INTRODUCTION
Among over 50 nominal species in the genus Paragonimus, Paragonimus westermani is the best-known because of its pathogenicity to humans and its wide distribution in Asia (Blair et al. Reference Blair, Xu and Agatsuma1999). This species shows a great deal of variation in morphology, biological properties and in DNA sequences (Blair et al. Reference Blair, Xu and Agatsuma1999; Nawa et al. Reference Nawa, Thaenkham, Doanh, Blair and Motarjemi2014; Blair et al. Reference Blair, Nawa, Mitreva and Doanh2016). Morphological and molecular variation of P. westermani is observed not only among geographically distant areas (Blair et al. Reference Blair, Agatsuma, Watanobe, Okamoto and Ito1997; Iwagami et al. Reference Iwagami, Ho, Su, Lai, Fukushima, Nakano, Blair, Kawashima and Agatsuma2000; Doanh et al. Reference Doanh, Shinohara, Horii, Habe and Nawa2009), but also within the same localities, as seen in Thailand, Sri Lanka and India (Binchai et al. Reference Binchai, Rangsiruji, Ketudat, Morishima and Sugiyama2007; Iwagami et al. Reference Iwagami, Rajapakse, Paranagama, Okada, Kano and Agatsuma2008; Devi et al. Reference Devi, Narain, Mahanta, Nirmolia, Blair, Saikia and Agatsuma2013). The first (snails) and second (crustaceans) intermediate hosts vary from place to place (Blair et al. Reference Blair, Xu and Agatsuma1999). Variation is also seen in ploidy: diploid, triploid and tetraploid forms have been reported from China, Japan, Taiwan and Korea. Elsewhere, such as in Malaysia, the Philippines and Thailand, only diploid forms are known (Blair et al. Reference Blair, Xu and Agatsuma1999).
In Vietnam, it had long been assumed that human paragonimiasis was caused by P. westermani (Landmann et al. Reference Landmann, Ngu and Thai1961). However, recent molecular studies of eggs from patients clearly showed that the causative species in northern Vietnam is Paragonimus heterotremus, but not P. westermani (Le et al. Reference Le, De, Blair, McManus, Kino and Agatsuma2006; Doanh et al. Reference Doanh, Dung, Thach, Horii, Shinohara and Nawa2011; De et al. Reference De, Murrell, Cong, Cam, Chau, Toan and Dalsgaard2003). More recently, we reported a high prevalence of P. westermani metacercariae in freshwater crab hosts, Vietopotamon aluoiense, in central provinces (Doanh et al. Reference Doanh, Shinohara, Horii, Habe and Nawa2009, Reference Doanh, Horii and Nawa2013). In this study, we report two additional crab species as new second intermediate hosts and a new locality for P. westermani in a northern province. Morphological and molecular comparisons of P. westermani in northern and central Vietnam are discussed herein.
MATERIALS AND METHODS
Study sites
The survey was conducted in two communes each in northern and central Vietnam (Fig. 1). In the north were Luong Son Commune, Bao Yen District, Lao Cai Province and An Lac Commune, Luc Yen District, Yen Bai Province, where P. heterotremus was reported as the commonest species and human patients have been detected (Le et al. Reference Le, De, Blair, McManus, Kino and Agatsuma2006; Doanh et al. Reference Doanh, Dung, Thach, Horii, Shinohara and Nawa2011; De et al. Reference De, Murrell, Cong, Cam, Chau, Toan and Dalsgaard2003). Two communes in central Vietnam were Huong Son and Tan Thanh Communes, Huong Hoa District, Quang Tri Province, close to Da Krong District, where heavy infection of P. westermani metacercariae in crabs was reported previously (Doanh et al. Reference Doanh, Shinohara, Horii, Habe and Nawa2009). The distance between the northern and central study areas is about 900 km (Fig. 1).

Fig. 1. Study sites in the north (Lao Cai and Yen Bai Provinces) and central (Quang Tri Province) Vietnam.
