Published online by Cambridge University Press: 03 February 2005
Trypanosomatids are early divergent parasites which include several species of medical interest. Trypanosoma rangeli is not pathogenic for humans but shows a high immunological cross-reactivity with Trypanosoma cruzi, the causative agent of Chagas' disease that affects more than 17 million people throughout the world. Recent studies have suggested that T. cruzi KMP-11 antigen could be a good candidate for the induction of immunoprotective cytotoxic responses against T. cruzi natural infection. In the present paper the genes coding for the T. rangeli kinetoplastid membrane protein-11 have been characterized. The results show that the locus encoding this protein is formed by 4 gene units measuring 550 nucleotides in length, organized in tandem, and located in different chromosomes in KP1(+) and KP1(−) strains. The gene units are transcribed as a single mRNA of 530 nucleotides in length. Alignment of the T. rangeli KMP-11 deduced amino acid sequence with the homologous KMP-11 protein from T. cruzi revealed an identity of 97%. Interestingly, the T and B cell epitopes of the T. cruzi KMP-11 protein are conserved in the T. rangeli KMP-11 amino acid sequence.
Trypanosomatids are flagellated protozoan parasites which cause severe diseases in humans such as African sleeping sickness caused by Trypanosoma brucei gambiense, and Trypanosoma brucei rhodesiense, Chagas' disease caused by Trypanosoma cruzi, and leishmaniasis caused by a variety of species of Leishmania, including those from Leishmania donovani complex, Leishmania mexicana complex, Leishmania tropica complex, and Leishmania braziliensis complex. Trypanosoma rangeli is apparently not pathogenic to humans and animals but shows similar morphology, and high immunological cross-reactivity with Trypanosoma cruzi (Afchain et al. 1979; Basso et al. 1991, 2004; Saldana & Sousa, 1996; Palau et al. 2003), interfering with the Chagas' disease diagnosis (Guhl & Vallejo, 2003). Moreover, these parasites are sympatric in some regions of America, share the same hosts range producing mixed infections, and often have identical insect vectors such as Rhodnius prolixus and Rhodnius colombiensis (D'Alessandro, 1976; D'Alessandro & Saravia, 1992, 1999; Guhl & Vallejo, 2003). Recent studies have shown that immunization of mice with T. rangeli protects animals against T. cruzi infection (Basso et al. 1991, 2004; Palau et al. 2003).
Two important epidemiological groups of T. rangeli have recently been described, KP1(−), and KP1(+) strains, based on the KP1 mini-circle presence in the parasite kinetoplast and the association with different adaptive lines of vectors. T. rangeli KP1(+) strains are related with Rhodnius species from the prolixus group (R. prolixus and Rhodnius neglectus) (Vallejo et al. 2003) and, T. rangeli KP1(−) strains are associated with Rhodnius species from the pallescens group (Rhodnius pallescens, R. colombiensis, and Rhodnius ecuadoriensis) (Vallejo et al. 2003).
Chagas' disease affects more than 17 million people in 15 endemic countries in Central and South America (WHO, 2002), and it represents a serious health problem. There is no immunoprophylaxis available and the current treatment is rather toxic, not very effective, and only indicated for patients in the acute phase of the disease or for T. cruzi-infected asymptomatic young people. Many attempts have been made in order to characterize new parasite antigens that are capable of eliciting protective immune responses and that could be employed to provide an immunoprophylaxis therapy against this sickness.
