Introduction
Eosinophilic meningitis caused by infection with Angiostrongylus cantonensis, the rat lungworm, is an emerging infectious disease, with increasing numbers of cases and recent expansion to new geographic areas (Wang et al., Reference Wang, Lai, Zhu, Chen and Lun2008; Lv et al., Reference Lv, Zhang, Steinmann, Yang, Yang, Zhou and Utzinger2011; Cowie, Reference Cowie2013a; Flerlage et al., Reference Flerlage, Qvarnstrom, Noh, Devicenzo, Madni, Bagga and Hysmith2017; Stockdale Walden et al., Reference Stockdale Walden, Slapcinsky, Roff, Calle, Goodwin, Stern, Corlett, Conway and McIntosh2017). Angiostrongylus cantonensis is a parasitic nematode with a complex life cycle (Alicata, Reference Alicata1965). Various species of rats are the definitive hosts (Yong and Eamsobhana, Reference Yong and Eamsobhana2015) and numerous gastropod species from widely different taxonomic groups have been recorded as intermediate hosts (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014). Accidental hosts such as humans and other mammals, as well as birds, are infected in the same manner as the definitive rat hosts, primarily by ingesting infected intermediate or paratenic hosts (Cowie, Reference Cowie2013b; Burns et al., Reference Burns, Bicknese, Qvarnstrom, DeLeon-Carnes, Drew, Gardiner and Rideout2014). However, in these accidental hosts, most larvae do not complete their natural life cycle. Instead they remain in the central nervous system and eventually die, primarily in the brain (Cowie, Reference Cowie2013a). Humans are usually infected through inadvertent consumption of infected snails on produce or wilful eating of raw or undercooked snails (Cowie, Reference Cowie2013b).
The first known human case of eosinophilic meningitis caused by A. cantonensis was in 1945 in Taiwan (Beaver and Rosen, Reference Beaver and Rosen1964). However, it was not until reports of cases in French Polynesia (Rosen et al., Reference Rosen, Laigret and Bories1961) and Hawaii (Horio and Alicata, Reference Horio and Alicata1961) that the link between the disease and infection by A. cantonensis became widely understood. Since then, cases have been reported in about 30 countries: in Asia, Australasia, Africa, South America and North America, and including many cases in the islands of the Pacific and the Caribbean (Wang et al., Reference Wang, Lai, Zhu, Chen and Lun2008). With increasing international trade and travel facilitating further spread of infected definitive and intermediate hosts, the range of A. cantonensis is likely to continue to expand, and although it is generally restricted to tropical and subtropical regions (Wang et al., Reference Wang, Lai, Zhu, Chen and Lun2008), global climate change may permit it to spread into currently more temperate areas (Lv et al., Reference Lv, Zhang, Steinmann, Yang, Yang, Zhou and Utzinger2011; York et al., Reference York, Butler and Lord2014, Reference York, Creecy, Lord and Caire2015).
Angiostrongylus cantonensis was first observed in the Hawaiian Islands in 1960 (Ash, Reference Ash1962), and the first definitive case of eosinophilic meningitis caused by A. cantonensis in Hawaii was in the same year (Horio and Alicata, Reference Horio and Alicata1961). Since then there have been cases on five of the six largest Hawaiian Islands (Kauai, Oahu, Maui, Lanai, Hawaii), with a possible increase in the frequency of cases since around 2004 (Hochberg et al., Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007; Cowie, Reference Cowie2017). Although there have been several studies identifying the intermediate hosts of A. cantonensis (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014), little is known about the factors affecting its distribution (e.g. Lv et al., Reference Lv, Zhang, Steinmann, Yang, Yang, Zhou and Utzinger2011).
