Published online by Cambridge University Press: 01 March 2004
The isolation and molecular characterization of the gene coding for L14 ribosomal protein from L. braziliensis is described. There are 2 copies of the gene per haploid genome, repeated in a head-to-tail tandem orientation and located in a single chromosome of approximately 950 kb. Northern blot analyses indicate the presence of a single transcript of 0·95 kb which is up-regulated when parasites reach the stationary growth phase. L. braziliensis L14 gene codes for a 175 amino acid long polypeptide showing 75–83% sequence identity with L14 proteins from trypanosomatids and approximately 25% with its counterparts from higher eukaryotic organisms. L14 ribosomal proteins from trypanosomatids and higher eukaryotes share along their molecules a similar distribution pattern of theoretically functional domains. L. braziliensis L14 recombinant protein is not recognized by sera from cutaneous leishmaniasis patients. Immunization of mice with one dose of L14 recombinant protein and a second dose of L14 protein covalently linked to the HSP70 from Trypanosoma cruzi induces a high antibody level against this L14 protein, which is mostly of the IgG2a subtype, as well as a strong increase in splenocyte proliferation index.
Trypanosomatid ribosomal proteins, like histones and heat-shock proteins, have a high sequence identity and a preserved cellular function along the evolutionary scale (Requena, Alonso & Soto, 2000). The L14 ribosomal protein is one of the molecules that form the large ribosomal subunit in eukaryotic organisms. The first complete amino acid sequence for an L14 ribosomal protein in eukaryotes was described in rats (Chan, Olvera & Wool, 1996). In spite of a high sequence identity, L14 proteins can differ significantly from each other in length. Thus, rat L14 is formed of 214 amino acids (Q63507) (Chan et al. 1996), its homologue in humans, of 213 (P50914) (Aoki et al. 1996), the Leishmania donovani protein of 175 (Q25278) (Campos-Neto et al. 1995) and Saccharomyces cerevisiae protein of 138 amino acids (P36105) (Boyer et al. 1993). Despite size differences, L14 ribosomal proteins present two highly conserved domains (Haynes, 1997). Since ribosome biosynthesis begins in the nucleus, ribosomal proteins (including L14) have to be translocated to the nucleus after their synthesis in the cytoplasm, in order to form the ribosome. The sequence that facilitates this protein transport is called the nuclear localization sequence (NLS), which is of the bipartite type formed by 2 separate clusters of basic regions of 3–4 residues (Miyamoto et al. 1997). Most of the L14 protein sequence variability is confined to the highly charged C-terminal region where basic amino acids predominate, mainly lysine and arginine. In L14, the presence of a series of amino-acid repetitions was detected. These are pentapeptides (reviewed by Requena et al. 2000) in the case of rat rL14 or tripeptides in Drosophila (Haynes, 1997) and humans (Tanaka et al. 1998).
Leishmania species belong to the Trypanosomatidae family and are causative agents of different pathologies in humans. During the natural infection by Leishmania species, a substantial Th1 and Th2-type cell response is produced by the host (Abbas, Murphy & Sher, 1996; Sjölander et al. 1998). However, there is evidence that protection against Leishmania infection is associated with a Th1 response (Sjölander et al. 1998; Pinto, de Mello Cortezia & Rossi-Bergmann, 2003). Many Leishmania proteins, including conserved family proteins such as histones, heat-shock proteins and ribosomal proteins, are recognized by the host immune system and elicit specific immune responses (reviewed by Requena et al. 2000). Immunization of BALB/c mice with the acid ribosomal protein LiP2a of L. infantum stimulates a Th1-type cellular proliferation and IFN-γ production (Soto, Alonso & Requena, 2000). However, immunization of mice with the L. major small subunit ribosomal protein, LmS3arp, causes inhibition of Th1 cytokine secretion (IFN-γ, IL-2 and IL12) and stimulates Th2-type cytokine secretion (IL-4 and IL-10) (Cordeiro-da-Silva et al. 2001).
Heat-shock protein Hsp70 in diverse organisms, eukaryotes and prokaryotes, is very effective at improving the immune response against the antigens with which it is complexed (reviewed by Srivastava, 2002). Direct participation of these proteins in antigen presentation is thus suggested. It has been shown that immunization of animals with haptens attached to Hsp proteins generates specific antibodies against the haptens (Barrios et al. 1992; Perraut et al. 1993).
