INTRODUCTION
Trematode life cycles are complex, usually employing multiple hosts, and often with low specificity in the definitive vertebrate host. Resolution of their life cycles is therefore challenging, requiring direct linkage of morphologically distinct larval stages such as cercariae with adults (Brant et al. Reference Brant, Morgan, Mkoji, Snyder, Rajapakse and Loker2006). Furthermore, life cycle elucidation in the laboratory can be technically and ethically problematic. DNA sequencing and the development of databases with species-specific reference DNA sequence data have enabled larval and adult trematodes to be matched and hosts accurately incriminated, thus informing taxonomy, biodiversity and epidemiology (Brant et al. Reference Brant, Morgan, Mkoji, Snyder, Rajapakse and Loker2006).
The Lecithodendriidae (Digenea: Plagiorchiida) are a prime example of taxonomic uncertainty due to missing links between larval and adult stages. These parasites infect insectivorous vertebrates and typically use prosobranch molluscs as first intermediate hosts. The emergent cercariae encyst as metacercariae in aquatic insect larvae, which are later ingested as adult insects by foraging definitive hosts. More detailed life cycle elucidation exists for only a few species (reviewed in Kudlai et al. Reference Kudlai, Stunžėnas and Tkach2015) making it difficult to assess the diversity of these parasites and their contribution to trematode communities in host populations. In addition, identification to species level is important as lecithodendriids are common hosts of intracellular endosymbiotic Neorickettsia bacteria (Rickettsiales, Anaplasmataceae), which can cause debilitating and sometimes fatal diseases in vertebrates, including humans (Greiman et al. Reference Greiman, Vaughan, Elmahy, Adisakwattana, Van Ha, Fayton, Khalil and Tkach2017).
Published reports on Lecithodendriidae in the UK are limited to early morphological studies (e.g. Nicoll, Reference Nicoll1923; Brown, Reference Brown1933) and a detailed study of gastrointestinal Lecithodendrium spp. in pipistrelle bats by Lord et al. (Reference Lord, Parker, Parker and Brooks2012) who used molecular analysis to revise phylogenetic relationships between lecithodendriid species. Otherwise, there are morphological reports of xiphidiocercariae under provisional names such as Cercaria helvetica XII Dubois, 1928 (Nasir and Erasmus, Reference Nasir and Erasmus1964), now known to be phylogenetically close to, but not identical to, L ecithodendrium linstowi (Kudlai et al. Reference Kudlai, Stunžėnas and Tkach2015), illustrating the importance of molecular confirmation. Here, we report the first identification of the cercariae of L. linstowi using molecular and morphological approaches and molecular incrimination of the snail intermediate host. The cercariae were collected during the UK Digenean Diversity Project, a molecular study of digeneans infecting freshwater snails in the UK.
MATERIALS AND METHODS
Collection and screening of snails
Eighty-three Radix sp. (Lymnaeidae) snails were collected by hand net from the Queen's River, Bushy Park, Surrey, England (51°24′42″N; 0°20′27″W) in September 2013. This artificial river was created in the 17th century to bring water from the Colne River to Hampton Court Palace (Bushy Park Management Plan, The Royal Parks, 2014, unpublished). Snails were individually placed in 50 mL glass beakers containing filtered, dechlorinated water and were screened for emergent cercariae by microscopy in the laboratory. Only one snail, identified as Radix peregra based on shell morphology, was observed to shed xiphidiocercariae.
Morphological description of cercariae
Cercariae were fixed in 4% formaldehyde solution and stored in 70% ethanol prior to processing. Cercariae examined by light microscopy were stained with acetocarmine, dehydrated in a graded ethanol series, cleared in HistoChoice (Sigma-Aldrich, UK) and mounted in Canada balsam. Image capture and measurements of cercariae were made using a Nikon Eclipse NiE microscope and NIS-Elements BR (Nikon Instruments, UK) software. Cercariae examined by scanning electron microscopy were dehydrated in a graded ethanol series, dried in hexamethyldisilazane, attached to stubs using double-sided tape, sputter coated with gold palladium and examined under a Zeiss EVO 50 scanning electron microscope. Upon confirmation of species, parasite reference material was deposited at the Natural History Museum, London, UK (accession numbers NHMUK 2017·6·15·1-2).