The kinetoplastid membrane protein-11 (KMP-11) is a ubiquitous and abundant protein, mainly located in the flagellum and the flagellar pocket of different species of kinetoplastids including Trypanosoma, Leishmania, Crithidia, Leptomonas and Phytomonas (Jardim et al. 1995; Stebeck et al. 1995, 1996; Berberich et al. 1997; Bridge et al. 1998; Ramirez et al. 1998; Thomas et al. 2000). Moreover, in T. cruzi, it has been demonstrated that 50% of the protein is associated with the cytoskeleton of the parasite (Thomas et al. 2000). KMP-11 protein is expressed during all parasite stages exhibiting higher levels in the insect stages (Stebeck et al. 1995). KMP-11 is well conserved among kinetoplastids and its predicted secondary structure consists of two α-helices separated by a random-coil segment. One third of each helical side is formed by hydrophobic residues, which are thought to interact with the lipidic bilayer in the kinetoplastid cell membrane (Stebeck et al. 1996). Interestingly, this protein presents a significant homology to the apolipoprotein B, as well as to the cytoskeleton-associated protein CIP1 from Arabidopsis thaliana (Thomas et al. 2000), and to calcium-binding proteins like LAV1-2, an EF-hand protein of 40 kDa from Physarium polycephalum (Fuertes et al. 2001). Based on these facts, it has been suggested that this protein may function in part to increase bilayer pressure, stabilizing molecules such as lipophosphoglycan within the parasite pellicular membrane (Jardim et al. 1995) as well as being implicated in the parasite mobility, and its attachment to the host cell (Thomas et al. 2000). On the other hand, it has been reported that KMP-11 protein is a potent inducer of humoral and cellular immune responses (Tolson et al. 1994) in infected animals and in leishmaniasis and chagasic patients (Berberich et al. 1997, 2003; Jensen et al. 1998; Ramirez et al. 1998, 2001; Mukhopadhyay et al. 1999; Trujillo et al. 1999; Marañon et al. 2001; Planelles et al. 2001, 2002; Thomas et al. 2001; de Carvalho et al. 2003). Moreover, it has recently been demonstrated that T. cruzi KMP-11 when fused to the heat shock protein HSP70 from this parasite elicits a specific cytotoxic and humoral immune response against the antigen and leads to protection in an experimental murine model (Planelles et al. 2001). In this context, herein we report the isolation and molecular characterization of the gene coding for KMP-11 protein from T. rangeli and its comparison to the homologous gene in T. cruzi.
Epimastigotes from KP1 (+) T. rangeli strains: H14 from Honduras (MHOM/Hond/H14, Acosta et al. 1991), Choachi from Colombia (IRHO/CO/82/Choachi), and Colombian KP1 (−) Tre strain (Morales et al. 2002) were grown at 28 °C in modified REI medium supplemented with 2% (v/v) heat-inactivated fetal bovine serum. T. cruzi epimastigotes, Munanta and Shubacbarina strains (Rodriguez et al. 1998) were grown at 28 °C in liver infusion triptone (LIT) medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum.
Genomic DNA (1–4 μg) from T. rangeli was digested with different restriction enzymes and also partially with HinfI enzyme for increasing periods of time, resolved on 0·8% agarose gels and transferred to nylon membranes (Bio-Rad) by standard procedures (Sambrook, Russell & Irwin, 2000). Full length KMP-11 coding sequence from T. cruzi and T. rangeli (KMP11Tr) were PCR amplified using, respectively, T. cruzi and T. rangeli genomic DNA and two primers KMP11F (5′-ATGGCCACCACTCTTGAG-3′) and KMP11R (5′-TTACTTTCCTGGGAACTG-3′) that map at the T. cruzi KMP-11 5′ end (including start codon) and at the T. cruzi KMP-11 3′ end (Thomas et al. 2000). Probes were labelled by the random primer method using [α-32P] dCTP (Feinberg & Vogelstein, 1983). Hybridizations were carried out using the methodology previously described by Puerta et al. (1994). Briefly, hybridization was performed overnight at 37 °C (heterologous probe) or 42 °C (homologous probe) in 50% formamide/5×SSC/0·1% SDS/5×Denhart's/0·05 M Na2HPO4/NaH2PO4 buffer/0·25 mg/ml heat-denatured herring sperm DNA. Post-hybridization washes were performed 4 times in 2×SSC/0·1% SDS at room temperature and once in 0·1×SSC/0·1% SDS at 65 °C for 1 h and exposed to Curix RP2 medical X-Ray film (Kodak).
For pulsed field gel electrophoresis (PFGE) analysis, agarose blocks containing about 5×107 parasites were prepared as described by Clark et al. (1990) and stored at 4 °C in 0·5 M EDTA, pH 9·5. Then 1/5 of each block was electrophoresed in an LKB 2015 Pulsaphor System apparatus (Pharmacia LKB, Sweden), using 1% agarose gels and 0·5×TBE buffer (40 mM Tris, 45 mM boric acid, 1 mM EDTA pH 8·3) at 13 °C with pulse times of 250, 500, 750 and 1000 s at 84 V for 80 h. Resolved chromosomes were transferred to a nylon filter and hybridized with the KMP11Tr probe as described above.