This study presents the isolation and molecular characterization of a gene coding for L14 ribosomal protein in L. braziliensis. The L14 protein recognition by sera of patients with cutaneous leishmaniasis is analysed, and the immune response (humoral and cellular) the protein generates in BALB/c mice immunized with the L14 recombinant protein is described.
L. braziliensis promastigotes (MOHN/PE/95/LQ-8) were grown with gentle shaking at 22 °C in RPMI 1640 medium (Gibco Paisley, UK), supplemented with 20% heat-inactivated bovine foetal serum. Cultures were initiated at 1×106 promastigotes/ml.
After L. braziliensis genomic DNA RAPD application (Williams et al. 1990), a series of different sized fragments was amplified and cloned in pBSKS vector (Stratagene). Sequence analysis of randomly selected clone revealed the existence of a 286 bp long ORF showing significant homology with a region of the gene coding for L14 ribosomal proteins of different trypanosomatids. This fragment was used as a probe to screen an L. braziliensis genomic library constructed in lambda phages, as described by Carmelo et al. (2000). Sequencing of a positive clone revealed the presence of the full-length L14 coding region. L. braziliensis L14 gene was subsequently PCR amplified using the isolated positive clone and the primer L14-S (5′-GGATCCGCATAATGGTCAAGTCCC-3′) and L14-A (5′-AAGCTTTTACTTCTTGGCCTTGGG-3′), which contain respectively the L14 start and stop codons and BamHI and HindIII restriction sites (underlined) and cloned in pGEMT vector (Promega Co., Madison, USA), to produce the pGEMT/L14 clone.
L. braziliensis genomic DNA was isolated by standard methods (Carmelo et al. 2000). Two μg of L. braziliensis genomic DNA was fully digested with Msp I and Sph I enzymes (Roche Diagnostics, Mannheim, Germany), which digest respectively twice and once inside the predicted ORF; then with Dde I restriction enzyme (Roche Diagnostics), which cuts once, 67 bp downstream of the stop codon at the non-coding 3′ end, before being size-resolved on 0·8% agarose gel. Total L. braziliensis RNA was purified by the guanidinium thiocyanate method (Sambrook, Fritsch & Maniatis, 1989) and 5 μg were size-fractionated on 1% agarose/formaldehyde gel. For PFGE analysis, agarose blocks containing approximately 80×106 promastigotes were prepared as previously described (Thomas & González, 1997) and stored at 4 °C in 0·5 M EDTA, pH 9·5. One fifth of each block was electrophoresed (1% agarose in 0·5× Tris–borate-EDTA buffer (TBE) at 12 °C. The running conditions were single pulses of 75 sec for 28 h, 100 sec for 18 h and 200 sec for 18 h at 50 V. DNA and RNA were transferred to nylon membranes (Bio-Rad, Richmond, CA, USA) using 10× SSC (for DNA) and 50 mM NaOH (for RNA) and hybridized with [α-32P]dCTP random-labelled L14 coding region. Hybridization conditions were undertaken overnight as previously described (Martínez et al. 2002). Stringency washes were performed in 0·1×SSC/0·1%SDS at 65 °C for 30 min. The membranes were exposed overnight to photographic films at −70 °C.
To generate the L. braziliensis L14 recombinant protein (LbrL14), pGEMT/L14 plasmid was digested with BamHI and HindIII enzymes (Roche Diagnostics) and the L. braziliensis ribosomal protein L14 coding region subsequently subcloned into pQE32 vector (Quiagen, Hilden, Germany) and digested with the same enzymes generating the pQE32LbrL14 expression vector. Sequencing was performed in order to check correct in-frame cloning and nucleotide composition. Purification of the LbrL14 protein was carried out by Ni2+ affinity chromatography after its overexpression by induction of vector transformed E. coli M15 strain with 0·1 mM isopropyl-β-D-thiogalacto-pyranoside (IPTG) for 2 h. The recombinant protein rLbrpL14 was extracted from the bacterial pellet in sonication buffer (300 mM NaCl, 50 mM Na2HPO4, 1 mM phenylmethylsulphonylfluoride) containing 0·05% SDS. The soluble protein extract was incubated with Ni-NTA agarose resin (Quiagen, Hilden, Germany) for 1 h at room temperature. The resin was washed twice with the sonication buffer supplemented respectively with 5 and 10 mM imidazole and the recombinant rLbrpL14 protein was finally eluted in the extraction buffer containing 15 mM imidazole. The LbrpL14 recombinant protein was extensively dialysed against PBS and the degree of purity was determined by SDS–PAGE and Coomassie blue staining (Laemmli et al. 1970). Protein concentration was measured by the Bradford method (Bio-Rad, Richmond, CA, USA). The purified L14 recombinant protein was tested by the E-Toxate reaction kit (Limulus amoebocyte lysate, Sigma, St Louis, MO, USA) and the endotoxin level was below the detection limit of the kit (<0·1 endotoxin units/mL).