Collection of metacercariae from freshwater crabs
Freshwater crabs caught in the study sites were identified based on the morphological appearances of the carapace and gonopods 1 of males (Dang and Ho, Reference Dang and Ho2012). The procedure of crab examination to collect Paragonimus metacercariae was described previously (Doanh et al. Reference Doanh, Shinohara, Horii, Habe, Nawa, The and Le2007). Metacercariae were initially identified based on morphological observation as described previously (Doanh et al. Reference Doanh, Horii and Nawa2013). Then P. westermani metacercariae from Yen Bai and Quang Tri Provinces were used for further morphological and molecular analyses. Specimens of Paragonimus metacercariae and crab hosts are available at Institute of Ecology and Biological Resources, Vietnam Academy of Sciences and Technology.
Molecular phylogenetic analyses
DNA sequences from three P. westermani metacercariae from Yen Bai Province and two each from morphologically different types from Quang Tri Province were used for molecular phylogenetic analyses. Nuclear ribosomal ITS2 regions were PCR-amplified using primers 3S and A28 (Bowles et al. Reference Bowles, Blair and McManus1995), and a partial 16S region of the mitochondrial DNA was amplified using primers T7-1 and SP6-1 (Agatsuma et al. Reference Agatsuma, Iwagami, Sato, Iwashita, Hong, Kang, Ho, Su, Kawashima and Abe2003). The PCR products were purified using Qiaquick PCR Purification Kit (Qiagen Inc., Tokyo, Japan). Both strands were directly sequenced in a Genetic Analyzer 3130 (Applied Biosystems Japan Ltd, Tokyo, Japan) using the PCR primers as sequencing primers and a Big-Dye terminator cycle-sequencing kit v3.1 (Applied Biosystems). For phylogenetic analyses, we downloaded from GenBank ITS2 and 16S sequences registered as P. westermani from different countries. The ITS2 and 16S sequence datasets for phylogenetic analyses were aligned using Clustal-W with default options. Evolutionary analyses were performed using the MEGA6 software package (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). The best-fit model of nucleotide substitution for each sequence dataset was determined. Phylogenetic trees were reconstructed using the maximum likelihood method based on the Kimura 2-parameter model with Gamma distribution (+G) for the ITS2 data, and the Hasegawa-Kishino-Yano model with Gamma distribution (+G) for the 16S data. Support for each clade was evaluated using the bootstrap test with 1000 replications.
RESULTS
Prevalence of Paragonimus metacercariae in crab hosts
In the north, only one species, Indochinamon tannanti (Fig. 2A), was found in mountain streams of both Luong Son and An Lac Communes. In central Vietnam, two crab species, V. aluoiense (Fig. 2B) and Donopotamon haii (Fig. 2C), were caught. The infection rates of Paragonimus metacercariae in crabs are shown in Table 1. In two communes in the northern provinces, the infection rates and intensities in I. tannanti crabs were similar to one another: 82·8% and 4–504 metacercariae/crab in Luong Son Commune, and 70·0% and 1–362 metacercariae/crab in An Lac Commune. In both locations, P. heterotremus metacercariae (about 200–300 µm in diameter with the inner cyst wall thickest at the two poles) were the most abundant while those of P. vietnamensis (large: about 700–800 µm in diameter) were rare. In addition, metacercariae of two other species, P. westermani (300–400 µm in diameter, with a thick inner cyst wall and the worm fully occupying the cyst) and Paragonimus bangkokensis (about 300–400 µm in diameter with a thin inner cyst wall and the worm forming a U-shape within the cyst), were detected in crabs in An Lac Commune, but with low infection rates (Table 1). Metacercariae of two or three of these species were often found in the same individual crabs.

Fig. 2. Fresh water crabs serve as second intermediate hosts of P. westermani in Vietnam. A. Indochinamon tannanti collected from Yen Bai Province; B. Vietopotamon aluoiense and C. Donopotamon haii collected from Huong Hoa district, Quang Tri Province. (upper: whole crab, and below: gonopods of males).
Table 1. Prevalence of Paragonimus metacercariae in crabs collected from studied sites

In Quang Tri Province, central Vietnam, the infection rate in V. aluoiense crabs in Huong Son Commune (100% and 7–500 metacercariae/crab) was much higher than that (10·0–12·0% and 1–6 metacercariae/crab in V. aluoiense and D. haii) in Tan Thanh Commune. Almost all metacercariae in these communes were identified as P. westermani (variable in size and shape, but mainly 300–400 µm in diameter with a thick inner cyst wall). A few P. proliferus metacercariae (large, excysted worms of about 2·5 mm in length) and P. bangkokensis were also found (Table 1). Co-infection of these Paragonimus species in the same individual crabs was occasionally observed.