Species distribution models (SDMs) that quantify patterns of habitat suitability by comparing species occurrences with environmental factors have been widely used to project future distributions (Guisan and Zimmermann, Reference Guisan and Zimmermann2000; Elith and Leathwick, Reference Elith and Leathwick2009). To inform management and adaptive responses, habitat suitability models are critical tools (Crase et al., Reference Crase, Vesk, Liedloff and Brendan2015). Determining how possible predictors such as temperature and precipitation may affect the distribution of A. cantonensis will permit better forecasting of its potential spread and facilitate better targeted public health intervention. The objectives of this study were to: (1) document the geographic distribution of A. cantonensis across the Hawaiian Islands; (2) develop a current habitat suitability model to estimate the probability of occurrence of A. cantonensis under current climate conditions; and (3) project future potential range shifts for A. cantonensis under climate change. We hypothesized that: (1) areas with higher precipitation will support higher levels of A. cantonensis than will drier areas, as the intermediate gastropod hosts are more abundant and active in such areas; (2) lower elevation areas will support higher levels of A. cantonensis than higher elevation areas, first because globally A. cantonensis is restricted to regions with tropical and subtropical climates and areas at higher elevations in Hawaii would be too cold, and second because of the greater numbers of rats at lower elevations (Amarasekare, Reference Amarasekare1994); and (3) as the climate changes, higher areas and areas in which precipitation increases will become suitable for A. cantonensis as well as for their snail hosts.
Materials and methods
Mollusc specimens and study area
As part of a survey of gastropods across the Hawaiian Islands starting in 2004, over 8000 live snails were collected from the six largest Hawaiian Islands and preserved in 75–95% ethanol. From these collections, 1271 specimens from 182 sites, encompassing all six islands, were selected to provide a broad coverage of species and locations. Geographical coordinates were recorded using hand-held global positioning system devices (Rino 520HCx; Garmin International, Olathe, Kansas, USA), and in some cases were estimated using Google Earth.
Total DNA was extracted from gastropod foot tissue, and the presence of A. cantonensis was determined following Qvarnstrom et al. (Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007, Reference Qvarnstrom, da Silva, Teem, Hollingsworth, Bishop, Graeff-Teixeira and da Silva2010), as described by Kim et al. (Reference Kim, Hayes, Yeung and Cowie2014).
Environmental data and exploratory analysis
We compiled and explored Geographic Information Systems (GIS) environmental grid layers for vegetation index (http://glcf.umd.edu/data/vcf/), soil type (http://sdmdataaccess.nrcs.usda.gov/), mean annual precipitation (Giambelluca et al., Reference Giambelluca, Chen, Frazier, Price, Chen, Chu, Eischeid and Delparte2013) and mean annual surface temperature (Giambelluca et al., Reference Giambelluca, Shuai, Barnes, Alliss, Longman, Miura, Chen, Frazier, Mudd, Cuo and Businger2014). From the Hawaiian Regional Climate Model (HRCM) based on Zhang et al. (Reference Zhang, Wang, Lauer and Hamilton2012), we explored mean annual precipitation, mean annual surface temperature, precipitation seasonality (coefficient of variation), precipitation of coldest quarter, precipitation of warmest quarter, temperature of coldest quarter and temperature of warmest quarter. Elevation was not included in the models as it is strongly correlated with temperature in the Hawaiian Islands. Some of the variables did not provide sufficient coverage of the study areas, nor support suitable class size and model complexity relative to the number of collection sites. Following exploratory investigations of all variables, only mean annual surface temperature and mean annual precipitation appeared to offer any predictive power, and only these two variables were used in the final analysis presented here.