The T. rangeli cytoplasmic RNA was isolated using standard procedures (Marañon et al. 2000). RNA (5 μg) was size-fractionated on 1% agarose/formaldehyde gels, transferred to nylon membrane using a 40 mM NaOH solution and hybridized with the radio-isotope labelled KMP11Tr probe as described above.
The T. rangeli KMP-11 coding region was PCR-amplified employing KMP11F and KMP11R primers and T. rangeli genomic DNA as template. Two different polymerases, standard Taq DNA polymerase (Promega) and a proof-reading Tli DNA polymerase (Promega) were used. Following ethidium bromide staining the product amplified by the Tli DNA polymerase and the 2 fragments produced by Taq DNA polymerase were gel extracted, purified using GFX Gel Band Purification kit (Amersham Biosciences) and cloned into the pGEM®-T Easy plasmid (Promega).
Both strands of the cloned inserts were sequenced by the Sanger method (Sanger et al. 1977) in a 373 Automatic DNA sequencer (Pharmacia LKB), using the universal and KMP11F and KMP11R primers.
Homology searches were performed using the GenBank and EMBL databases and FASTA program (Pearson, 1990). Sequence alignments were performed using MULTALIN (Corpet, 1988) and LALIGN programs (Pearson, 1990).
In order to verify the presence of the KMP-11 gene in T. rangeli, we took advantage of the close phylogenetic relationship between T. rangeli and T. cruzi. Thus, T. rangeli genomic DNA was digested with different restriction enzymes and hybridized with the sequence coding for KMP-11 protein from T. cruzi (Fig. 1A). Several hybridization bands were observed in Southern blot analysis indicating the existence of homologous KMP-11 genes in T. rangeli. As a first approach for isolating the homologous genes in T. rangeli, primers KMP11F and KMP11R with map, respectively, at the 5′ and 3′ ends of T. cruzi KMP-11 coding gene were used in a PCR reaction using T. rangeli genomic DNA as template and two different DNA polymerases, Tli and Taq. When Tli polymerase was employed, a single band of approximately 280 bp was observed (data not shown). When Taq polymerase was used, 2 bands of approximately 830 bp and 280 bp were amplified (Fig. 1B). All the amplified fragments were cloned into pGEM®-T Easy plasmid (Promega) and sequenced by use of the Sanger method.

Fig. 1. (A) Southern blot of Trypanosoma rangeli genomic DNA. (A) Samples of 4 μg from epimastigotes of the T. rangeli Tre strain were digested with BsteII, NarI, Sau3A, StyI and XhoI restriction endonucleases and hybridized to the T. cruzi KMP-11 coding sequence. HindIII digested λ Phage DNA was used as molecular weight marker. (B) Ethidium bromide-stained 1% agarose gel showing the PCR amplified products using TaqI DNA polymerase, KMP11F/R primers and T. rangeli Tre strain genomic DNA (lanes 1 and 2) or no DNA (lane 3). A 100 bp ladder (Promega) was used as molecular weight marker.
Sequence analysis of the small amplified band (Accession number AY147904) showed correspondence with the T. rangeli KMP-11 coding sequence (Fig. 2A) which has an identity of 88% with the T. cruzi KMP-11 coding region. The G/C content of this ORF is slightly lower than the A/T content (50·2%). In addition, a strong preference for codons ending in G or C for charged amino acids was observed. For example, 4/5 aspartic acid residues, 13/15 glutamic acid residues, 5/5 histidine residues, 14/15 lysine residues, 3/3 asparagine residues, and 4/5 glutamine residues present in the T. rangeli KMP-11 protein are encoded by GAC, GAG, CAC, AAG, AAC, and CAG codons, respectively.

Fig. 2. Nucleotide sequence and deduced amino acid sequence from the Trypanosoma rangeli KMP-11 coding genes. (A) Sequence of the DNA fragment amplified using Tli enzyme. Numbers to the left of the sequence indicate the nucleotide and amino acid position. The stop codon is marked by an asterisk. The XhoI restriction site is in bold. [bull ] indicates the two protein kinase C phosphorylation sites, [lozf ] marks the casein kinase II phosphorylation sites, and [squf ] denotes the O-glycosylation site. Amino acids that comprise the two theoretical α-helices are underlined. (B) Sequence from the 829 bp amplified fragment. Numbers to the left of the sequence indicate the nucleotide and amino acid positions. Stop codons are marked by an asterisk. The XhoI restriction site is in bold and, XmnI restriction site is both in bold and in italic. The polypyrimidine track is underlined inside the intergenic region. [lozf ] indicates the putative spliced leader acceptor sites.