In total, 46 sera from individuals suffering different pathologies were tested. Thus, 24 sera corresponded to Peruvian cutaneous leishmaniasis (CL) patients whose infection was diagnosed by culture and microscopical visualization of parasites by the Microbiology Laboratory, Faculty of Biology, San Antonio Abad University of Cusco (Peru). Ten sera were from Brazilian Chagas' disease patients in the chronic phase of the infection, diagnosed by ELISA and the presence of complement-fixing antibodies (Zurita et al. 2003). Four sera, from Peruvian individuals living in the same area as the former and without history of contact with Leishmania, and 8 sera from individuals in the Canary Islands (Spain) without previous contact with the parasite, who had never travelled to Leishmania-endemic areas, were found negative by ELISA against a Leishmania total antigen extract (Pedrosa et al. 1999) and employed as negative controls in the ELISA assays.
BALB/c female mice (6–8 weeks old) were obtained from IFFA-CREDO (CRIFFA, Lyon, France) and held under clean conventional conditions. Groups of 4 mice received 3 subcutaneous immunizations on days 0, 21 and 63. Group 1 received three 20 μg doses of purified LbrL14. Group 2 was immunized 3 times with 20 μg of LbrL14 emulsified with incomplete Freund's adjuvant (IFA) (1[ratio ]1, v/v). Group 3 was first immunized with 20 μg of LbrL14 and subsequently two 20 μg doses of LbrL14 cross-linked to T. cruzi Hsp70 protein in molar proportion (4[ratio ]1) using 0·2% glutaraldehyde solution (Marañón et al. 2001). As a control group, mice were immunized with saline solution.
The ELISA test for L. braziliensis-specific antibody was performed as described by González et al. (2002), using as secondary antibody either affinity-purified goat anti-mouse IgG (Fab specific) (Sigma, St Louis, MO, USA), IgG1 (subclass specific) or IgG2a (subclass specific) (Nordic) peroxidase-conjugated antibodies at a dilution of 1[ratio ]2000, 1[ratio ]8000 and 1[ratio ]1000, respectively. Protein was fixed with carbonate buffer, pH 9·6 at 5 μg/ml. All sera were diluted to 1/100 and 1/200 in order to confirm the reaction linearity at this antigen concentration, and the reaction was maintained for 2 h at 37 °C in PBS–Tween-20 with skimmed milk at 5%. Sera from pre-immune mice were used as negative controls. Absorbance was determined as the difference between the arithmetic mean absorbance from each serum diluted to 1/100 and the arithmetic mean obtained from pre-immune sera. All assays were performed in duplicate.
For cell proliferation assays, as described by Marañón et al. (2000), spleen cells were split in flat-bottom 96-well plates (4×105 cells/well) in the presence of 20, 4 and 0·2 μg/ml of antigen or Con A (1 μg/well, Sigma, St Louis, MO, USA) in triplicate wells. The final volume was adjusted to 200 μl/well in Dulbecco's modified Eagle medium (Gibco BRL, Paisley, Scotland) supplemented with 10% foetal calf serum (Life Technologies, Eggenstein, Germany), 2 mML-glutamine (Gibco BRL), 0·25 mM arginine (Merck, Darmstadt, Germany), 0·55 mM asparagine (Merck, Darmstadt, Germany), 50 μM 2-mercaptoethanol (Sigma, St Louis, MO, USA), 100 IU/ml penicillin (Sigma, St Louis, MO, USA), 100 μg/ml streptomycin (Sigma, St Louis, MO, USA), 10 mM HEPES (Sigma, St Louis, MO, USA) and 1 mM sodium pyruvate (Riedel-de Haen, Seelze, Germany). Plates were incubated at 37 °C in a 5% CO2 atmosphere for 3 days. After addition of [methyl-3H] thymidine (0·5 μCi/well), cells were incubated for another 6 h at 37 °C. Genomic DNA was immobilized in glass-fibre filtermats using an Inotech harvester. The 3H incorporation was measured in a Wallac 1450 microbeta counter device.
