Introduction
Recent efforts to better understand the diversity of the parasites of freshwater fishes in North America have revealed considerable gaps in our knowledge and the existence of taxonomic problems in many groups, including caryophyllidean tapeworms (Scholz and Choudhury, Reference Scholz and Choudhury2014; Scholz and Oros, Reference Scholz, Oros, Caira and Jensen2017). These tapeworms are a dominant group of endoparasitic helminths in catostomid fishes (Cypriniformes: Catostomidae), with about 60 described species in 22 genera (Scholz and Kuchta, Reference Scholz and Kuchta2017; Kuchta et al., Reference Kuchta, Řehulková, Francová, Scholz and Šimková2020).
To provide a robust taxonomic baseline for future ecological, evolutionary and biogeographical studies, individual genera of North American caryophyllideans are being revised, based on a critical examination of type material and newly collected and properly processed specimens, including hologenophores, from North American catostomids (Scholz et al., Reference Scholz, Oros, Choudhury, Brabec and Waeschenbach2015; Oros et al., Reference Oros, Brabec, Kuchta, Choudhury and Scholz2016, Reference Oros, Uhrovič and Scholz2018). One of the more species-rich genera of caryophyllideans is Biacetabulum Hunter, 1927 with 10 recognised species (Scholz and Oros, Reference Scholz, Oros, Caira and Jensen2017). This genus was erected by Hunter (Reference Hunter1927) based on B. infrequens from the silver redhorse, Moxostoma anisurum (Rafinesque), from the Rock River in Illinois, USA, and at the time, as its type and only species.
None of the earlier molecular phylogenetic studies included species of Biacetabulum (see, e.g., Olson et al., Reference Olson, Scholz, Poddubnaya and Littlewood2008; Brabec et al., Reference Brabec, Scholz, Králová-Hromadová, Bazsalovicsová and Olson2012; Scholz et al., Reference Scholz, Oros, Choudhury, Brabec and Waeschenbach2015). More recently, Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021) provided sequences of two nuclear and one mitochondrial gene for several taxa of Biacetabulum, but did not identify most of them to species level or did so only tentatively. Therefore, a taxonomic revision of Biacetabulum is pending because many species are known only from original, often very brief and insufficient, descriptions (Hunter, Reference Hunter1927, Reference Hunter1929). Species composition of the genus, its monophyly and host associations of its species should also be critically assessed using molecular data.
Over the last decade, specimens of caryophyllidean tapeworms fixed using a standardized method (see Oros et al., Reference Oros, Scholz, Hanzelová and Mackiewicz2010) were freshly collected from a variety of fish hosts, including suckers (Catostomidae), in Canada and the USA. This new material has enabled us to critically examine the validity of several genera of North American caryophyllideans (Scholz et al., Reference Scholz, Oros, Choudhury, Brabec and Waeschenbach2015; Oros et al., Reference Oros, Brabec, Kuchta, Choudhury and Scholz2016, Reference Oros, Uhrovič and Scholz2018). Furthermore, molecular data were generated for specimens of Biacetabulum from different species of redhorses (Moxostoma spp.) and spotted sucker (Minytrema melanops (Rafinesque)). These data revealed the existence of three genetically distinct, morphologically similar, and closely related lineages of tapeworms with a conspicuously long neck. In addition, examination of specimens from Hypentelium nigricans (Lesueur), has shown they also represent a new species, although no ethanol-preserved material was available for molecular analyses. These four new species are described to document this case of diversification of similar and closely related tapeworms in different, often congeneric fish hosts.
Materials and methods
Molecular study
The following sequence data (28S rDNA) were used to assess phylogenetic relationships of the three newly described species of the long-necked Biacetabulum-species complex: (i) two unpublished 28S rRNA gene sequences of specimens from Moxostoma collapsum (Cope) (US 274b), South Carolina, and M. melanops (US 209d), Mississippi; the sequences were obtained by Alec Perkins (USA) during his internship at the Institute of Parasitology (Czech Republic) in 2016, following the protocol described by Brabec et al. (Reference Brabec, Scholz, Králová-Hromadová, Bazsalovicsová and Olson2012); and (ii) eight new 28S rDNA sequences (see Table 1) as follows: Genomic DNA was isolated using the E.Z.N.A. Tissue DNA Kit (Omega Bio-tek, Inc., Norcross, USA) following the manufacturer's instructions. A 1350 nucleotide (nt) long fragment of the 28S rRNA gene (D1–D3 regions) was amplified by polymerase chain reaction (PCR) following the protocol described by Brabec et al. (Reference Brabec, Scholz, Králová-Hromadová, Bazsalovicsová and Olson2012). PCR amplicons were purified using exonuclease I and shrimp alkaline phosphatase enzymes (Werle et al., Reference Werle, Schneider, Volker and Fiehn1994) and sequenced from both strands using the PCR primers and additional internal sequencing primer 300F (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000). Sequences were assembled and edited using Geneious version 11 (Biomatters, Auckland, New Zealand).