Morphology of P. westermani metacercariae
Paragonimus westermani metacercariae collected from Yen Bai Province were uniform in morphology; round/spherical in shape, measuring 350·3 × 348·0 µm on average, with a thick inner cyst wall (Fig. 3). In contrast, metacercariae from Quang Tri Province varied in size, shape and thickness of the inner cyst wall, and were divided into five different types (Fig. 4A–E). The dimensions of metacercariae are given in Table 2. Type 1 (Fig. 4A) was spherical and smallest in size, measuring 282 × 278 µm on average, with a thin inner cyst wall; type 2 (Fig. 4B) was elongate oval, measuring 413·3 × 296·7 µm, with a thick inner cyst wall; type 3 (Fig. 4C) was spherical, measuring 380·4 × 376·8 µm, with a thin inner cyst wall; type 4 (Fig. 4D) was similar to type 3 in shape and size (386·3 × 376·9 µm), but with a thicker inner cyst wall; and type 5 (Fig. 4E) was spherical in shape and the largest in size (444·7 × 437·5 µm) with a thin inner cyst wall. These five types differed significantly from one another in size (P < 0·001), except for types 3 and 4. Metacercariae from Yen Bai and type 4 from Quang Tri Province are morphologically similar to the typical type reported from East Asia (Japan, Korea, China) and from Southeast Asia (Thailand, Malaysia and the Philippines), and was the most common type, the other types were much less common than type 4. There was no relationship between morphological characteristics of P. westermani metacercariae and the isolation sites in crabs.
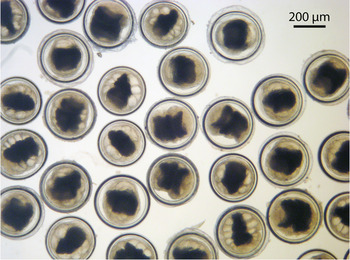
Fig. 3. Paragonimus westermani metacercariae collected from Yen Bai Province.
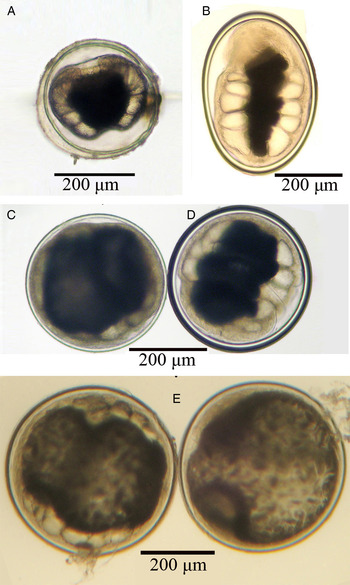
Fig. 4. Different types of P. westermani metacercariae collected from Quang Tri Province. A. type 1, B. type 2, C. type 3, D. type 4 and E. type 5.
Table 2. Measurement of different types of P. westermani metacercariae

Molecular analyses
The ITS2 and 16S sequences obtained from P. westermani metacercariae from Yen Bai and Quang Tri Provinces were submitted to Genbank (accession numbers LC144895-LC144910). Of the 464 sites in the ITS2 alignment, 1–2 (0·2–0·4%) sites were variable among P. westermani samples from Vietnam regardless of locality or morphology. In the phylogenetic tree (Fig. 5), all Vietnamese samples were placed in a group close to sequences from East Asia (Japan, Korea, China and Taiwan). One ITS2 sequence from Thailand (AB354218) and one from India (JN656182) were also included in this group, while the majority of ITS2 sequences of P. westermani from Thailand were grouped with those from Malaysia and the Philippines in a distinct clade to form the Southeast Asia group. Likewise, the majority of Indian samples fell into a separate group and P. westermani from Sri Lanka also constituted a distinct lineage. ITS2 sequences of a morphologically distinct species, Paragonimus siamensis, within the P. westermani complex formed a well-supported clade that rendered ‘P. westermani’ paraphyletic.