For current climate variables, we used temperature (Giambelluca et al., Reference Giambelluca, Shuai, Barnes, Alliss, Longman, Miura, Chen, Frazier, Mudd, Cuo and Businger2014) and precipitation (Giambelluca et al., Reference Giambelluca, Chen, Frazier, Price, Chen, Chu, Eischeid and Delparte2013) estimates with 250 m grid cell resolution. For future climate, we used the 2100 projections of the HRCM from Zhang et al. (Reference Zhang, Wang, Lauer and Hamilton2012), based on the Weather Research and Forecasting model V3.3. Original spatial resolution was 3 km for all islands except Maui, with 1 km resolution. The HRCM is a regional dynamical downscaling of climate changes based on averaging multiple CMIP3 global circulation models (GCMs) and future climate forcing of the Special Report on Emissions Scenarios (SRES) A1B (Nakicenovic and Swart, Reference Nakicenovic and Swart2000), a conservative emission pathway. New future forcing scenarios have been developed to replace SRES. CMIP5 Representative Concentration Pathways (RCPs) are designed to cover a wider range of possible magnitudes of climate change in models (Collins et al., Reference Collins, Knutti, Arblaster, Dufresne, Fichefet, Friedlingstein, Gao, Gutowski, Johns, Krinner, Shongwe, Tebaldi, Weaver, Wehner, Stocker, Qin, Plattner, Tignor, Allen, Boschung, Nauels, Xia, Bex and Midgley2013). However, for small, extremely diverse climate regions with steep precipitation gradients and trade wind inversions such as Hawaii, GCM projections may be unsuitable. All environmental variables were resampled to a consistent projection (geographic WGS1984) with 250 m grid cell resolution in ArcGIS 10.0. Final resolution used for modelling was 250 m.
SDMs and forecasting change
SDMs were generated based on the detected presence and absence of A. cantonensis in the gastropod specimens screened, with mean annual surface temperature and mean annual precipitation as the explanatory variables. We developed SDMs with two correlative approaches, generalized linear models (GLMs) and boosted regression tree (BRT) models, and compared their performance.
GLMs have great utility in statistical analyses either when variances are not equal, when error structure is non-normal or both (Crawley, Reference Crawley2007). For the logistic regression framework, we built models using binomial error distributions and default link logit functions (Agresti, Reference Agresti1996; Manning, Reference Manning2007) to predict habitat suitability as probability of occurrence. We used a backward simplification approach from a full model with all predictors, generating seven models in the glmulti package (Calcagno, Reference Calcagno2013) in R version 3.2.0. We first compared individual model fit using the relative Akaike information criterion and model weight. We tested model performance using area under the curve (AUC) of the receiver operating characteristic. An AUC score above 0.7 may indicate model performance as fair, and above 0.9 as excellent (Swets, Reference Swets1988). With the package cvAUC (LeDell et al., Reference LeDell, Petersen and van der Laan2013) in R ver 3.2.0, we performed 10-fold cross-validation (cv) on each of the seven models to calculate cvAUC scores and standard errors. Goodness of fit between modelled and observed values was evaluated by deviance explained, D 2, as null deviance minus residual deviance, divided by null deviance (Guisan and Zimmermann, Reference Guisan and Zimmermann2000). Mean cvAUC scores with mean standard errors and deviance explained were compared to select the optimal predictive model.
BRT models (De'ath, Reference De'ath2007; Elith et al., Reference Elith, Leathwick and Hastie2008) are machine-learning approaches that automatically model multiway interactions among explanatory variables and can capture complex, non-linear response curves that are often more representative of species responses (Gaston, Reference Gaston2003). We compared BRT models with tree complexity from 1 to 5, varying learning rates and constant bag fractions (0.75) using the gbm functions in the dismo package (Ridgeway, Reference Ridgeway2015) in R ver 3.2.0 and code from Elith et al. (Reference Elith, Leathwick and Hastie2008). To assess the predictive performance of BRT modelling strategies and to select optimal models, we used 10-fold cv to compare cvAUC scores and standard error with the gbm functions (Lu et al., Reference Lu, Schölkopf and Zhao2011; Crase et al., Reference Crase, Vesk, Liedloff and Brendan2015). Goodness of fit between modelled and observed values was evaluated by deviance explained (D 2).
We selected the best model generated between the GLM and BRT approaches by comparing cvAUC scores and D 2. We used the best model to estimate the probabilities of A. cantonensis occurrence across the study area and generate a current habitat suitability map. We forecasted potential suitability changes under one future projected climate scenario for mean annual surface temperature and mean annual precipitation (2100) using the HRCM CMIP3 SRES A1B emission scenario (based on Zhang et al., Reference Zhang, Wang, Lauer and Hamilton2012), in R ver 3.2.0 using the raster package (Hijmans et al., Reference Hijmans, van Etten, Cheng, Mattiuzzi, Sumner, Greenberg, Lamigueiro, Bevan, Racine, Shortridge and Hijmans2015).