Table 1. Host, geographical origin and GenBank accession data for taxa included in the phylogenetic analyses

Novel 28S rDNA sequences of species of the Biacetabulum-species complex was aligned with those previously generated from species of this genus and of the genus Archigetes Leuckart, 1878 (Table 1) using MUSCLE (Edgar, Reference Edgar2004) as implemented in Geneious vs 11. The alignment was trimmed to match the shortest sequence and ambiguously aligned positions were manually excluded. The final alignment that was used for the analyses (after trimming and excluding gaps) was 1326 nucleotides long. A sequence of Hunterella nodulosa Mackiewicz et McCrae, 1962 (JQ034127) was selected as the outgroup based on the results of the phylogenetic analyses by Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Phylogenetic relationships were estimated using maximum likelihood (ML) and Bayesian inference (BI) methods. The best-fitting model for the analyses was estimated with jModelTest 2.1.2 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) and was found to be the Hasegawa–Kishino–Yano model + invariant sites (HKY + I). BI analysis was conducted using MrBayes software (ver. 3.2.3) (Ronquist et al., Reference Ronquist, Teslenko, Van Der Mark, Ayres, Darling, Höhna and Huelsenbeck2012). Markov chain Monte Carlo (MCMC) chains were run for 3 000 000 generations, log-likelihood scores were plotted, and only the final 75% of trees were used to produce the consensus tree. The ‘burn-in' period was determined as the point when the average standard deviation of split frequency values reached <0.01. ML analysis was conducted using PhyML version 3.0 (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010) run on the ATGC bioinformatics platform (http://www.atgc-montpellier.fr/) with a nonparametric bootstrap validation based on 100 pseudoreplicates. FigTree ver. 1.4 software (Rambaut, Reference Rambaut2012) was used to visualise the trees. Genetic distances (uncorrected p-distance) were calculated in MEGA ver. 6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
Morphological study
The present study is based on the evaluation of newly collected specimens of the long-necked Biacetabulum-species complex from four species of redhorses (Moxostoma spp.), spotted sucker (M. melanops) and northern hog sucker (H. nigricans) (see the list of material studied below and Table 2). Tapeworms collected by the present authors were obtained from the intestine of freshly killed fish, rinsed with saline and fixed in hot, near-boiling, 4% formaldehyde (see Appendix in Oros et al., Reference Oros, Scholz, Hanzelová and Mackiewicz2010). For light microscopy, specimens were stained in Mayer's carmine, dehydrated in an ascending ethanol series, cleared with eugenol, and mounted in Canada balsam. Line drawings were made using a Leica DM 5000 Blight microscope (Leica Microsystems, Wetzlar, Germany). For histological studies, the material was embedded in paraplast and 12–15 μm sections were taken and stained with Weigert hematoxylin.
Table 2. Comparative measurements of tapeworms of the long-necked Biacetabulum-species complex (Caryophyllidea)

For scanning electron microscopy (SEM), samples (scoleces and the posterior part of the body with gonopores) were dehydrated in ethanol and amylacetate series, infiltrated with HMDS (hexamethyldisilazane), following which the HMDS was allowed to evaporate off the specimens. Subsequently, samples were mounted on stubs, sputter-coated with gold (20–25 nm) and observed using a JEOL JSM 6510LA scanning electron microscope (JEOL Ltd., Tokyo, Japan). The terminology of microtriches follows Chervy (Reference Chervy2009) and that of scoleces follows Mackiewicz (Reference Mackiewicz, Khalil, Jones and Bray1994) and Oros et al. (Reference Oros, Uhrovič, Choudhury, Mackiewicz and Scholz2020). In morphological descriptions, measurements are in micrometres (μm) unless otherwise stated.
Scientific and common names of fish follow Froese and Pauly (Reference Froese and Pauly2020). The newly collected material is deposited in the Harold W. Manter Laboratory, University of Nebraska, Lincoln, USA (HWML), Helminthological Collection of the Institute of Parasitology of the Biology Centre of the Czech Academy of Sciences in České Budějovice, Czech Republic (IPCAS), and the Smithsonian National Museum of Natural History, Washington, D.C., USA (USNM).
Results
Phylogeny
Both BI and ML analyses yielded trees with identical, generally strongly supported topologies (Fig. 1). Ten novel sequences of Biacetabulum spp. and three sequences retrieved from GenBank formed a strongly supported monophyletic group comprising the long-necked Biacetabulum-species complex. Within this clade, three sister species with their own unique host associations were recognised (Fig. 1).

Fig. 1. Bayesian phylogram of 28S rDNA for Biacetabulum spp. Nodal support from BI and ML bootstrap support are indicated as BI/ML; values <0.90 (BI) and <70 (ML) are not shown. The scale bar indicates the expected number of substitutions per site. The newly generated sequences are highlighted in bold. The dotted rectangle indicates the clade of the long-necked Biacetabulum-species complex. Labels along the vertical bars reflect the species within this complex. FL, Florida; MB, Manitoba; MS, Mississippi; SC, South Carolina; WI, Wisconsin; WV, West Virginia.
Sequences of three novel isolates (US 272b, 272c, and 274b) and two previously published isolates (US 274c/PBI-469 and 275c/PBI-420) collected from M. collapsum in South Carolina, USA formed a strongly supported subclade. Sequence divergence among these isolates (intraspecific divergence) was 0–0.1% (0–1 nt).
Sequences of five novel isolates from M. anisurum (CAN 38c and 38d) and M. erythrurum (Rafinesque) (CAN 36a and 36b) in Manitoba, Canada, and from M. erythrurum (US 979/WV_FR19_641-2) in West Virginia, USA formed another strongly supported subclade. Sequence divergence among individual isolates in this subclade was 0–0.2% (0–2 nt).
Sequences of remaining isolates, two novel (US 209d and US 839a) and one previously published (US 233b/PBI-416), collected from M. melanops in Mississippi, USA, formed the third subclade, albeit without strong support. These isolates represented a third species within the long-necked Biacetabulum-species complex. Sequence divergence among individual isolates was 0–0.1% (0–1 nt).
The interspecific divergence among the three new species ranged between 0.3 and 1.0% (4–13 nt). Phylogenetic estimates based on sequence data also indicated notable host specificity – every species of Biacetabulum is restricted to one or two congeneric hosts (Fig. 1).
Morphological study
All newly collected worms of the long-necked Biacetabulum-species complex from different species of redhorses, spotted sucker, and northern hog sucker are phenotypically rather uniform, being characterised by having a long body (maximum width represents only 2–3% of total body length, see Table 3) with a long, slender neck (its length represents ≥30% of total body length, see Table 3), and a globular scolex that is much wider than the neck. The scolex bears a central, large, deep acetabulum-like loculus on the ventral and dorsal sides, shallow but distinct lateral loculi, and a distinct apical disc. All have numerous testes, a cirrus-sac that is situated between the anterior arms of ovarian wings, and a uterus that extends anteriorly well beyond the thick-walled cirrus-sac (Figs 2–8).

Fig. 2. Line drawings of Biacetabulum isaureae n. sp. from Moxostoma collapsum, South Carolina, USA (US 275d, IPCAS C-591/3). (A) Total view, (B) anterior part with scolex, and (C) posterior part, ventral view. cs, cirrus sac; esv, external seminal vesicle; fgp, female genital pore; mg, Mehlis' glands; mgp, male genital pore; ov, ovary; rs, seminal receptacle; te, testes; ut, uterus; vd, vas deferens; vf, vitelline follicles; vtd, vitelline duct.