The positive clone from the genomic library, with an insert of 13 kb, was isolated and partially sequenced. Nucleotide sequence analysis of the fragment revealed the presence of an ORF of 528 nucleotides (Accession number AF233642). The deduced amino acid sequence of this ORF codes for a 175 amino acid long polypeptide, denominated LbrL14, with a theoretical molecular mass of 20·08 kDa and pI of 10·90. BLAST analysis of the L. braziliensis L14 with other L14 sequences of different trypanosomatids (Fig. 1) demonstrated 82·9% identity with the homologous L. donovani protein, 76·4% with the L14 protein from Trypanosoma congolense and 74·7% identity with the L14 from T. brucei. However, the homology detected with other L14 sequences belonging to different non-trypanosomatid organisms, was significantly lower: 22·85% identity with human L14 (Homo sapiens), 27·2% with the rat (Rattus norvegicus) and 20·05% with the yeast Saccharomyces cerevisiae.

Fig. 1. Alignment of L14 ribosomal protein sequence from eukaryotes and prokaryotes. L. braz: Leishmania braziliensis (AAF73072); L. dono: Leishmania donovani (Q25278); T. bruc: Trypanosoma brucei (P55842); T. cong: Trypanosoma congolensis (T11856); Human: Homo sapiens (JC5954); Rat: Rattus norvegicus (JC4808); S. cere: Saccharomyces cerevisiae (S46797); identical residues to the LbrL14 deduced amino-acid sequence in L14 ribosomal proteins are boxed. The 7 conserved amino acids described in all L14 ribosomal proteins are in bold. The 2 proposed conserved domains of these proteins are double-underlined and named as A and B.
The ribosomal L14 from L. braziliensis contains a high percentage of basic amino acids (18·2% arginine and 17·5% lysine). These residues confer a high level of positive charge to the protein, a common characteristic of ribosomal and histone proteins, involved in nucleic acid interactions in eukaryotic organisms (Asland et al. 1994). The 7 conserved amino acids described in the L14 ribosomal proteins (Haynes, 1997) are also present in LbrL14 at similar positions (Fig. 1, in bold). Moreover, the two conserved domains proposed as critical sequences for the function of the L14 proteins (Haynes, 1997) are also present in L. braziliensis L14 indicated as A and B in Fig. 1. The characteristic DF(D/E) R(F/Y) motif contained in the B domain of L14 ribosomal protein is found in the L. braziliensis sequence as DFERY.
A theoretical analysis of the putative regulation domains of L. braziliensis L14 ribosomal protein showed the existence of one N-glycosylation site (68–71 residues), a protein kinase C phosphorylation site (located at position 80–82) and one casein-kinase II phosphorylation site (106–109 residues). All these domains are present in the L14 proteins from other trypanosomatids (Fig. 2). Moreover, in the carboxyl-terminal region of the L. braziliensis L14 a potential bipartite nuclear targeting sequence is observed (149–163 residues).
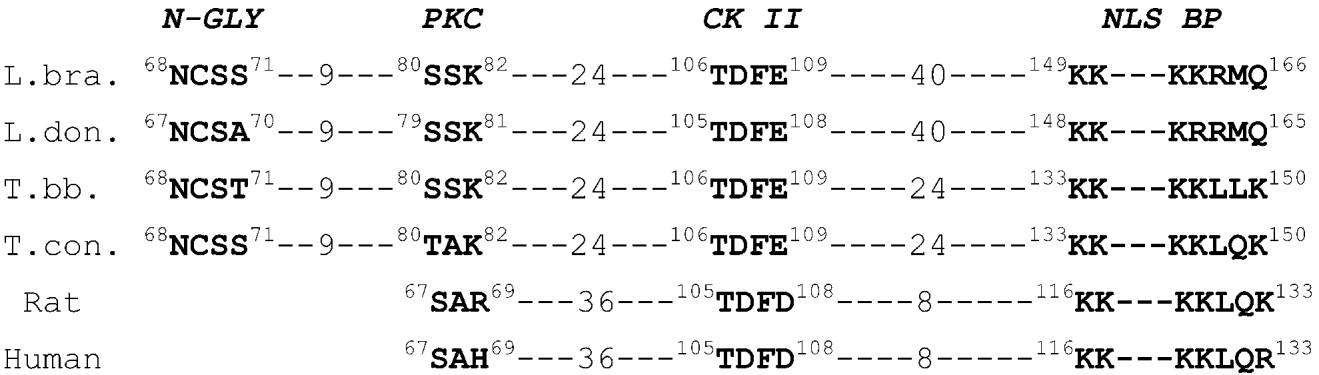
Fig. 2. Schematic representation of the theoretical functional domains of L14 ribosomal protein from different species. The amino acid sequence of the putative domains, N-glycosylation site (N-GLY), protein kinase C phosphorylation site (PKC), casein-kinase II phosphorylation site (CK II) and bipartite type nuclear localization signal sequence (NLS BP) is shown for all the proteins. The position of the motifs inside the sequences and the number of amino acids separating them is indicated.