Results
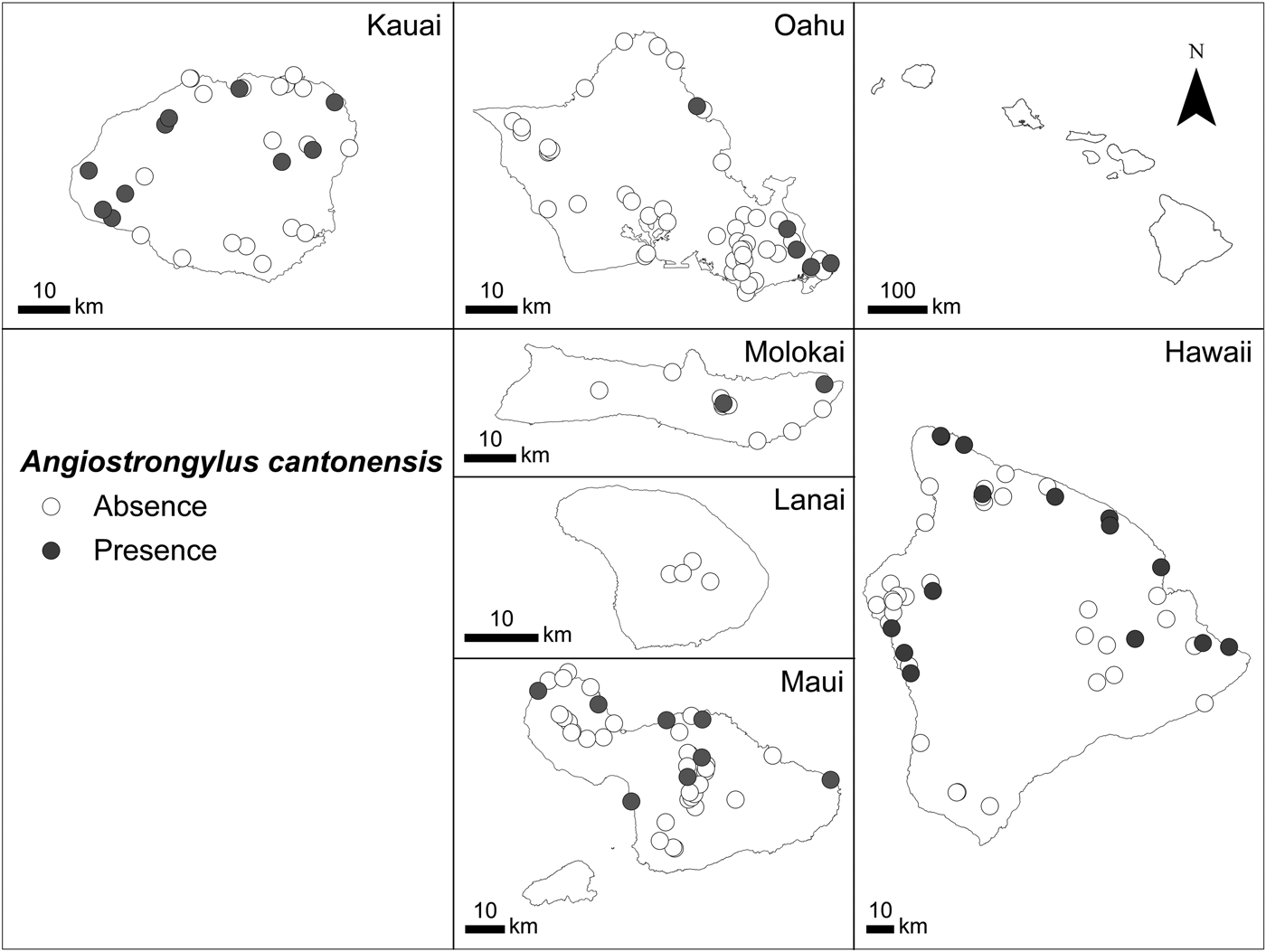
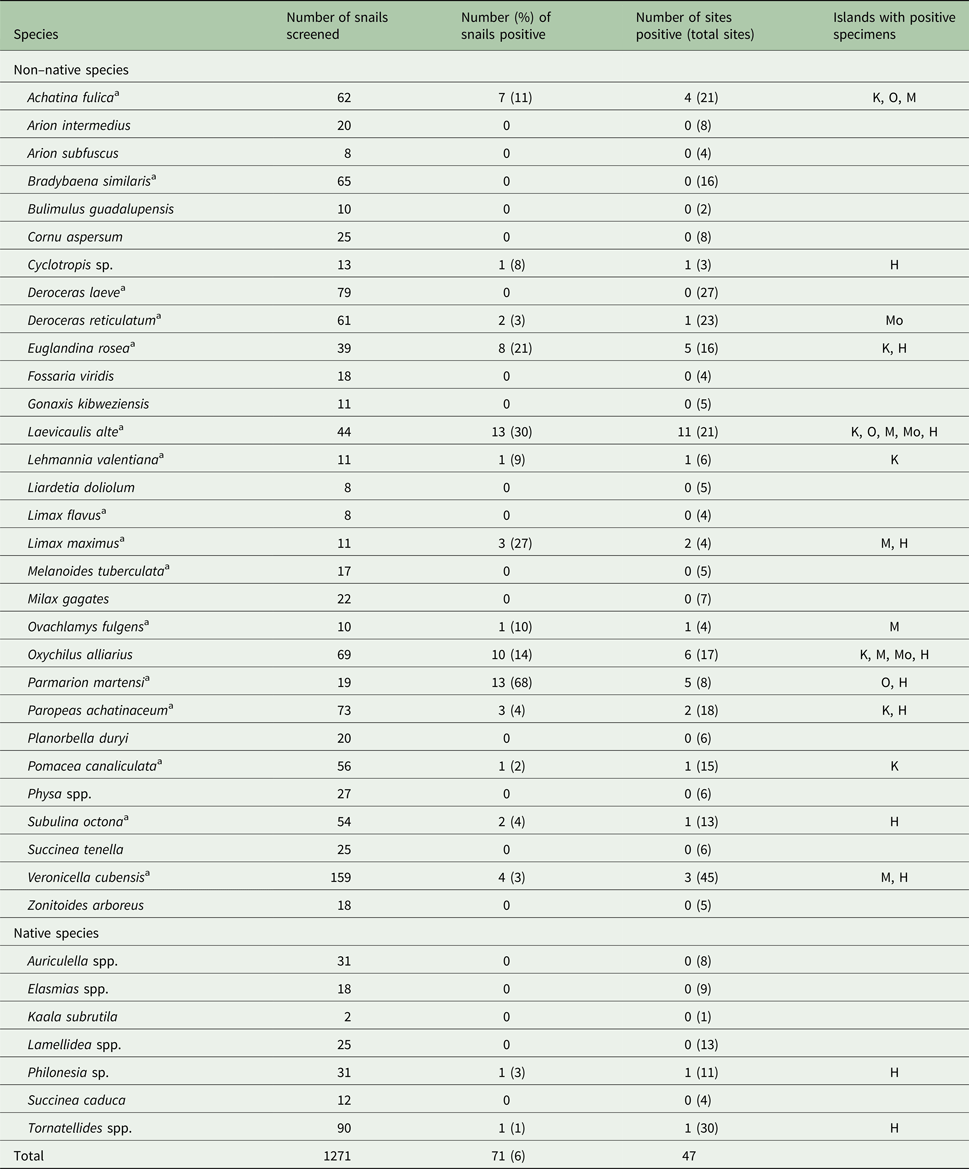
Of the 37 gastropod species screened, 16 (71 specimens) tested positive for A. cantonensis (Table 1). Of the 182 sites, 40 sites located on five of the six islands (not Lanai) had infected gastropods (Fig. 1). Kauai and Hawaii had the highest percentages of sites with A. cantonensis (34 and 33%, respectively), followed by Molokai, Maui and Lanai combined as Maui Nui (18%) and Oahu (10%) (χ 2 = 10.6, d.f. = 5, P = 0.014). More windward sites (northern and eastern sites influenced by the trade winds and therefore with higher precipitation) had more A. cantonensis than leeward (southern and western sites with generally less precipitation) (28 and 16%, respectively; χ 2 = 4.0, d.f. = 1, P = 0.047).