Fig. 3. Histological sections of Biacetabulum isaureae n. sp. from Moxostoma collapsum (US 275d), South Carolina, USA. (A) Sagittal section of the posterior part of body, (B, C) longitudinal sections of scolex, and (D) sagittal section of genital pores; E, cross-section of middle portion of body. cs, cirrus-sac; esv, external seminal vesicle; fgp, female genital pore; ilm, internal longitudinal muscles; mg, Mehlis' glands; mgp, male genital pore; orc, osmoregulatory canals; ov, ovary; rs, seminal receptacle; te, testes; ut, uterus; vd, vas deferens; vf, vitelline follicles.

Fig. 4. Scanning electron micrographs of Biacetabulum isaureae n. sp. from Moxostoma collapsum, South Carolina, USA (US 272b, US 275d, IPCAS C-591/3). (A) Anterior part with scolex, (B) posterior part with genital pores, (C) scolex, (D) detail of common genital atrium, (E) microtriches surrounding the common genital atrium, and (F) microtriches on the body. Small letters in A and B indicate position of E and F.
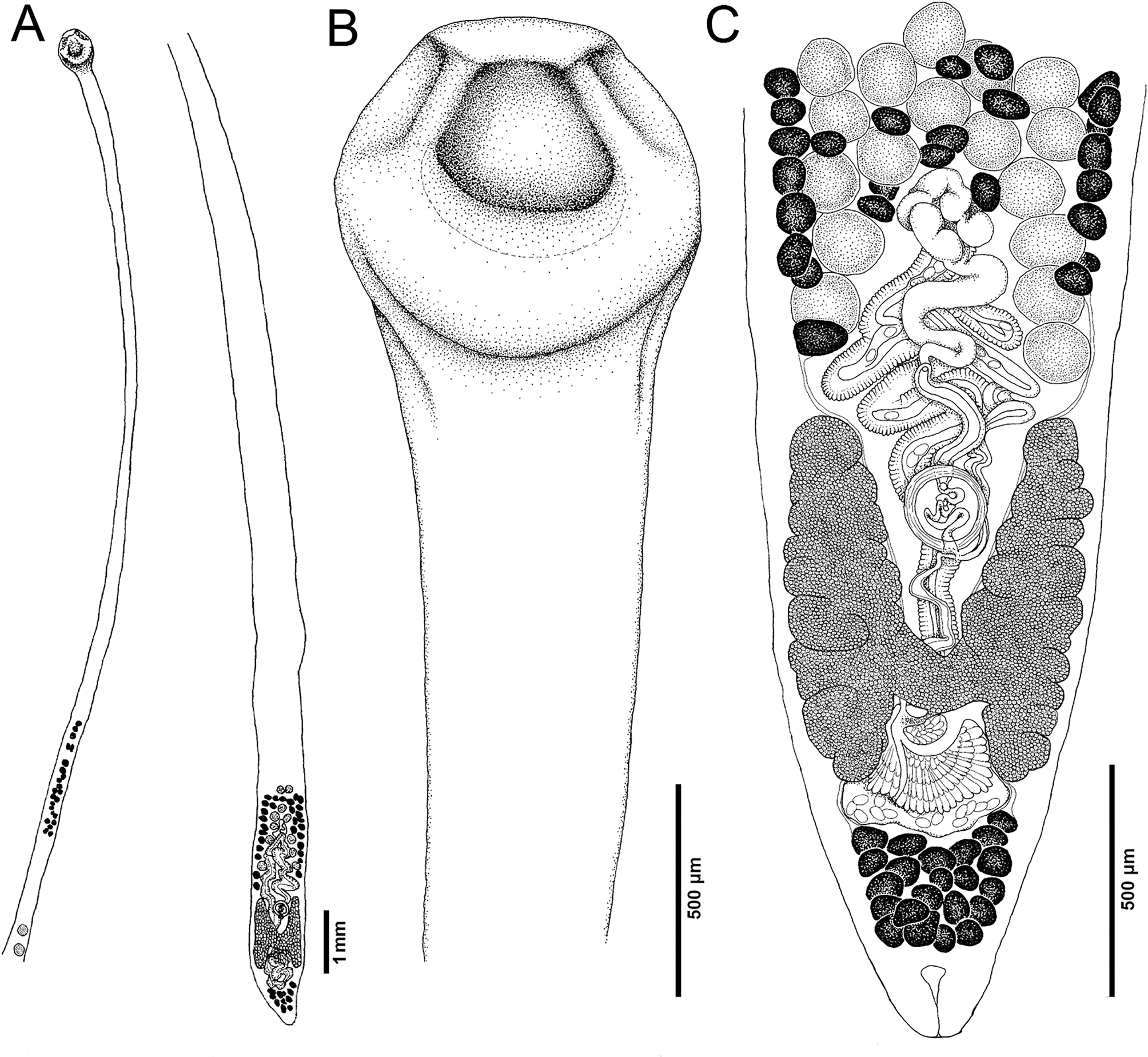
Fig. 5. Line drawings of Biacetabulum longicollum n. sp. from Moxostoma anisurum, Red River, St. Vital Park, Manitoba, Canada (CA 38), and Assiniboine River, Manitoba, Canada (SR-I-96M). (A) Total view, (B) anterior part with scolex, and (C) posterior part, ventral view.

Fig. 6. Scanning electron micrographs of Biacetabulum longicollum n. sp. from Moxostoma anisurum, Red River, Manitoba, Canada (CA 38). (A) Anterior part with scolex, (B) posterior part with genital pores, (C) scolex, (D) detail of common genital atrium, and (E) microtriches surrounding the common genital atrium. Small letters in B indicate position of E and F.

Fig. 7. Line drawings of Biacetabulum overstreeti n. sp. from Minytrema melanops, Mississippi, USA (US 209d, US 233b, US 839a). (A) Total view, (B) anterior part with scolex, and (C) posterior part, ventral view.

Fig. 8. Histological sections of Biacetabulum overstreeti n. sp. from Minytrema melanops, Mississippi, USA (US 209d, US 224a). (A) Sagittal section of the posterior part of body, (B, C) longitudinal section of scolex, (D) sagittal section of genital pores, and (E) cross-section of the middle portion of body.
Table 3. Comparative measurements of body proportion and neck length across the genus Biacetabulum (new species emboldened).

