thank Mike Bruford and two anonymous reviewers for their helpful comments on the manuscript.