The genomic organization of this gene was studied by Southern blot and chromosomal blotting of genomic DNA using the L. braziliensis L14 coding region as a probe. Fig. 3A shows the pattern of bands observed after digestion of L. braziliensis genomic DNA with 3 restriction enzymes which cut inside (Sph I and Msp I) and outside (Dde I) the L14 coding region (Accession number AF233642). The presence of 2 hybridization bands of similar intensity in all the lanes, one of them with the same size (1·6 kb), indicated that 2 copies of the L14 gene are arranged in tandem. Fig. 3B shows the proposed organization for the L. braziliensis L14 locus. To determine the chromosomal location of the ribosomal L14 gene cluster, L. braziliensis chromosomes were separated by pulsed-field gel electrophoresis and hybridized with the L14 coding region. The results shown in Fig. 3C indicate that the L14 locus is indeed located in a single chromosome of about 900 kb.

Fig. 3. (A) Southern blot analysis, 2 μg of Leishmania braziliensis promastigote genomic DNA was digested with Msp I (M), Sph I (S) and Dde I (D) restriction enzymes and separated on 0·8% agarose gel electrophoresis. After blotting the filter was hybridized with L14 coding region labelled with [α-32P]dCTP. The size of the hybridized bands (Kb) is indicated on the left of the panel. (B) Map representing the L14 locus from L. braziliensis. The coding regions are schematized (hatched boxes). S, Sph I; D, Dde I and M, Msp I. (C) PFGE analysis, chromosomes from 16×106 promastigotes were size resolved on 1% agarose gel electrophoresis, transferred to nylon membrane and hybridized with [α-32P]dCTP labelled L. braziliensis L14 coding region. MW, Yeast DNA-PFGE markers in Mb (Amersham Pharmacia Biotech Inc.).
When Northern blots containing total RNA from L. braziliensis promastigote forms in the logarithmic and stationary phases of growth were probed with the L. braziliensis L14 coding region, a single hybridization band of approximately 0·97 kb was detected in both growth phases (Fig. 4). Densitometric analysis of the hybridization bands revealed that the L14 RNA level was up-regulated when the parasites reached the stationary growth phase, with a 3-fold increase in the L14 RNA level.

Fig. 4. Northern blot analysis of Leishmania braziliensis L14 messenger. Five μg of ethidium bromide stained total RNA from Trypanosoma cruzi epimastigotes and L. braziliensis promastigotes in logarithmic (L) and stationary (S) phase of growth were separated on 1% agarose/formaldehyde gel (A), transferred to a nylon membrane and hybridized to the radio-isotope labelled L. braziliensis L14 coding region (B). Size of T. cruzi ribosomal messengers is indicated on the left-hand side of panel A and size of LbL14 messenger with an arrow in panel (B).
The overexpressed protein profile is shown in Fig. 5 (lane 2), where an intensely stained band of approximately 22 kDa corresponding to the expected size of the LbrL14 protein is observed. Fig. 5 (lane 3) shows the purified rLbL14 recombinant protein obtained by passing the soluble fraction of E. coli M15 strain overexpressing LbrL14 (see Fig. 5, lane 4) through a Ni2+ affinity chromatography column. The degree of purity was found to be higher than 90% as assessed by Coomassie blue staining.