Examination of stained specimens (whole mounts, hologenophores or paragenophores in most cases) revealed some slight, but consistent differences between specimens of individual clades (newly described species) in the following morphological characteristics (see Table 2 for comparative measurements): (i) number of postovarian vitelline follicles; (ii) posterior extent of preovarian vitelline follicles; (iii) relative width of the cirrus sac (ratio of the cirrus-sac width to the width of the body at its level); (iv) position of the cirrus sac in relation to the anterior wings of the ovary; (v) posterior extent of the testes; (vi) relative size of the testes; and (vii) relative extent of the uterus.
Based on molecular and morphological differences, they are considered to represent four distinct species of the long-necked Biacetabulum-species complex, each with its own unique host association. These new species are described below.
1. Biacetabulum isaureae n. sp. Figs 2–4
Material studied: 15 stained specimens (whole mounts), 2 specimens for scanning electron microscopy (SEM) and histological sections of two worms, all from notch lip redhorse, Moxostoma collapsum (US 272b, 274b, 275d; IPCAS C-591/3), Congaree River at Columbia, South Carolina, USA, collected by R. Kuchta and M. Oros on 29 March 2012.
Description (based on whole mounts of 15 specimens; for measurements – see Table 2): Caryophyllidea, Capingentidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate and slender, with maximum width at level of anterior vas deferens or ovary, tapering towards neck region (Fig. 2A); covered with acicular fillitriches (filiform microtriches) (Fig. 4F).
Scolex spherical, wider than neck, with one pair of large acetabulum-like loculi, two pairs of shallow lateral loculi, and slightly convex apical disc (Figs 2B, 3B, C, 4A, C). Neck narrow, very long. Internal and external longitudinal muscles well-developed. Osmoregulatory canals narrow, in cortex (Fig. 3E).
Testes medullary, subspherical to widely oval (Fig. 3E). Anterior-most testes begin posterior to anterior-most vitelline follicles. Posteriorly, testes reach anterior-most loops of uterus, slightly anterior to posterior-most preovarian vitelline follicles (Fig. 2C). Cirrus-sac subspherical, thick walled. External seminal vesicle elongate, thick walled. Male and female genital pores open to distinct genital atrium (corresponding to Fig. 5.24 of Mackiewicz, Reference Mackiewicz, Khalil, Jones and Bray1994; Figs 2C, 3A, D, 4B, D).
Ovary compact (non-follicular), H-shaped, with deep lobes (Fig. 2C). Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus, joins with uterus to form uterovaginal canal, opens separately from male gonopore into distinct genital atrium (Figs 3A, D, 4D). Preovarian vitelline follicles numerous, in medullary parenchyma (Fig. 3E). Preovarian vitelline follicles reach posteriorly slightly anterior to external seminal vesicle, not reaching close to ovarian wings (Fig. 2C). Postovarian vitelline follicles relatively few (Fig. 2C; Table 2).
Uterus forms several loops, extending markedly anterior to cirrus-sac (Figs 2C, 3A); uterine glands well developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without fully formed oncosphere.
Taxonomic summary
Type host: Notchlip redhorse, Moxostoma collapsum (Cypriniformes: Catostomidae).
Site of infection: Usually in the anterior part of the intestine, firmly attached with the scolex, but not buried deeply into the intestinal mucosa.
Type locality: Congaree River at Columbia (33°49′48.9″N, 80°54′42.8″W), South Carolina, USA.
Distribution: USA (South Carolina).
Type material: Holotype (IPCAS C-889); two paratypes (IPCAS C-889); two paratypes (HWML 216478, 216479); two paratypes (USNM 1655788, 1655789).
Representative DNA sequences: Sequences of five individuals from M. collapsum (US 272b, 272c, 274b, 274c, 275c) in South Carolina were submitted to GenBank MZ031042–MZ031044.
Etymology: The species name honours Isaure de Buron, South Carolina, for her significant contribution to fish parasitology and help with sampling fishes in South Carolina in 2012.
Differential diagnosis: The new species belongs to one of three closely related, but distinct lineages of the long-necked Biacetabulum-species complex. Like other species of this complex described below, the new species is typified by a long body (up to 46 mm long) with a long (9–17 mm in length), slender neck, a globular scolex that is much wider than the neck region, bearing a large, deep acetabulum-like loculus on the ventral and dorsal sides, numerous testes, a cirrus sac situated between the anterior arms of ovarian lobes, and a uterus with loops extending far anterior to the thick-walled cirrus sac.
Remarks
Oros et al. (Reference Oros, Uhrovič, Choudhury, Mackiewicz and Scholz2020) provided an illustration (Fig. 3C) and SEM micrograph (Fig. 6D) of the scolex of B. isaureae n. sp. from M. collapsum, but the tapeworm was misidentified as B. infrequens because of a resemblance of their scoleces. However, a closer examination of this tapeworm revealed that it is B. isaureae. In addition, the worm in Oros et al. (Reference Oros, Uhrovič, Choudhury, Mackiewicz and Scholz2020) was collected from M. collapsum and not the spotted sucker, M. melanops, as was reported.
Material studied: 12 stained specimens (whole mounts) from Moxostoma anisurum, Assiniboine River, Manitoba, Canada, collected by P.A. Nelson on 22 May 1996; 23 specimens from M. anisurum [host field code CA ( = Canada) 38, 39, 52], Red River, St. Vital Park, Manitoba, Canada, A. Choudhury and M. Oros, 26 and 27 June 2013; 7 specimens from Moxostoma erythrurum (CA 36), Red River, St. Vital Park, Manitoba, Canada, A. Choudhury and M. Oros, 26 June 2013; 1 specimen from M. erythrurum [FR19_641 ( = Florian Reyda's collection)], Kanawha River, Montgomery, West Virginia, F. Reyda, 9 August 2019.
Description (based on whole mounts of 13 specimens from M. anisurum in Canada; for measurements – see Table 2): Caryophyllidea, Capingentidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate, slender, with maximum width at level of anterior vas deferens, or ovary, tapering towards neck region (Fig. 5A), covered with acicular fillitriches (filiform microtriches) (Fig. 6F).
Scolex spherical, wider than neck, with pair of large, central acetabulum-like loculi, two pairs of shallow lateral loculi, and slightly convex apical disc (Figs 5B, 6A, C). Neck narrow, very long. Internal and external longitudinal muscles well developed. Osmoregulatory canals narrow, in cortex.