Fig. 5. Maximum Likelihood trees reconstructed from ITS2 sequences. Bootstrap scores expressed as percentages of 1000 replications are given at each node. New sequences obtained in this study are indicated with sample code and number of identical samples in parentheses, and others from DNA database are indicated with their accession no., species name, and 3-letter country code (Japan = JPN, Korea = KOR, China = CHN, Taiwan = TWN, India = IND, Sri Lanka = LKA, the Philippines = PHL, Malaysia = MYS, Thailand = THA, and Vietnam = VNM).
In the 16S alignment (760 bp), Vietnamese P. westermani differed among themselves by 0–1·4%. In the phylogenetic tree (Fig. 6) they formed a clade closest to the East Asian group. Sequences from Thailand and the Philippines constituted another group while two sequences from Malaysia and India stood separately. An Indian sequence was far distant (18·3–20·5%) from other groups. The alignment of 16S sequences revealed constant differences between diploid and triploid/tetraploid types at two nucleotides, A/G and C/T at positions 238 and 523, respectively. These sites are equivalent to the positions 236 and 521 in the alignment analyzed by Agatsuma et al. (Reference Agatsuma, Iwagami, Sato, Iwashita, Hong, Kang, Ho, Su, Kawashima and Abe2003). According to the bases exhibited at these sites, all Vietnamese specimens from Yen Bai and Quang Tri Provinces are supposed to be the diploid type.

Fig. 6. Maximum Likelihood trees reconstructed from 16S region of mitochondrial DNA. Bootstrap scores expressed as percentages of 1000 replications are given at each node. New sequences obtained in this study are indicated with sample code and number of identical samples in parentheses, and others from DNA database are indicated with their accession no., species name, and 3-letter country code as noted in Fig. 5.
DISCUSSION
It has long been known that P. westermani shows a large diversity of morphology, genetic markers, biology and pathogenicity (Blair et al. Reference Blair, Xu and Agatsuma1999). At the metacercarial stage, variations in size and cyst wall thickness have been reported (Miyazaki, Reference Miyazaki1991; Blair et al. Reference Blair, Xu and Agatsuma1999). The ‘typical’ metacercariae of this species (i.e. from China, Korea, Japan and elsewhere) are round or spherical in shape with a thick inner cyst wall (Miyazaki, Reference Miyazaki1991). In East Asia, triploid metacercariae (348–412 × 328–396 µm) are larger than diploids (276–368 × 272–360 µm) (Miyazaki, Reference Miyazaki1978; Kawashima et al. Reference Kawashima, Hua, Ho and Kawashima1989; Park et al. Reference Park, Lee, Im, Park and Yong2001). Paragonimus westermani metacercariae from Sri Lanka (656 × 465 µm) and India (420·6–1012·7 µm) were more oval and much larger in size with a thin inner cyst wall (Iwagami et al. Reference Iwagami, Rajapakse, Paranagama, Okada, Kano and Agatsuma2008; Devi et al. Reference Devi, Narain, Agatsuma, Blair, Nagataki, Wickramasinghe, Yatawara and Mahanta2010, Reference Devi, Narain, Mahanta, Nirmolia, Blair, Saikia and Agatsuma2013). Striking variation in size was also reported from Thailand (Sugiyama et al. Reference Sugiyama, Morishima, Binchai, Rangsiruji and Ketudat2007). In Vietnam, we found uniform morphology of P. westermani metacercariae in Da Krong District, Quang Tri Province (Doanh et al. Reference Doanh, Shinohara, Horii, Habe and Nawa2009) and in Yen Bai Province, but considerable morphological variation in Huong Hoa District, Quang Tri Province, central Vietnam. While most were similar to the typical metacercariae from East and Southeast Asia, some resembled the small metacercariae of China and Thailand (Kawashima et al. Reference Kawashima, Hua, Ho and Kawashima1989; Sugiyama et al. Reference Sugiyama, Morishima, Binchai, Rangsiruji and Ketudat2007), or had an elongate-oval shape, similar to, but smaller than, those of Sri Lanka and India. However, they showed high genetic similarities despite morphological variation, except for slight genetic differences between the geographically distant populations of Quang Tri and Yen Bai Provinces.