Molecular analysis
Total genomic DNA was isolated from a pool of ten 96% ethanol-fixed morphologically identical cercariae using the Qiagen DNeasy Blood and Tissue Kit (Qiagen Inc.) following the manufacturer's protocol. PCR was performed to amplify partial fragments of the large ribosomal subunit (LSU) using primers LSU28S (forward; TAGGTCGACCCGCTGAAYTTAAGCA) and 1500R (reverse; GCTATCCTGAGGGAAACTTCG) as described by Olson et al. (Reference Olson, Cribb, Tkach, Bray and Littlewood2003). The internal transcribed spacer region (ITS) was amplified using primers; p1 (forward; GTCGTAACAAGGTTTCCGTAGGTG) and p2 (reverse; TATGCTTAAATTCAGCGGGTAATC) according to Wang et al. (Reference Wang, Li, Ni, Zhai, Chen, Chen and Zhu2009).
In order to accurately identify the Radix species acting as an intermediate host, DNA was also extracted from a tissue snip from the foot of the infected snail using the same methods described above, but with an extended 24 h initial digest. A partial fragment of the mitochondrial cytochrome c oxidase 1 gene (cox1) was amplified with PCR using primers LCO1490 (GGTCAACAAATCATAAAGATATTGG) and HCO2198 (TAAACTTCAGGGTGACCAAAAAATCA) using protocols described by Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and the ITS2 region was amplified using primers NEWS (TGTGTCGATGAAGAACGCAG) and RIXO (TTCTATGCTTAAATTCAGGGG) (Almeyda-Artigas et al. Reference Almeyda-Artigas, Bargues and Mas-Coma2000).
PCR amplicons generated from both the cercariae and the snail were visualized in 1% agarose gels stained with gel red (Bioline™) prior to sequencing using the same PCR primers with Fluorescent Dye Terminator Sequencing Kits (Applied Biosystems™) run on an Applied Biosystems™ 3730XL automated sequencer. Resultant sequences were assembled in BioEdit (Hall, Reference Hall1999) and corrected manually to resolve ambiguous base calls. BLASTn searches were performed at NCBI (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) to provide initial identification and to ensure no contamination and sequences were submitted to GenBank (accession numbers: MF498820- MF498823).
Phylogenetic analysis
The MUSCLE algorithm (http://www.ebi.ac.uk) was used to align the generated sequences with retrieved GenBank sequences: (i) for lecithodendriid spp., Maritrema spp., Microphallus spp. and Collyriclum faba were used as out-groups; (ii) for Radix spp., Lymnaea stagnalis was used as the outgroup. Since most of the available lecithodendriid sequences on GenBank were ITS2, the complete ITS sequence from this study and other retrieved sequences were trimmed to the ITS2 fragment prior to analysis.
Neighbour-joining (NJ) and maximum-likelihood (ML) methods were employed to perform phylogenetic reconstruction for the parasite and the snail species using MEGA v6 (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). For the xiphidiocercariae, NJ trees based on ITS2 and LSU were constructed under the conditions of the Kimura 2 parameter model (K2P). Based on the lowest Bayesian information criterion, MEGA6 identified that the K2P model with a gamma distribution best fit the ITS2 and LSU data; thus, both ML analyses were performed under the conditions of this model. For Radix spp., the NJ analysis for both the ITS2 and cox1 were performed under the conditions of the K2P model, but the ML analysis was performed using the Tamura 3 parameter with gamma distribution for ITS 2 and the Hasegawa–Kishino–Yano with gamma distribution for cox1. In all analyses, nodal support values were estimated using 1000 bootstrap replicates.
RESULTS
Morphological description of L. linstowi cercariae
The body was oval-elongate and very contractile, usually longer than the tail (Table 1, Fig. 1A–C). The oral sucker was sub-terminal, round-oval with a small central stylet (Fig. 1B and D). The ventral sucker was round-oval, located posterior to the mid-body (Fig. 1C and E). Fine spines and type 1 sensory papillae with tegumental collars covered the body tegument (Fig. 1D–F). The tail was simple with indented margins, without a finfold, spines or sensory papillae (Fig. 1G). Three pairs of penetration gland cells filled with granules were located anterior to the ventral sucker with ducts opening anteriorly either side of stylet. The pharynx was small and the intestinal tract was indistinct. The v-shaped excretory vesicle was thin-walled ending in a sub-terminal excretory pore. Numerous cystogenous cells and refractile granules obscured structures in the body (Fig. 1B).