Testes medullary, subspherical to widely oval. Anterior-most testes begin posterior to anterior-most vitelline follicles. Posteriorly, testes reach halfway down anterior uterine loops, relatively close to ovary, slightly short of posterior-most preovarian vitelline follicles. Cirrus-sac subspherical, thick walled. External seminal vesicle elongate, thick walled. Male and female genital pores open to distinct genital atrium (corresponding to figure 5.24 of Mackiewicz, Reference Mackiewicz, Khalil, Jones and Bray1994; Figs 5C, 6D), area around gonopores covered with gladiate spinitriches (Fig. 6E).
Ovary compact (non-follicular), H-shaped, with deep lobes (Fig. 5C). Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus, joins uterus to form uterovaginal canal, opening separately from male gonopore in distinct genital atrium. Preovarian vitelline follicles numerous, in medullary parenchyma. Preovarian vitelline follicles extend beyond testes, terminating slightly anterior to external seminal vesicle, relatively close to ovarian wings (Fig. 5C). Postovarian vitelline follicles present, numerous (Fig. 5C; Table 2).
Uterus forms several loops, extending markedly anterior to cirrus-sac; preovarian uterine loops occupy roughly triangular space (Fig. 5C); uterine glands well developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without fully formed oncosphere.
Taxonomic summary
Type host: Silver redhorse, Moxostoma anisurum (Cypriniformes: Catostomidae).
Additional definitive host (verified by molecular data): Golden redhorse, M. erythrurum (Cypriniformes: Catostomidae).
Site of infection: Usually in the anterior part of the intestine, firmly attached with the scolex, but not buried deeply into the intestinal mucosa.
Type locality: Red River, St. Vital Park (49°49′49.9″N, 97°8 53.8″W), Manitoba, Canada.
Distribution: Canada (Manitoba), USA (West Virginia).
Type material: Holotype (IPCAS C-890/1); two paratypes (IPCAS C-890/1); two paratypes (HWML 216480); two paratypes (USNM 1655790).
Representative DNA sequences: Sequences of two individuals from M. anisurum (CA 36a, 36b) in Manitoba, Canada and three individuals from M. erythrurum (CA 38c, 38d, FR19_641) in Manitoba, Canada and West Virginia, USA were submitted to GenBank MZ031045–MZ031049.
Etymology: The species is named after the typical, very long and narrow neck – longi = long, colli = neck.
Differential diagnosis: The new species differs from B. isaureae n. sp. by (i) the extent of the ovarian wings in relation to the extent of the area occupied by the uterus, 49–61% in B. longicollum n. sp. vs 39–52% in B. isaureae; (ii) the extent of preovarian vitelline follicles, which reach relatively close to the ovarian wings in B. longicollum vs slightly anterior to the external seminal vesicle in B. isaureae; (iii) position of the cirrus sac, which is situated more posteriorly between the anterior ovarian wings in B. longicollum compared to B. isaureae; and (iv) more numerous postovarian follicles in B. longicollum (12–35) compared to B. isaureae (8–14 follicles).
Remarks
Molecular data provide evidence for tapeworms from M. anisurum and M. erythrurum in Canada and West Virginia to belong to a separate lineage than those from M. collapsum in South Carolina, differing in 8–13 nucleotides (interspecific divergence 0.6–1.0%). Morphological differences between B. longicollum n. sp. and B. isaureae n. sp. are subtle, but consistent. Taken together, the molecular and morphological data indicate that they represent closely related, but distinct species. Biacetabulum longicollum and B. isaureae both occur in congeneric hosts (Moxostoma spp.). Future surveys should provide more data on the actual host spectrum and distribution of this newly described species, which is currently known from Manitoba (Canada) and West Virginia (USA).
In addition to B. longicollum n. sp., another species, B. infrequens Hunter, Reference Hunter1927, was described from M. anisurum by Hunter (Reference Hunter1927). Even though the original description of B. infrequens was brief and no illustration of the whole worm was provided by Hunter (Reference Hunter1927), it is possible to differentiate this species from B. longicollum n. sp. by the following characteristics (compare Figs 5, 6 in the present paper with figs. 2, 3, 13–15 in Hunter, Reference Hunter1927 and figs. 15, 16 in Calentine, Reference Calentine1965): (i) total body length (16–22 mm in B. infrequens vs up to 48 mm in B. longicollum n. sp.); (ii) length of the neck (short, only 0.5 mm in length in B. infrequens vs very long, 5.5–20.9 mm in the new species); (iii) position of the cirrus sac (anterior to the ovary in the former species vs between the anterior ovarian arms in B. longicollum n. sp.); and (iv) scolex morphology (see Hunter, Reference Hunter1927 and Calentine, Reference Calentine1965).
SEM examination of B. longicollum has revealed the presence of two different types of microtriches around the genital pores: numerous, dense acicular filitriches are intermingled with relatively few gladiate spinitriches (Fig. 6E, F). As far as the present authors are aware, detailed information about the surface ultrastructure (microthrix morphology) of caryophyllideans around the gonopore is not available, thus further research is needed, including SEM and detailed transmission electron microscopy observations, to clarify the different types of microtriches. Overall, the surface of caryophyllidean tapeworms has been reported to be uniformly covered by the acicular or capilliform filitriches (see, e.g., Ash et al., Reference Ash, Scholz, Oros and Kar2011; Scholz et al., Reference Scholz, Oros, Choudhury, Brabec and Waeschenbach2015; Oros et al., Reference Oros, Brabec, Kuchta, Choudhury and Scholz2016; Barčák et al., Reference Barčák, Oros, Hanzelová and Scholz2017).
3. Biacetabulum overstreeti n. sp. Figs 7–9
Material studied: One specimen (hologenophore, comprising the anterior and posterior part of the specimen) including its cross-sections from Minytrema melanops (US 209d), Big Lake near Benndale, Pascagoula River, Mississippi, collected by R. Kuchta and M. Oros on 20 March 2012; one specimen (hologenophore – PBI 416) from M. melanops (US 233b), Benndale, Pascagoula River, Mississippi, R. Kuchta and M. Oros, 23 March 2012; one specimen (hologenophore) from M. melanops (US 839a), Poticaw Landing & Moon Lake, Pascagoula River, Mississippi, T. Scholz, R. Kuchta and M. Oros, 20 June 2019; longitudinal sections of a specimen from M. melanops (US 224a), Pearl River, Mississippi (30°52′20.2′′N, 88°46′21.8′′W), R. Kuchta and M. Oros, 22 March 2012.