Fig. 5. SDS–polyacrylamide gel electrophoresis of E. coli whole lysate and LbrL14 protein. Lane 1, whole lysate from non-induced culture from pQE32LbrL14 transformed E. coli; Lane 2, whole lysate from E. coli overexpressing LbrL14 protein; Lane 3, soluble protein fraction from E. coli extract of bacteria overexpressing LbrL14 protein; Lane 4, LbrL14-purified recombinant protein after Ni-NTA chromatography. Molecular size marker (MW) is shown in kDa.
The reactivity against L. braziliensis L14 protein of a collection of 24 sera from leishmaniasis patients was determined by ELISA using it as antigen. The sera from 10 chagasic patients were also assayed and 12 healthy individuals were used as controls. The cut-off value, established at 1[ratio ]100 sera dilution, was defined as the mean absorbance value of the healthy control sera plus 3 standard deviations (cut-off=0·055). The results indicated that the recombinant protein is not recognized by sera from leishmaniasis or chagasic patients, in either native or denaturing conditions.
The antibody response (IgG) generated against the rLbL14 protein is shown in Fig. 6. The animals immunized with the LbrL14 protein alone did not produce any significant level of anti-L14 antibodies. However, those immunized with the LbrL14 protein emulsified in IFA or linked to Hsp70 presented a high titre of anti-L14 IgG antibodies, slightly higher in BALB/c mice immunized with LbrL14-IFA. In both cases, immunogens enhanced the humoral immune response against the LbrL14 antigen in a dose-dependent manner. Six weeks after the third immunization (15 weeks in Fig. 6A) a positive response against LbrL14 could still be detected, with OD values of 0·62 and 0·5 at 1/100 sera dilution for mice immunized respectively with LbrL14-IFA and LbrL14-Hsp70. Analysis of the IgG subclasses in the pooled sera revealed that immunization with LbrL14 linked to HSP70 induced a clear IgG2a antibody bias 6 weeks after the third immunization (Fig. 6B), with an IgG2a/IgG1 ratio of 1·6. However, the mice immunized with LbrL14 emulsified in IFA showed an anti-L14 IgG2a/IgG1 balance of approximately 1 at 3 and 6 weeks after the third immunization. The antibodies generated against the LbrL14 recognized the native protein in parasite extracts by Western blot at dilution 1/20 (figure not shown).

Fig. 6. Analysis of IgG (A), IgG2a (B1) and IgG1 (B2) antibody responses of BALB/c mice immunized with LbrL14 alone (hatched bars), LbrL14 protein emulsified with IFA (grey bars), or with 1 dose of LbrL14 protein alone and 2 doses of LbrL14 recombinant protein cross-linked to Trypanosoma cruzi HSP70 protein (solid bars). The plot shows the average of the optical density readings obtained at 490 nm in sera as a function of time after the first immunization.
In order to study the effect of T. cruzi Hsp70 carrier on the cell response induced by the L14, lymphoproliferation assays were carried out. The results shown in Fig. 7 indicate that splenocytes from mice immunized twice with LbrL14 protein alone produce a low level of cellular proliferation, detected only at a protein concentration of 20 μg/ml. However, splenocytes from mice whose second immunization was with LbrL14 protein linked to T. cruzi Hsp70 protein, showed significant cell proliferation, with a stimulation index of 3 in the presence of 0·2 μg/ml LbrL14 protein. As expected, in splenocytes from saline solution immunized control mice, no lymphocyte stimulation was detected.

Fig. 7. Lymphoproliferative response to LbrL14 protein in mice immunized with recombinant LbL14 protein. Spleen cells from mice immunized with saline solution ([squf ]), LbrL14 protein alone ([utrif ]) or LbrL14 protein linked to Trypanosoma cruzi Hsp70 protein ([bull ]) were removed 4 weeks after the second immunization and cultured for 3 days in the presence of 0·2, 4 and 20 μg/ml of LbrL14 purified protein. After addition of [methyl-3H] thymidine (0·5 μCi/well), cells were incubated for another 6 h at 37 °C and 3H incorporation was measured. Stimulation index was calculated as (arithmetic mean of c.p.m. (stimulated culture) – arithmetic mean of c.p.m. (control culture))/arithmetic mean of c.p.m. (control culture).