Sequence analysis of the large band (Accession number AY325812) revealed 2 copies of the KMP-11 coding gene separated by an intergenic region of 270 bp (Fig. 2B). In this intergenic region there is a polypyrimidine track followed by 4 putative splicing acceptor sites (AG dinucleotides). Alignment of the sequences of the two ORFs within the 829 bp band showed an identity at nucleotide level of 90·3% whereas the comparison among the Tli amplified band and these two ORFs showed that they shared a higher percentage of similarity with the first ORF (98%) than with the second one (91%). Interestingly, the percentage of identity among the KMP-11 coding gene copies is higher between T. rangeli and T. cruzi (Accession numbers AJ000077, AF167435, and AF167434) than between T. rangeli and T. brucei (Accession number AF028726) genes. Comparison between the T. rangeli and T. cruzi intergenic regions revealed an identity of 58% suggesting that higher homology is focused on coding sequences.
The genomic organization of the T. rangeli KMP-11 genes was studied by Southern blot analysis of genomic DNA using the T. rangeli KMP-11 coding region as probe. The presence of a highly intense hybridization band of 550 bp in length in lane XhoI (Fig. 1A) and lanes AgeI and HinfI (Fig. 3A, fragment I), restriction endonucleases which cut once within the KMP-11 unit, is consistent with the existence of several copies of the KMP-11 gene separated by their intergenic regions of similar size in the T. rangeli genome. Digestion of genomic DNA with HaeIII and EaeI enzymes, which cut twice inside most KMP-11 units produces, as expected, an intense hybridization band of approximately 415 bp (fragment II in Fig. 3A). A slightly hybridization band of 800 bp was also generated (fragment III in Fig. 3A). This fragment corresponds to the KMP-11 unit located at the 3′ end of the cluster and part of its flanking region. In addition, nucleotide polymorphism in the second copy of the KMP-11 locus creates a new HaeIII restriction site at nucleotide 219 (as seen by DNA sequencing of the larger amplified band, shown in Fig. 2B). This polymorphism produces an additional slight hybridization fragment of approximately 520 bp which originated due to the absence of the EaeI/HaeIII restriction site located at the intergenic region comprised between the second and third copy of the KMP-11 locus.

Fig. 3. (A) Samples of 4 μg from the Trypanosoma rangeli Tre strain genomic DNA were digested with different restriction endonucleases and hybridized with the T. rangeli KMP-11 coding sequence (KMP11Tr). Molecular weight markers, HindIII digested λ Phage DNA and HaeIII digested Φ X174 RF DNA were used. (B) Samples of 1 μg of T. rangeli Tre strain genomic DNA were partially digested with the HinfI restriction endonuclease for 5 min, 10 min, 20 min, 30 min, 1 h and 2 h at 37 °C. HindIII digested λ Phage DNA and HaeIII digested Φ X174 RF DNA were used as molecular weight markers. The approximately 630 bp hybridization band observed after 20 and 30 min of enzyme digestion corresponds to the fragment formed by 1 gene unit plus the HinfI short fragment located at the locus 5′ end. (C) Schematic representation of the KMP-11 locus. The coding regions (open boxes), the intergenic regions, the 5′ and 3′ non-coding regions of the cluster (thick black lines) and restriction endonuclease cleaving sites, HaeIII (H), AgeI (A), HinfI (Hf) and EaeI (E) are indicated. (D) Pulsed-field gel electrophoresis of chromosomes from T. rangeli H14, Choachí and Tre, T. cruzi Shubacbarina and Munanta strains. The size of the hybridization bands is indicated.