Description (based on whole mounts of three specimens from M. melanops in Mississippi, USA; for measurements, see Table 2): Caryophyllidea, Capingentidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate and slender, with maximum width at level of anterior vas deferens or ovary, tapering continuously towards neck region, covered with acicular fillitriches (filiform microtriches) (Fig. 9C).

Fig. 9. Scanning electron micrographs of Biacetabulum overstreeti n. sp. from Minytrema melanops, Mississippi, USA (US 224a). (A) Posterior part with genital pores, (B) detail of common genital atrium, and (C) microtriches surrounding the common genital atrium.
Scolex spherical, wider than neck, with pair of central, large acetabulum-like loculi, two pairs of shallow lateral loculi, and slightly convex apical disc (Figs 7A, B, 8B, C). Neck narrow, very long. Internal and external longitudinal muscles well developed. Osmoregulatory canals narrow, in cortex (Fig. 8E).
Testes medullary, subspherical to widely oval (Fig. 8E). Anterior-most testes begin posterior to anterior-most vitelline follicles. Posteriorly, testes reach anterior-most loops of uterus, slightly anterior to posterior-most preovarian vitelline follicles (Fig. 7C). Cirrus-sac subspherical, thick-walled. External seminal vesicle elongate, thick-walled. Male and female genital pores open into distinct genital atrium (corresponding to figure 5.24 of Mackiewicz, Reference Mackiewicz, Khalil, Jones and Bray1994; Figs 7C, 8A, D, 9A, B).
Ovary compact (non-follicular), H-shaped, with deep lobes (Fig. 7C). Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus, joins with uterus to form uterovaginal canal opening posterior to male gonopore into distinct genital atrium (Figs 8A, D, 9A, B). Preovarian vitelline follicles numerous, in medullary parenchyma (Fig. 8E). Preovarian vitelline follicles reach posteriorly anterior to external seminal vesicle, not extending to ovarian wings (Fig. 7C). Postovarian vitelline follicles few (Fig. 7C; Table 2).
Uterus forms several loops extending markedly anterior to cirrus-sac (Figs 7C, 8A); uterine glands well developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without a fully formed oncosphere.
Taxonomic summary
Type and only host: Spotted sucker, Minytrema melanops (Cypriniformes: Catostomidae).
Site of infection: Usually in the anterior part of the intestine; firmly attached with the scolex, but not buried deeply into the intestinal mucosa.
Type locality: Pascagoula River at Benndale (30°52′20.2″N, 88°46′21.8″W), George County, Mississippi, USA, R. Kuchta and M. Oros, 20 March 2012, USA.
Distribution: USA (Mississippi).
Type material: Holotype (IPCAS C-891); one paratype (longitudinal sections) (IPCAS C-891); two paratypes (whole mount – hologenophore and longitudinal sections) (USNM 1655791, 1655792).
Representative DNA sequences: Sequences of three individuals from M. melanops (US 209d, 233b and 839a) in Mississippi were submitted to GenBank MZ031050, MZ031051.
Etymology: The species name is dedicated to Robin M. Overstreet from the Gulf Coast Research Laboratory, Ocean Springs, Mississippi, for his extraordinary contribution to fish parasitology and for supporting the present authors in their sampling trips to southern Mississippi in 2012 and 2019.
Differential diagnosis: The new species corresponds in its morphology to other species of the long-necked Biacetabulum-species complex characterised above. It differs from B. isaureae n. sp. and B. longicollum n. sp. mainly by the size and position of the cirrus-sac. The width of the cirrus-sac represents more than one-third (37–39%) of the body width at the level of the cirrus-sac in B. overstreeti (B. isaureae 27–34%; B. longicollum 24–28%). The cirrus-sac is situated slightly before the anterior end of the ovarian wings in B. overstreeti vs being situated more posterior, i.e., between the ovarian arms in B. isaureae and B. longicollum.
In addition, B. overstreeti differs in the length of the ovarian wings in relation to the length of the uterus area, which is 35–41% in B. overstreeti vs 39–52% in B. isaureae and 49–61% in B. longicollum. The new species can also be differentiated from B. longicollum by a lower number of postovarian vitelline follicles (usually 8–16 follicles) compared to 12–35 follicles in the latter species (see also Table 2 for comparative measurements and the key for identifying species of the ‘long-necked’ species complex provided below).
Molecular data provide additional evidence that tapeworms from M. melanops collected in Mississippi, USA belong to a separate lineage and differ from B. isaureae in 4–8 nucleotides (interspecific divergence 0.3–0.6%) and from B. longicollum in 6–9 nucleotides (0.5–0.7%).
Remarks
Only three hologenophores of B. overstreeti n. sp. were available for morphological descriptions of this species. Therefore, the total length of the body could not be measured. Despite this limitation, we are fully convinced that other morphological and biometrical characteristics are available to sufficiently characterize the new species, which is well characterized also genetically (Fig. 1).
Another species of Biacetabulum, B. banghami Mackiewicz, Reference Mackiewicz1968, was described from M. melanops in Alabama, but this species has also been reported from Moxostoma erythrurum and H. etowanum (Jordan) in Oklahoma and Kentucky (Mackiewicz, Reference Mackiewicz1968). This species differs from B. overstreeti n. sp. most conspicuously by scolex morphology (the scolex of B. banghami bears very shallow loculi and two pairs of auricular projections on its anterior edge – see Mackiewicz, Reference Mackiewicz1968).
Material studied: 15 stained specimens (whole mounts) and 2 specimens for SEM examination from Hypentelium nigricans (851, 852), Hiwassee River downstream Apalachia Dam, Tennessee, USA, collected by Price, Sewell, Entier, Bouchard and J.S. Mackiewicz on 5 May 1968.
Description (based on whole mounts of 15 specimens from H. nigricans, Tennessee, USA; for measurements – see Table 2): Caryophyllidea, Capingentidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate, slender, with maximum width at level of anterior vas deferens or ovary, tapering towards neck region (Fig. 10A). Body covered with acicular fillitriches (filiform microtriches) (Fig. 11F).