Fig. 1. Cercariae of Lecithodendrium linstowi from Radix balthica. (A–C) Entire cercaria. (A) Line drawing, scale bar = 25 µm. (B) Photomicrograph, stylet (black arrow), penetration glands (stippled arrows), ventral sucker (white arrow), scale bar = 25 µm. (C) Scanning electron micrograph, scale bar = 20 µm. (D–G) Scanning electron micrographs showing characteristic features, including spinose body tegument. (D) Subterminal oral sucker, stylet detached during processing, scale bar = 2 µm. (E) Ventral sucker, sensory papillae (arrows), scale bar = 2 µm. (F) Junction of body with tail, sensory papillae (arrows), scale bar = 5 µm. (G) Simple tail, scale bar = 10 µm.
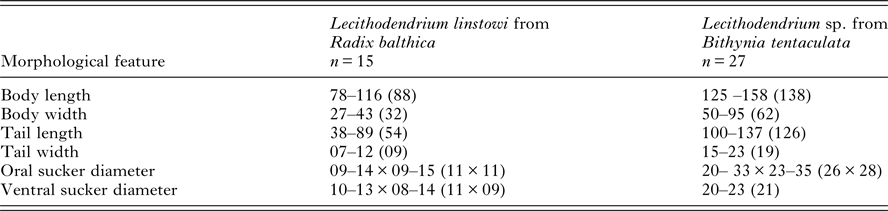
Table 1. Comparison of morphometric features of Lecithodendrium linstowi and Lecithodendrium sp. [syn. Cercaria helvetica XII Dubois, 1928 (Kudlai et al. Reference Kudlai, Stunžėnas and Tkach2015)] cercariae
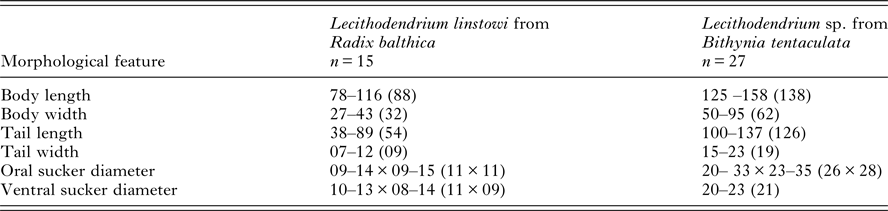
Measurements (μm) are given as the range followed by the mean value in parentheses.
Molecular and phylogenetic analysis
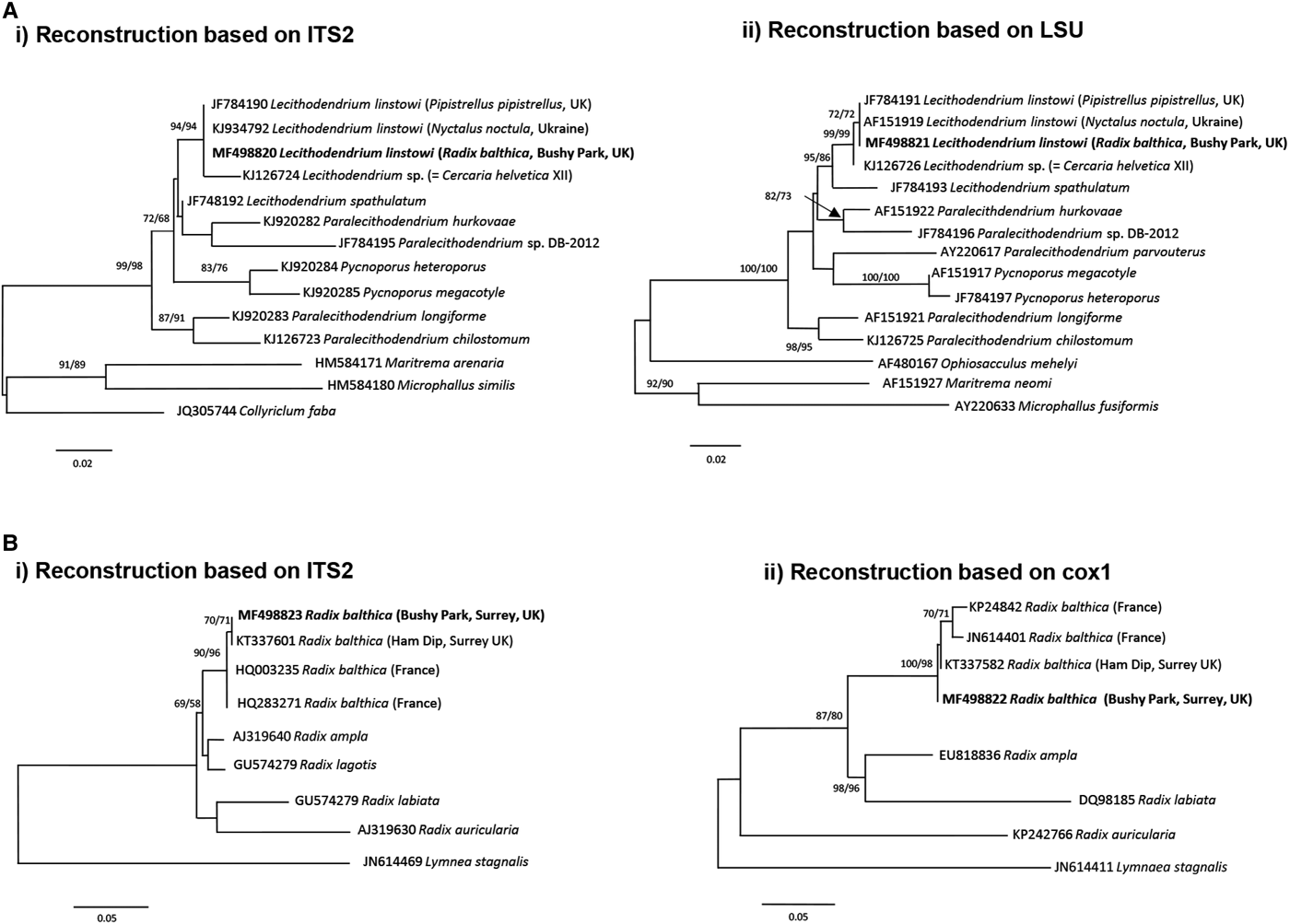
The xiphidiocercariae sequences were 930 base pairs (bp) long for the complete ITS (365 bp after trimming to the ITS2 fragment) and the partial fragments of the LSU were 1110 bp. BLAST searches on GenBank and pairwise p-distance comparisons of ITS2 and LSU sequences demonstrated that the cercariae were an exact match to