Fig. 1. The presence (grey, 40 sites) and absence (white, 142 sites) of Angiostrongylus cantonensis (rat lungworm) at the study sites in the Hawaiian Islands. Dots for some sites overlap and may not be visible.
Table 1. Angiostrongylus cantonensis (rat lungworm) infection rates in gastropod species screened and distribution of positive infection among the Hawaiian Islands

K, Kauai; O, Oahu; M, Maui; Mo, Molokai; H, Hawaii.
a Previously recorded as a host in the Hawaiian Islands and/or elsewhere (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014).
Individuals that were negative for A. cantonensis did not occur at sites with mean annual surface temperatures as low as 10.3 °C, whereas positive specimens occurred at sites with temperatures only as low as 15.2 °C, essentially being restricted to warmer, lower elevation sites than negative specimens (Fig. 1). Both negative and positive specimens occurred at sites with temperatures up to 23.9 °C. Specimens that were negative for A. cantonensis were from sites with mean annual precipitation of 224–8615 mm (Fig. 1), whereas those testing positive were from sites with a narrower range of mean annual rainfall (291–5960 mm). The average mean annual temperature for sites with A. cantonensis was higher (21.3 °C) than for sites where it was absent (20.5 °C). Sites with A. cantonensis also had higher mean annual precipitation (1816 mm) than sites without it (1699 mm).
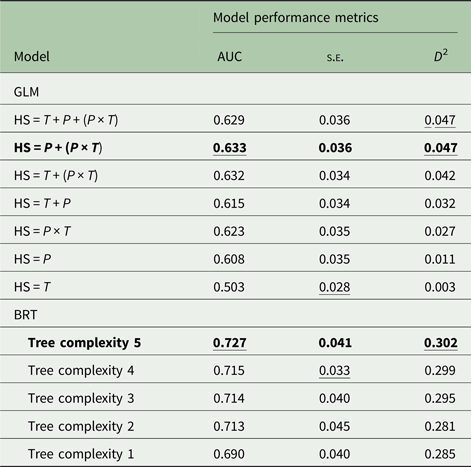
Among the seven habitat suitability models produced with the GLM approach (Table 2), the optimal model was precipitation + (precipitation × temperature) with coefficients as follows: intercept, −3.846; precipitation, −1.096e−03; precipitation × temperature, 8.206e−05. This model had the highest mean cvAUC score (0.633) though not the lowest cvAUC standard error (±0.036). The cvAUC score indicates poor model performance. The model shared the highest D 2 (0.047); however, this value is very low compared with BRT values.
Table 2. Model performance metrics for models of Angiostrongylus cantonensis habitat suitability

AUC, 10-fold cross-validated area under the curve; s.e., standard error of the mean; D 2, deviance explained; GLM, generalized linear models; HS, habitat suitability; T, mean annual surface temperature; P, mean annual precipitation; BRT, boosted regression tree models.
Boldface indicates the optimal model in each approach. Underline indicates the optimal scores for each statistic.
Of the five models generated with the BRT approach (Table 2), the optimal model had a tree complexity of 5, learning rate of 0.025 and bag fraction of 0.75. This model had the highest mean cvAUC score (0.727), though not the lowest cvAUC standard error (±0.041). The cvAUC score indicates fair model performance. The model had the highest D 2 (0.302). This BRT model was selected as best overall and used to generate habitat suitability maps for current and future projected climates in 2100.