To determine the copy number of T. rangeli KMP-11 gene Southern blotting and hybridization with the KMP11Tr coding region employing genomic DNA partially digested with HinfI was carried out. The obtained results, shown in Fig. 3B, indicate that there are 4 copies of KMP-11 genes in T. rangeli. Copy number estimation was confirmed by densitometric analysis of the HinfI-hybridized bands. The 550 bp fragment obtained after 30 min of digestion with HinfI was used as reference in order to compare with the same fragment obtained after 1 and 2 h of digestion. A map representing the genomic organization of the KMP-11 cluster is shown in Fig. 3C. To identify the chromosome that contains the KMP-11 cluster, pulsed-field gel electrophoresis was carried out with different T. rangeli strains (Fig. 3D). Analysis of the hybridization bands revealed the existence of divergence in the cluster location among strains. Thus, the KMP-11 cluster is located in two chromosomes of 3·1 and 3·4 Mb in the KP1(−) Tre strain while in the KP1(+) Choachi strain it is located in two chromosomes of 1·6 and 1·4 Mb. In KP1(+) H14 strain the KMP-11 cluster is contained in a single chromosome of approximately 1·6 Mb. In the Shubacbarina and Munanta strains, which belong to group I of T. cruzi, the KMP-11 genes are positioned on a chromosomal band of 1·49 Mb.
The expression product of the T. rangeli KMP-11 genes was analysed by Northern blotting using total RNA from T. rangeli hybridized to the T. rangeli KMP-11 coding region. As shown in Fig. 4, a single hybridization band of 530 nt in length was detected in the epimastigote form of the parasite.

Fig. 4. Northern blot analysis of total RNA of epimastigotes from Trypanosoma rangeli Tre strain in the logarithmic phase of growth. The filter was hybridized with the radio-isotope labelled KMP11Tr probe.
The T. rangeli KMP-11 deduced amino acid sequence corresponds to a protein of 92 amino acids (Fig. 2A) with a molecular weight of 11 kDa and a theoretical isoelectric point of 5·96. Prediction of post-translational modifications shows the existence in KMP-11 protein, of a single O-glycosylation site at threonine 39 as well as 4 phosphorylation sites, 2 of them depending on protein kinase C and 2 on casein kinase II (Fig. 2A). In addition, theoretical analysis of the secondary structure reveals the presence of 2 α-helices separated by a random-coil segment, like that found in other KMP-11 proteins (Stebeck et al. 1995; Thomas et al. 2000). All of these characteristics are conserved among the deduced amino acid sequence of the 3 sequenced KMP-11 ORFs with the exception of the isoelectric point of the second KMP-11 copy found in the 829 bp fragment which is 6·52. Alignment of the T. rangeli KMP-11 deduced amino acid sequences revealed that the ORF amplified by Tli has an identity of 98% with the first ORF contained in the 829 bp fragment and 96% with the second (Fig. 5). Moreover, comparison of the Tli amplified band with T. cruzi, T. brucei and Leishmania panamensis KMP-11 sequences showed an identity of 97%, 92·4% and 88%, respectively. The first ORF of the 829 bp clone revealed an identity of 96% with T. cruzi, 91% with T. brucei and 86% with L. panamensis KMP-11 sequences, whereas the second ORF showed an identity of 98%, 93%, and 85% with T. cruzi, T. brucei and L. panamensis KMP-11 proteins, respectively. Besides, it is important to note that the homology shared between T. cruzi KMP-11 and the cytoskeleton associated protein CIP1 from Arabidopsis thaliana (Thomas et al. 2000) is also present in all T. rangeli KMP-11 deduced sequences. In the same way, the homology between KMP-11 proteins and calcium-binding proteins is also kept in the T. rangeli KMP-11 protein (Fuertes et al. 2001). Finally, the B and T cell epitopes of T. cruzi KMP-11 are conserved in the T. rangeli KMP-11 protein.

Fig. 5. Alignment of the deduced amino acid sequences from Trypanosoma rangeli KMP-11 coding sequences (Tr2tliA: Accession number Q8IS88, Tr1: Accession number AAP88967, and Tr2: Accession number AAP88968), T. cruzi (Tc: Accession number Q9U6Z1), T. brucei (Tb: Accession number Q26773), and L. panamensis (Lp: Accession number Q9NHU4). Dots represent identical amino acids.