L. linstowi. Phylogenetic analyses based on the ITS2 and LSU alignments for NJ and ML showed that the novel sequences clustered with L. linstowi adult sequences from bats and formed a clade with Lecithodendrium sp. cercariae (syn. C. helvetica XII Dubois, 1928) (Fig. 2A). Comparison of uncorrected pairwise genetic distance (p-distance) between both species using MEGA v6 revealed greater genetic divergence in ITS2 (0·014, 1·4%) than LSU (0·006, 0·6%).
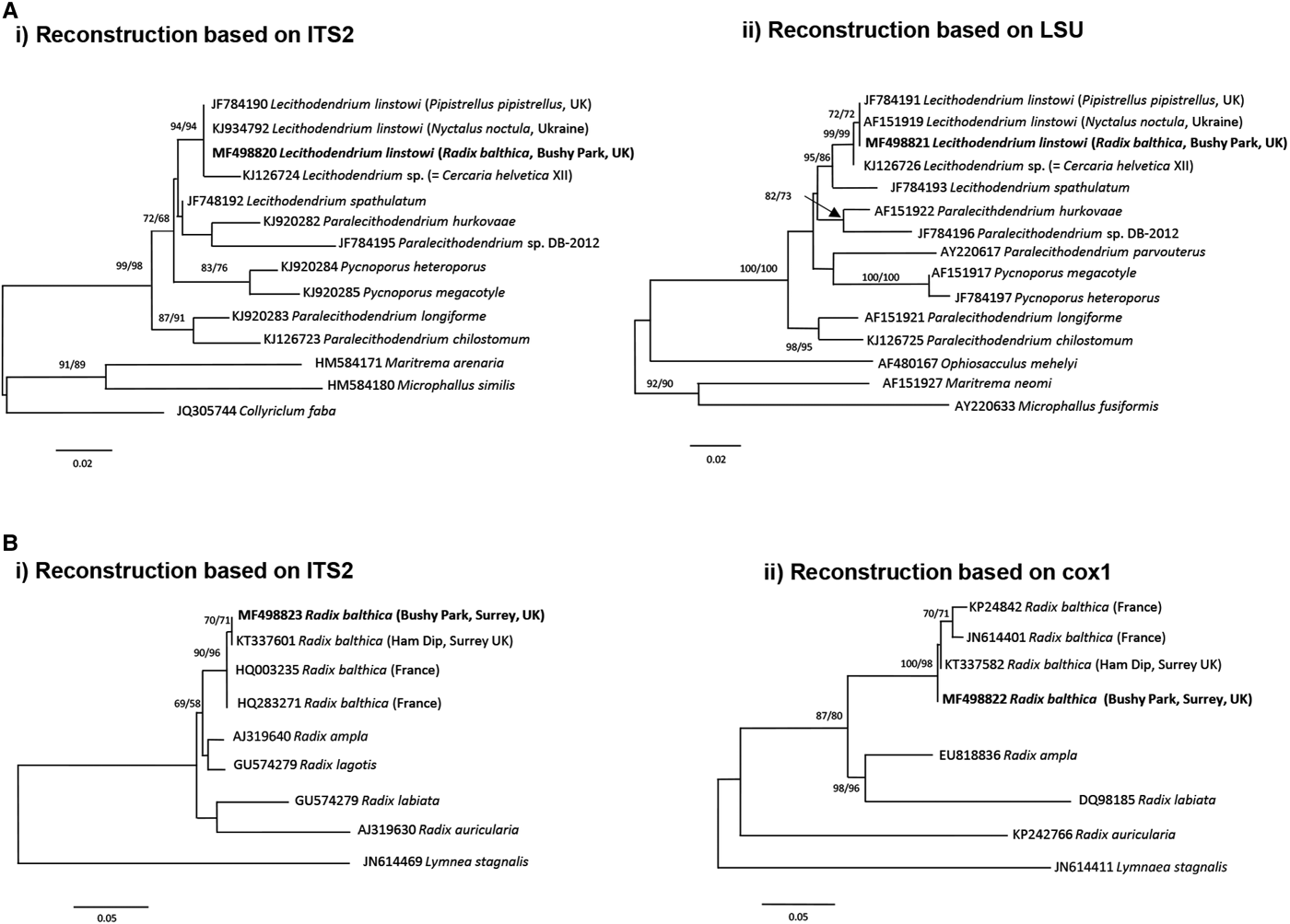
Fig. 2. Phylogenetic identification of Lecithodendrium linstowi and Radix balthica. (A) Phylogenetic reconstructions based on (i) ITS2 and (ii) LSU sequences of Lecithodendriidae used for the identification of xiphidiocercariae infecting R. balthica from Bushy Park, Surrey, UK. (B) Phylogenetic reconstructions based on (i) ITS2 and (ii) cox1 sequences used for identification of R. balthica from Bushy Park, Surrey, UK. Trees were constructed using the ML method. The scale shows the number of nucleotide substitutions per site between sequences. The nodal support is given in NJ and ML bootstraps respectively and shows values >50%. Sequences from this study are indicated in bold.
The generated ITS2 and cox1 sequences for the snail were 440 and 570 bp, respectively. Phylogenetic analysis based on both molecular markers and NJ/ML methods (Fig. 2B) produced congruent hypotheses regarding the placement of the novel sequences from this study. Three main sub-clades of