Little is known about the basic ribosomal proteins, such as L14, in the lower eukaryotic protozoan parasites belonging to the Trypanosomatidae family. A few trypanosomatid L14 ribosomal proteins have previously been described but there are no reported data describing their molecular and immunological characteristics. Analysis of amino acid sequence homology reveals that L14 ribosomal protein is highly conserved among trypanosomatids but substantially differs from the L14 proteins of higher eukaryotes. However, L14 ribosomal proteins from trypanosomatids and higher eukaryotes share a similar pattern of theoretically active domains. Interestingly, the distances between these motifs are also maintained within the genus, which indicates that although there are yet no experimental data to confirm that all these motifs are functionally active, their presence with conserved sequences and distribution in trypanosomatids and higher eukaryotic organisms might be an indication of their importance for L14 protein activity. An equivalent situation has recently been described for L25 ribosomal protein from L. braziliensis, where 3 rRNA binding-site motifs that maintain the same amino acid distribution among distantly evolved organisms have been reported (González et al. 2002).
Molecular characterization of the L. braziliensis L14 gene revealed the presence of 2 copies per haploid genome repeated in a head-to-tail tandem orientation, located in a single chromosome of approximately 900 kb. The copy number of the gene coding for the L14 in the genome of eukaryotic organisms is variable. Thus, as in L. braziliensis, Xenopus laevis shows 2 genes coding for L14 (Beccari et al. 1986), while the rat genome contains from 6 to 8 copies of the L14 gene unit (Chan et al. 1996). The 2 L14 gene units from L. braziliensis show polymorphism at nucleotide level that affects endonuclease restriction sites. A similar polymorphism has been described in the locus coding for the L. braziliensis L25 ribosomal protein (González et al. 2002). Northern blot analysis using RNA from L. braziliensis promastigotes during the different growth phases of the parasite indicates that the L14 mRNA level increases when the parasite culture enters the stationary growth phase. These data could suggest that the half-life of the L14 messenger in the stationary growth phase is higher than in the logarithmic growth phase as described for H2A mRNA from T. cruzi (Marañón et al. 2000b).
Antigenicity analysis of the L. braziliensis recombinant L14 revealed that this protein is not recognized by sera from cutaneous leishmaniasis patients. Furthermore, immunization of mice with 2 doses of the purified L14 recombinant protein does not generate a detectable antibody level against L14 protein in contrast to other Leishmania ribosomal proteins such as P0 (Soto et al. 2000) or the Leishmania protein homologous to mammalian ribosomal protein S3a, named LmS3arp (Cordeiro-da-Silva et al. 2001). In addition, the data presented in this paper reveal that when the second immunization is carried out with the ribosomal L14 covalently linked to Hsp70 from T. cruzi, a strong specific humoral response is induced. Interestingly, the antibodies generated against L14 were mostly of the IgG2a subtype, as an indication of a Th1 type induced response (Coffman, Lebman & Rothman, 1993). Moreover, splenocytes of mice immunized with a second dose of L14 linked to Hsp70 revealed an increase in the cell proliferation index compared to those from mice immunized solely with L14. This is not the first time that non-immunogenic molecules change their behaviour after fusion to a heat-shock protein. A covalent linkage between the Hsp70 from Mycobacterium tuberculosis and the p24 HIV-1 protein was thus necessary to develop a humoral and cellular response against p24 viral protein in BALB/c mice (Suzue & Young, 1996). Furthermore, mice immunized with KMP11 protein fused to T. cruzi Hsp70, but not with KMP11 alone, generate a strong humoral and cell response with the induction of IgG2a antibodies and specific CTLs against KMP11 antigen (Marañón et al. 2001; Planelles et al. 2001). The 70 kDa heat-shock protein immunomodulative activity could be related with its ability to interact with antigen-presenting cells (APC) (Singh-Jasuja et al. 2001).
This study suggests that L. braziliensis L14 ribosomal protein is not perceived by the host immune system in either natural infection or experimental immunization. However, with an appropriate carrier it does generate an immune response.
This work was supported by Grant FIS 99/1038 and Grant FIS PI021511, Spain. Dr M. C. Thomas and Dr M. C. López were supported by Grants 00/145 SAS and FIS 011084, Spain. We thank Dr Rosa Pacheco for kindly providing the Peruvian cutaneous leishmaniasis patients' sera.