Isolation and characterization of the Trypanosoma rangeli KMP-11 gene has allowed us to compare it with the sequence of the homologous protein in other trypanosomes. Comparison of genomic organization between KMP-11 from T. rangeli and T. cruzi demonstrated a similar organization of 4 tandemly repeated copies separated by intergenic regions of 270 bp in length. In contrast, Leishmania KMP-11 ORFs are separated by longer intergenic regions which exhibit different sizes. KMP-11 transcripts in both trypanosomes have approximately 500 nt in length, whereas in Leishmania the KMP-11 messenger is longer, with a length of 1300 nt. Thus, all of these KMP-11 genes transcribe a high portion of their intergenic regions. These differences can be the result of different regulatory mechanisms operating in these trypanosomatids. In fact, whereas in T. cruzi the level of the KMP-11 transcripts is the same through the different life-stages (Thomas et al. 2000), in Leishmania infantum the KMP-11 transcripts are upregulated in promastigotes (Berberich et al. 1998). Thus, it is possible that intergenic regions could include specific regulatory sequences responsible for operating at different levels, post-transcriptional and translational.
Two important epidemiological groups of T. rangeli have recently been described, KP1(−) strains associated with R. pallescens group, and KP1(+) strains associated with R. prolixus group (Vallejo et al. 2002, 2003). Therefore, the differences observed in the KMP-11 locus location between KP1(+) and KP1(−) strains could be the result of an evolution process that facilitated an increase in the distance between these two lineages of T. rangeli. Indeed, there are several lines of evidence that indicate that these two groups of T. rangeli differ not only by using molecular markers such as KP1 mini-circle and PCR mini-exon amplifications, but also in their biological behaviour (Vallejo et al. 2002, 2003). Thus, the chromosomal location of KMP-11 genes constitutes another molecular marker that can differentiate these T. rangeli subpopulations. It is interesting to note that KMP-11 location in T. cruzi is also different among strains belonging to groups I and II. Thomas et al. (2000) reported the location of the KMP-11 gene in a chromosome of 1900 kb in the Y strain, which belongs to group II whereas in Munanta and Shubacbarina group I strains these genes are located in a chromosome of 1490 kb. Since the KMP-11 locus is located at different chromosomes depending on species and strains, it could be employed as a tool for diagnostic purposes. On the other hand, since KMP-11 genes are organized in a single locus, the presence of two chromosomal hybridization bands in T. rangeli Choachi and Tre strains can be the result of differences in size between homologous chromosomes. These polymorphisms have been also described in other trypanosomatids (Henriksson et al. 1995; Toaldo et al. 2001).
Sequence analysis also showed the existence of polymorphism at nucleotide level inside the T. rangeli KMP-11 coding sequences. For instance, the second unit of the 829 bp fragment lacks an XhoI restriction site and, however, contains a new XmnI restriction site. This polymorphism was also detected by PCR-RFLP analysis of Tre and other KP1(−) strains (laboratory data). In addition, the HaeIII and EaeI restriction enzymes, besides cutting at the beginning of the coding region of all genes, they also cut only inside the intergenic regions located between the first and second copies and the third and fourth copies. This finding could be indicative of a duplication event that has produced 4 copies from the originally existent 2 copies.
Polymorphism also affects the protein amino acid composition. Indeed, there are 4 changes among the known deduced amino acid sequences, 3 of them being conservative and 1 being non-conservative. These results contrast with the absence of polymorphism in the T. brucei KMP-11 proteins (Bridge et al. 1998). In T. cruzi KMP-11 gene units only the absence of the lysine residue located just before the protein stop codon in the deduced amino acid sequence of the first copy of the T. cruzi KMP-11 cluster is observed (Thomas et al. 2000).
Comparative sequence analysis showed that the intergenic region of T. rangeli KMP-11 genes has a greater identity with T. cruzi (58% in 269 nts) than with T. brucei (65% in 77 nts) region. The polypyrimidine tract, located upstream from the 4 putative splicing acceptor sites, is not preceded by the 9 GT dinucleotide repetitions observed in T. cruzi and T. brucei KMP-11 genes.
All the above-mentioned analyses demonstrated that the T. rangeli KMP-11 protein is phylogenetically closer to the T. cruzi homologous protein than to the T. brucei homologous protein. In fact, one copy from T. rangeli KMP-11 shares a higher homology with the T. cruzi than with its other own copies. This high degree of similarity between T. cruzi and T. rangeli should be corroborated with other molecular markers. Interestingly, Stevens et al. (1999), using the ssrRNA sequences, found a close evolutionary relationship between T. rangeli and T. cruzi, both of which are placed in the same clade.