Radix spp. were observed: R. ampla + R. lagotis; R. labiata + R. auricularia and Radix balthica in the ITS2 tree and R. ampla + R. labiata; R. auricularia and R. balthica in the cox1 tree. In both trees the sequence from this study clustered within the R. balthica clade (Fig. 2B).
DISCUSSION
We report the first molecular and morphological identification of the cercariae of L. linstowi and incrimination of R. balthica as the molluscan first intermediate host. The rDNA LSU and ITS2 data confirm that the xiphidiocercariae in this study were L. linstowi based on 100% sequence similarity to adults from Nyctalus noctula (common noctule) in the Ukraine (Tkach et al. Reference Tkach, Pawlowski and Mariaux2000) and Pipistrellus pipistrellus in the UK (Lord et al. Reference Lord, Parker, Parker and Brooks2012). Phylogenetic analysis of ITS2 and cox1 identified the snail host of L. linstowi as R. balthica, although it morphologically resembled R. peregra, and therefore further supports synonymy of R. balthica with R. peregra as proposed by Bargues et al. (Reference Bargues, Vigo, Horak, Dvorak, Patzner, Pointer, Jackiewicz, Meier-Brook and Mas-Coma2001) and Lawton et al. (Reference Lawton, Lim, Dukes, Kett, Cook, Walker and Kirk2015). The data emphasize the need for molecular identification of lymnaeid snails to determine their role as intermediate hosts in the life cycles of digeneans, particularly those of medical and veterinary importance.