Fig. 1. Alignment of L14 ribosomal protein sequence from eukaryotes and prokaryotes. L. braz: Leishmania braziliensis (AAF73072); L. dono: Leishmania donovani (Q25278); T. bruc: Trypanosoma brucei (P55842); T. cong: Trypanosoma congolensis (T11856); Human: Homo sapiens (JC5954); Rat: Rattus norvegicus (JC4808); S. cere: Saccharomyces cerevisiae (S46797); identical residues to the LbrL14 deduced amino-acid sequence in L14 ribosomal proteins are boxed. The 7 conserved amino acids described in all L14 ribosomal proteins are in bold. The 2 proposed conserved domains of these proteins are double-underlined and named as A and B.

Fig. 2. Schematic representation of the theoretical functional domains of L14 ribosomal protein from different species. The amino acid sequence of the putative domains, N-glycosylation site (N-GLY), protein kinase C phosphorylation site (PKC), casein-kinase II phosphorylation site (CK II) and bipartite type nuclear localization signal sequence (NLS BP) is shown for all the proteins. The position of the motifs inside the sequences and the number of amino acids separating them is indicated.
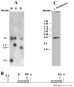
Fig. 3. (A) Southern blot analysis, 2 μg of Leishmania braziliensis promastigote genomic DNA was digested with Msp I (M), Sph I (S) and Dde I (D) restriction enzymes and separated on 0·8% agarose gel electrophoresis. After blotting the filter was hybridized with L14 coding region labelled with [α-32P]dCTP. The size of the hybridized bands (Kb) is indicated on the left of the panel. (B) Map representing the L14 locus from L. braziliensis. The coding regions are schematized (hatched boxes). S, Sph I; D, Dde I and M, Msp I. (C) PFGE analysis, chromosomes from 16×106 promastigotes were size resolved on 1% agarose gel electrophoresis, transferred to nylon membrane and hybridized with [α-32P]dCTP labelled L. braziliensis L14 coding region. MW, Yeast DNA-PFGE markers in Mb (Amersham Pharmacia Biotech Inc.).
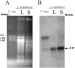
Fig. 4. Northern blot analysis of Leishmania braziliensis L14 messenger. Five μg of ethidium bromide stained total RNA from Trypanosoma cruzi epimastigotes and L. braziliensis promastigotes in logarithmic (L) and stationary (S) phase of growth were separated on 1% agarose/formaldehyde gel (A), transferred to a nylon membrane and hybridized to the radio-isotope labelled L. braziliensis L14 coding region (B). Size of T. cruzi ribosomal messengers is indicated on the left-hand side of panel A and size of LbL14 messenger with an arrow in panel (B).
Fig. 5. SDS–polyacrylamide gel electrophoresis of E. coli whole lysate and LbrL14 protein. Lane 1, whole lysate from non-induced culture from pQE32LbrL14 transformed E. coli; Lane 2, whole lysate from E. coli overexpressing LbrL14 protein; Lane 3, soluble protein fraction from E. coli extract of bacteria overexpressing LbrL14 protein; Lane 4, LbrL14-purified recombinant protein after Ni-NTA chromatography. Molecular size marker (MW) is shown in kDa.

Fig. 6. Analysis of IgG (A), IgG2a (B1) and IgG1 (B2) antibody responses of BALB/c mice immunized with LbrL14 alone (hatched bars), LbrL14 protein emulsified with IFA (grey bars), or with 1 dose of LbrL14 protein alone and 2 doses of LbrL14 recombinant protein cross-linked to Trypanosoma cruzi HSP70 protein (solid bars). The plot shows the average of the optical density readings obtained at 490 nm in sera as a function of time after the first immunization.

Fig. 7. Lymphoproliferative response to LbrL14 protein in mice immunized with recombinant LbL14 protein. Spleen cells from mice immunized with saline solution ([squf ]), LbrL14 protein alone ([utrif ]) or LbrL14 protein linked to Trypanosoma cruzi Hsp70 protein ([bull ]) were removed 4 weeks after the second immunization and cultured for 3 days in the presence of 0·2, 4 and 20 μg/ml of LbrL14 purified protein. After addition of [methyl-3H] thymidine (0·5 μCi/well), cells were incubated for another 6 h at 37 °C and 3H incorporation was measured. Stimulation index was calculated as (arithmetic mean of c.p.m. (stimulated culture) – arithmetic mean of c.p.m. (control culture))/arithmetic mean of c.p.m. (control culture).