Fig. 10. Line drawings of Biacetabulum hypentelii n. sp. from Hypentelium nigricans, Tennessee, USA (851). (A) Total view, (B) anterior part with scolex, and (C) posterior part, ventral view.

Fig. 11. Scanning electron micrographs of Biacetabulum hypentelii n. sp. from Hypentelium nigricans, Tennessee, USA (851). (A) Anterior part with scolex, (B) posterior part with genital pores, (C) scolex, (D) detail of common genital atrium, and (E) microtriches surrounding the common genital atrium.
Scolex spherical, wider than neck, with pair of large, central acetabulum-like loculi, two pairs of shallow lateral loculi, and slightly convex apical disc (Figs 10B, 11A, C). Neck narrow, very long. Internal and external longitudinal muscles well-developed.
Testes medullary, subspherical to widely oval. Anterior-most testes begin posterior to anterior-most vitelline follicles. Posteriorly, testes reach halfway down anterior uterine loops, relatively close to ovary, slightly short of posterior-most preovarian vitelline follicles. Cirrus-sac subspherical, thick walled. External seminal vesicle elongate, thick walled. Male and female genital pores open to distinct genital atrium (corresponding to figure 5.24 of Mackiewicz, Reference Mackiewicz, Khalil, Jones and Bray1994), (Fig. 11D), area around gonopores covered with gladiate spinitriches (Fig. 11E).
Ovary compact (non-follicular), H-shaped, with deep lobes (Fig. 10C). Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus, joins uterus to form uterovaginal canal, opening separately from male gonopore in distinct genital atrium. Preovarian vitelline follicles numerous, in medullary parenchyma. Preovarian vitelline follicles extend beyond testes, terminating slightly anterior to external seminal vesicle, relatively far to ovarian wings (Fig. 10C). Postovarian vitelline follicles in low number, sometimes absent (Fig. 10C; Table 2).
Uterus forms several loops, extending markedly anterior to cirrus-sac; preovarian uterine loops occupy roughly triangular space (Fig. 10C); uterine glands well-developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without a fully formed oncosphere.

Fig. 12. Map of the distribution of the newly described species of Biacetabulum. Circle – B. longicollum n. sp.; triangle – B. isaureae n. sp.; square – B. overstreeti n. sp.; empty square – B. hypentelii n. sp.
Taxonomic summary
Type host: Northern hog sucker, Hypentelium nigricans (Cypriniformes: Catostomidae).
Site of infection: Intestinal lumen (more precise data not available).
Type locality: Hiwassee River downstream Apalachia Dam, Tennessee, USA (35°10′07.0″N 84°17′54.9″W).
Distribution: USA (Tennessee).
Type material: Holotype (IPCAS C-892); two paratypes (IPCAS C-892); two paratypes (HWML 216481); two paratypes (USNM 1655793, 1655794).
Etymology: The species is named after the type host.
Differential diagnosis: The new species differs from the other three new species by (i) less numerous postovarian follicles, even absent in B. hypentelii (0–13 follicles) vs 8–14 in B. isaureae, 12–35 in B. longicollum and 8–16 in B. overstreeti; (ii) extent of preovarian vitelline follicles, which reach relatively far from the ovarian wings in B. hypentelii vs slightly anterior to the external seminal vesicle in B. isaureae and B. overstreeti, and relatively close to the ovarian wings in B. longicollum; (iii) relative size of the cirrus sac in relation to the body with in B. hypentelii 21–29% vs 27–34% in B. isaureae, 15–33% in B. longicollum, and 37–39% in B. overstreeti; (iv) length of neck in relation to total boly length in B. hypentelii 24–35% vs 30–47% in B. isaureae and 34–47% in B. longicollum; (v) the extent of the ovarian wings in relation to the extent of the area occupied by the uterus, 28–50% in B. hypentelii vs 39–52% in B. isaureae 49–61% in B. longicollum and 35–41% in B. overstreeti; (vi) position of the genital atrium from posterior margin in relation to total body length, 3–4% in B. hypentelii vs 4–5% in B. longicollum and 4–6% in B. isaureae; and (vii) size of testes in relation to body width in B. hypentelii 14–19% vs 18–24% B. isaureae, 18–21% in B. longicollum and 23–29% in B. overstreeti.
Remarks
Mackiewicz (Reference Mackiewicz1972) provided a microphotograph (see his fig. 6) of a long-necked tapeworm identified as B. infrequens that he found in two northern hogsuckers, H. nigricans, from the Hiwassee River in Tennessee in May 1968. These specimens were kindly provided by J.S. Mackiewicz and examined; they resemble those of the long-necked Biacetabulum-species complex described in this study. Although no ethanol-preserved material was available for the present molecular analyses, these specimens differ from the three recently described species (see above), and represent a new species.
A key to the identification of species of the long-necked Biacetabulum-species complex
Because of close morphological similarity in the sibling species of the long-necked Biacetabulum-species complex characterised above, a key to their identification is provided. In some cases, measurements of individual species may overlap; therefore, it is necessary to combine several characteristics.
1a. Postovarian follicles numerous, up to 35 (mean > 10) ……… ……………………………………………………………… 2.
1b. Postovarian follicles few, <13 (mean 7); preovarian vitelline follicles and testes reach the level of the external seminal vesicle, i.e., relatively far from the anterior margin of the ovary (Fig. 5C); cirrus-sac situated more posteriorly between the ovarian wings (Fig. 5C); parasite of Hypentelium nigricans …………………………………………… B. hypentelii n. sp.
2a. Preovarian vitelline follicles and especially testes do not reach the level of the external seminal vesicle, i.e., they are at distance from the anterior margin of the ovary (Figs 2C, 7C); cirrus-sac situated more anterior between the ovarian wings (Figs 2C, 7C)………………………………………………… 3
2b. Preovarian vitelline follicles and testes reach more posteriorly, up to the level of the external seminal vesicle, i.e., near the anterior margin of the ovary (Fig. 5C); cirrus-sac situated more posterior between the ovarian wings (Fig. 5C); parasite of Moxostoma anisurum and M. erythrurum………………… ………………………………………… B. longicollum n. sp.
3a. Width of the cirrus-sac <35% of the body width at the same level (Fig. 2C); cirrus-sac does not extend anterior to the ovarian wings, situated more posteriorly between the ovarian wings (Fig. 2C); parasite of M. collapsum …………………… ……………………………………………… B. isaureae n. sp.