Lecithodendrium linstowi is a generalist trematode species that is one of the most prevalent and abundant helminths of Eurasian bats (Esteban et al. Reference Esteban, Amengual and Cobo2001; Lord et al. Reference Lord, Parker, Parker and Brooks2012) and also infects the Hungarian harvest mouse (Micromys minutus pratensis) (Matskási, Reference Matskási1971). Its prevalence can be partly explained by the ubiquity of R. balthica. Adults of L. linstowi were first reported in the UK by Lord et al. (Reference Lord, Parker, Parker and Brooks2012) from the duodenum and upper jejunum of pipistrelle bats (P. pipistrellus and P. pygmaeus). Bushy Park is an important bat habitat with nine bat species recorded since 2004 (Bushy Park Management Plan, The Royal Parks, 2014 unpublished) so further lecithodendriid species are likely to exist at this location, particularly since L. linstowi is commonly associated with L. spathulatum, which probably shares aquatic insect larvae hosts (Lord et al. Reference Lord, Parker, Parker and Brooks2012). There is no evidence available for negative health impacts of lecithodendriid species on bat hosts.
Phylogenetic reconstruction illustrates a well-supported relationship between cercariae and adults of L. linstowi (Tkach et al. Reference Tkach, Pawlowski and Mariaux2000; Lord et al. Reference Lord, Parker, Parker and Brooks2012) and confirms the separate Lecithodendrium clade proposed by Lord et al. (Reference Lord, Parker, Parker and Brooks2012). Analysis of p-distance estimates of divergence verify that L. linstowi and Lecithodendrium sp. (syn. C. helvetica XII Dubois, 1928) from Bithynia tentaculata (Kudlai et al. Reference Kudlai, Stunžėnas and Tkach2015) are closely related separate species. The observed differences were within levels usually recorded among closely related congeneric species such as Echinostoma caproni and E. paraensei (Vilas et al. Reference Vilas, Criscione and Blouin2005). Both species have non-virgulate, morphologically similar xiphidiocercariae, although L. linstowi is smaller (Table 1). The lack of a virgula organ in L. linstowi demonstrates that this trait is not an absolute synapomorphy for lecithodendriids (Lotz and Font, Reference Lotz, Font, Bray, Jones and Gibson2008) and cannot be used as a broad phylogenetic characteristic.
The application of molecular approaches in the current study has enabled taxonomic linkage of cercariae of L. linstowi to adult stages without attempting life cycle elucidation, and accurate incrimination of the snail host, thus emphasizing the essential role of DNA sequencing in understanding digenean life cycles. Future molecular studies will be required to identify the second intermediate hosts of L. linstowi to achieve resolution of its life cycle. The intermediate host species for many bat parasites are unknown and the lack of reference material and DNA sequence data hinders an understanding of parasite biodiversity in bats. As highlighted by Lord and Brookes (Reference Lord, Brooks, Klimpel and Mehlhorn2014), protected species status in the UK means that bats, unless dead or euthanized due to injury, cannot be directly examined. Molecular-based surveys of first and second intermediate hosts are therefore important for long-term monitoring of parasitic infections in endangered bat populations and other vertebrates and the identification of emerging zoonoses. Lecithodendriidae in bats have been identified as hosts of Neoricketssia in Egypt, the Philippines, Thailand, North and South America. Neoricketssia are vertically transmitted through the parasite life cycle, but can be horizontally transmitted to vertebrate hosts and cause disease (Greiman et al. Reference Greiman, Vaughan, Elmahy, Adisakwattana, Van Ha, Fayton, Khalil and Tkach2017). Lecithodendrium sp. harbours Neoricketssia risticii, which causes the debilitating and sometimes fatal disease equine monocytic ehrlichiosis (Potomac horse fever) in the Americas. Horses are probably infected through inadvertently consuming metacercariae in insect hosts, while grazing or drinking (reviewed in Vaughan et al. Reference Vaughan, Tkach and Greiman2012). It is therefore important to screen accessible intermediate hosts for both digeneans and their endosymbiont bacteria to provide new insights into neorickettsial-digenean epidemiology.
ACKNOWLEDGEMENTS
We thank The Royal Parks Commission for permission to collect snails from Bushy Park, Surrey, Richard Giddens for assistance with scanning electron microscopy and the Molecular Sequencing Facility at the Natural History Museum.
FINANCIAL SUPPORT
This work was supported by the Tertiary Education Trust Fund, Nigeria (E.E.E., PhD studentship).