Finally, it is important to remark that T. rangeli KMP-11 protein as T. cruzi protein conserves the predicted secondary structure as well as the motifs implicated in the hypothetical functions assigned to these proteins, including those for phosphorylation and calcium binding. The high degree of conservation of this protein and its presence in all trypanosomatids render KMP-11 an important protein for these parasites.
This work was supported by Colciencias and Fondo para la Promoción de la Investigación y la Tecnología del Banco de la República de Colombia, contracts No. 190-2000 and 200115, respectively. The work carried out by M. C. Thomas and M. C. López was supported by Grants FIS PI020862 and PI020565 and RICET C03-04 (MSC), Spain. The authors give special thanks to A. López-Barajas for her technical assistance.
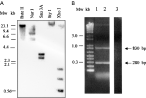
Fig. 1. (A) Southern blot of Trypanosoma rangeli genomic DNA. (A) Samples of 4 μg from epimastigotes of the T. rangeli Tre strain were digested with BsteII, NarI, Sau3A, StyI and XhoI restriction endonucleases and hybridized to the T. cruzi KMP-11 coding sequence. HindIII digested λ Phage DNA was used as molecular weight marker. (B) Ethidium bromide-stained 1% agarose gel showing the PCR amplified products using TaqI DNA polymerase, KMP11F/R primers and T. rangeli Tre strain genomic DNA (lanes 1 and 2) or no DNA (lane 3). A 100 bp ladder (Promega) was used as molecular weight marker.

Fig. 2. Nucleotide sequence and deduced amino acid sequence from the Trypanosoma rangeli KMP-11 coding genes. (A) Sequence of the DNA fragment amplified using Tli enzyme. Numbers to the left of the sequence indicate the nucleotide and amino acid position. The stop codon is marked by an asterisk. The XhoI restriction site is in bold. [bull ] indicates the two protein kinase C phosphorylation sites, [lozf ] marks the casein kinase II phosphorylation sites, and [squf ] denotes the O-glycosylation site. Amino acids that comprise the two theoretical α-helices are underlined. (B) Sequence from the 829 bp amplified fragment. Numbers to the left of the sequence indicate the nucleotide and amino acid positions. Stop codons are marked by an asterisk. The XhoI restriction site is in bold and, XmnI restriction site is both in bold and in italic. The polypyrimidine track is underlined inside the intergenic region. [lozf ] indicates the putative spliced leader acceptor sites.

Fig. 3. (A) Samples of 4 μg from the Trypanosoma rangeli Tre strain genomic DNA were digested with different restriction endonucleases and hybridized with the T. rangeli KMP-11 coding sequence (KMP11Tr). Molecular weight markers, HindIII digested λ Phage DNA and HaeIII digested Φ X174 RF DNA were used. (B) Samples of 1 μg of T. rangeli Tre strain genomic DNA were partially digested with the HinfI restriction endonuclease for 5 min, 10 min, 20 min, 30 min, 1 h and 2 h at 37 °C. HindIII digested λ Phage DNA and HaeIII digested Φ X174 RF DNA were used as molecular weight markers. The approximately 630 bp hybridization band observed after 20 and 30 min of enzyme digestion corresponds to the fragment formed by 1 gene unit plus the HinfI short fragment located at the locus 5′ end. (C) Schematic representation of the KMP-11 locus. The coding regions (open boxes), the intergenic regions, the 5′ and 3′ non-coding regions of the cluster (thick black lines) and restriction endonuclease cleaving sites, HaeIII (H), AgeI (A), HinfI (Hf) and EaeI (E) are indicated. (D) Pulsed-field gel electrophoresis of chromosomes from T. rangeli H14, Choachí and Tre, T. cruzi Shubacbarina and Munanta strains. The size of the hybridization bands is indicated.

Fig. 4. Northern blot analysis of total RNA of epimastigotes from Trypanosoma rangeli Tre strain in the logarithmic phase of growth. The filter was hybridized with the radio-isotope labelled KMP11Tr probe.

Fig. 5. Alignment of the deduced amino acid sequences from Trypanosoma rangeli KMP-11 coding sequences (Tr2tliA: Accession number Q8IS88, Tr1: Accession number AAP88967, and Tr2: Accession number AAP88968), T. cruzi (Tc: Accession number Q9U6Z1), T. brucei (Tb: Accession number Q26773), and L. panamensis (Lp: Accession number Q9NHU4). Dots represent identical amino acids.