Phylogenetically, P. westermani was previously considered to be divided into two groups: East and Southeast groups (Blair et al. Reference Blair, Agatsuma, Watanobe, Okamoto and Ito1997). With accumulation of molecular data, subsequent studies, including this one, found that P. westermani is much more diverse and is divided into several groups: East Asia (Japan, Korea, China and Taiwan), South Asia (India and Sri Lanka), and Southeast Asia (Malaysia, Philippines and Thailand). The two latter groups include several sub-groups from different countries. Vietnamese P. westermani is closer to the East Asia group than the Southeast Asia group, suggesting their origin from the former group.
In relation to pathogenicity, human paragonimiasis caused by P. westermani is confirmed to East Asia (Japan, Korea, China and Taiwan) and the Philippines (Blair et al. Reference Blair, Xu and Agatsuma1999). Recently, a single case of human P. westermani infection has been reported in India based on molecular evidence (Singh et al. Reference Singh, Hiromu, Devi and Singh2015). In Vietnam, P. heterotremus has been confirmed as the pathogen of human paragonimiasis in northern provinces (Le et al. Reference Le, De, Blair, McManus, Kino and Agatsuma2006; Doanh et al. Reference Doanh, Dung, Thach, Horii, Shinohara and Nawa2011, Reference Doanh, Horii and Nawa2013). In support of this, metacercariae of P. heterotremus have been commonly found in Potamid crabs in northern provinces (Doanh et al. Reference Doanh, Horii and Nawa2013). Recent surveys have reported very high infection rates of P. westermani metacercariae in crab hosts (Potamiscus sp., which was later identified as Vietopotamon aluoiense) in central provinces (Doanh et al. Reference Doanh, Shinohara, Horii, Habe and Nawa2009, Reference Doanh, Horii and Nawa2013; Dang and Ho, Reference Dang and Ho2012). However, it remains unclear whether Vietnamese P. westermani might be infective to humans (Doanh et al. Reference Doanh, Horii and Nawa2013). In this study we also found high infection rates of P. westermani in Huong Hoa District, Quang Tri Province, central Vietnam and detected a new locality in Yen Bai Province in the north, suggesting that P. westermani is widely distributed in the country. In addition, we found two new crab hosts, D. haii and I. tannanti (the latter previously known as Potamon tannanti (Dang et al. Reference Dang, Thai and Pham1980)). Metacercariae of P. westermani are found to be the most prevalent in central area while P. heterotremus is the most common species in northern provinces. This distribution pattern may depend on many ecological/environmental factors, such as the presence of potential intermediate and final hosts, as well as host specificity of Paragonimus species. Regarding to the second intermediate hosts, all 7 Paragonimus species in Vietnam use Potamid crabs (Doanh et al. Reference Doanh, Horii and Nawa2013), however, the same crabs are usually infected with two or more Paragonimus species, suggesting low specificity among Paragonimus species. The finding of the present results suggest that special attention should be paid to human paragonimiasis caused by P. westermani in central provinces and co-infection with P. heterotremus in northern Vietnam. Highly specific molecular and immunodiagnostic methods, such as monoclonal antibody or inhibition ELISA assays, should be developed to identify the pathogen causing human paragonimiasis and to differentially diagnose between P. heterotremus and P. westermani infections.
In conclusion, we found P. westermani metacercariae for the first time in northern Vietnam and in two new second intermediate crab hosts, I. tannanti and D. haii. Metacercariae of P. westermani in Vietnam showed great morphological variation, but slight genetic variation. Paragonimus westermani metacercariae often co-occur with other Paragonimus species, including P. heterotremus, in the same crab hosts, suggesting possibility of co-infection in humans. Highly specific molecular and immunodiagnostic methods are required to make a differential diagnosis for P. heterotremus and P. westermani infections.
ACKNOWLEDGEMENTS
We would like to express our deep gratitude to Vietnam National Foundation for Science and Technology Development (NAFOSTED).
FINANCIAL SUPPORT
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.12-2012.52 to Pham Ngoc Doanh.