3b. Width of the cirrus-sac >35% of the body width at the same level (Fig. 7C); cirrus sac situated more anteriorly between the ovarian wings (Fig. 7C); parasite of Minytrema melanops ……………………………………………B. overstreeti n. sp.
Discussion
Molecular and morphological evaluation of newly collected material of Biacetabulum tapeworms with an extraordinarily long neck from several catostomids, especially redhorses, in North America provided evidence of the existence of a species complex in this genus (Fig. 12). It is possible that this species complex represents an example of recent speciation of tapeworms in different, partly congeneric fish hosts, as documented by subtle, but consistent morphological differences between tapeworms of individual genetic lineages/morphotypes. A similar case of likely recent speciation in different fish hosts has been documented only in the caryophyllidean genus Caryophyllaeus Gmelin, 1790, parasites of cyprinids in Eurasia (see Barčák et al., Reference Barčák, Oros, Hanzelová and Scholz2014, Reference Barčák, Oros, Hanzelová and Scholz2017; Bazsalovicsová et al., Reference Bazsalovicsová, Králová-Hromadová, Brabec, Hanzelová, Oros and Scholz2014; Hanzelová et al., Reference Hanzelová, Oros, Barčák, Miklisová, Kirin and Scholz2015). It is also possible that the small genetic and morphological changes in this species complex reflect slow diversification accompanied by host-shifting and geographical range expansion.
The existence of a species complex has also been revealed by molecular data in Paracaryophyllaeus Kulakovskaya, 1961, parasites of Eurasian loaches. However, no clear host-related pattern of speciation was detected in this group of Eurasian caryophyllideans by Scholz et al. (Reference Scholz, Oros, Bazsalovicsová, Brabec, Waeschenbach, Xi, Aydogdu, Besprozvannykh, Shimazu, Králová-Hromadová and Littlewood2014). Future phylogenetic and biogeographical analyses using a more comprehensive data set may provide evidence of the diversification mode in this long-necked Biacetabulum species complex.
The present data provide another evidence that the actual species diversity of North American caryophyllideans is higher than previously thought. Biacetabulum contained 10 species recognised by Scholz and Oros (Reference Scholz, Oros, Caira and Jensen2017), of which 9 occur in the Nearctic region (the taxonomy of B. tandoni Johnston et Muirhead, 1950 from Australia remains questionable). Based on the present study, the total number of species of the genus increases to 14, but the actual species richness of Biacetabulum is undoubtedly much higher, as indicated by a recent molecular phylogenetic study (Scholz et al., Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021) as well as our unpublished results from caryophyllideans recently collected in the southern USA. For example, tapeworms found in grey redhorse, M. congestum (Baird and Girard, 1854) from the Pedernales River, Texas, and those from shorthead redhorse, M. macrolepidotum (Lesueur) in the Cedar River, Wisconsin, found by the present authors may also belong to yet undescribed species of Biacetabulum (unpublished data).
The high diversity of scolex types in Nearctic caryophyllideans (Mackiewicz, Reference Mackiewicz1972) was highlighted in a recent study by Oros et al. (Reference Oros, Uhrovič, Choudhury, Mackiewicz and Scholz2020) that also drew attention to the relatively high variation in scolex shape among some congeneric species, including those of Biacetabulum. In contrast, the present study of four species of the long-necked Biacetabulum-species complex from disparate hosts revealed a rather uniform scolex morphology. The newly described species of the long-necked Biacetabulum-species complex exhibit a relatively high/strict (oioxenous or stenoxenous) host specificity because they occur in a single host species or in two (B. longicollum) congeneric species of definitive hosts. However, new material is needed to confirm this strict host specificity.
The present study provides yet more evidence for the high, yet poorly known species diversity of caryophyllidean tapeworms in North America. It also points out the need to apply methods of integrative taxonomy to properly collected and processed specimens of these fish parasites. It is obvious that future studies should critically scrutinise existing information – in the form of literature and museum depositions – on host associations and distribution of caryophyllideans, which represent a dominant component of communities of intestinal helminths in catostomid fishes in North America (Kuchta et al., Reference Kuchta, Řehulková, Francová, Scholz and Šimková2020).
Acknowledgements
Three reviewers provided insightful comments and valuable suggestions that helped us to improve the manuscript. We thank the late John S. Mackiewicz for providing numerous specimens from his private collection, including specimens from H. nigricans, and for helpful comments, and Eric Hoberg and Pat Pilitt, both of the former U.S. National Parasite Collection (USNPC) in Beltsville, MD, USA, for enabling one of the present authors (T.S.) to study specimens of North American caryophyllideans including B. infrequens. Thanks are also due to Anirban Ash, India, who took photomicrographs of caryophyllideans in USNPC in 2008, Alec Perkins, USA, for providing two unpublished sequences of B. isaureae generated during his internship at the Institute of Parasitology in 2016, Florian Reyda, New York, for providing specimens of B. longicollum from West Virginia, Roman Kuchta, Czech Republic, Megan Bean, Texas, Isaure de Buron, South Carolina, and Steve Curran, Eric Pulis and Robin M. Overstreet, Mississippi, for help with collecting fish cestodes in the United States, and to Patrick Nelson, Winnipeg, Canada, and Florian Reyda, Oneonta, New York, USA, for donating additional specimens from Manitoba and New York, respectively. MO and AC thank Doug Watkinson and Patrick Nelson for help with sampling in Manitoba, Canada.
Author contribution
MO and AC collected material. TS and MO designed the study. DU, MO, and TS conceived morphological study (DU made line drawings). OK conceived molecular phylogenetic analyses. TS drafted the text and all other authors contributed to writing the manuscript.
Financial support
This study was partly supported by the Grant Agency VEGA (no. 2/0126/20), Ministry of Education, Sports and Youth (project LTAUSA18010) and the Institute of Parasitology (RVO: 60077344). Stays of M.O. and T.S. in North America in 2013 and 2017, respectively, were enabled by the Fulbright Commission. AC acknowledges Faculty Development Grants and other funding from St. Norbert College.
Conflicts of interest
The authors declare there are no conflicts of interest.
Ethical standards
Fish examined for parasite were euthanised humanely, following the Ethical Standards of individual research institutions.