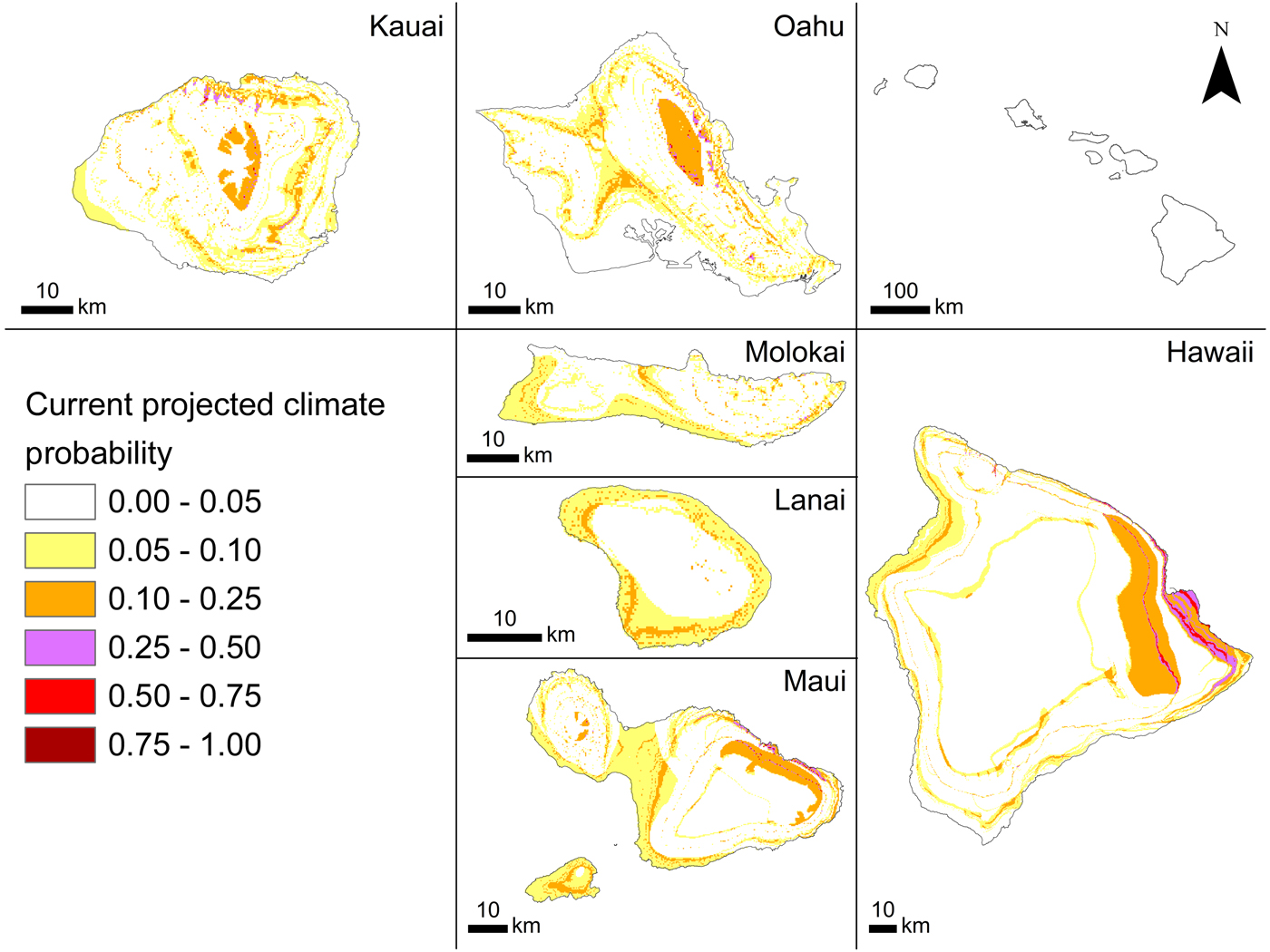
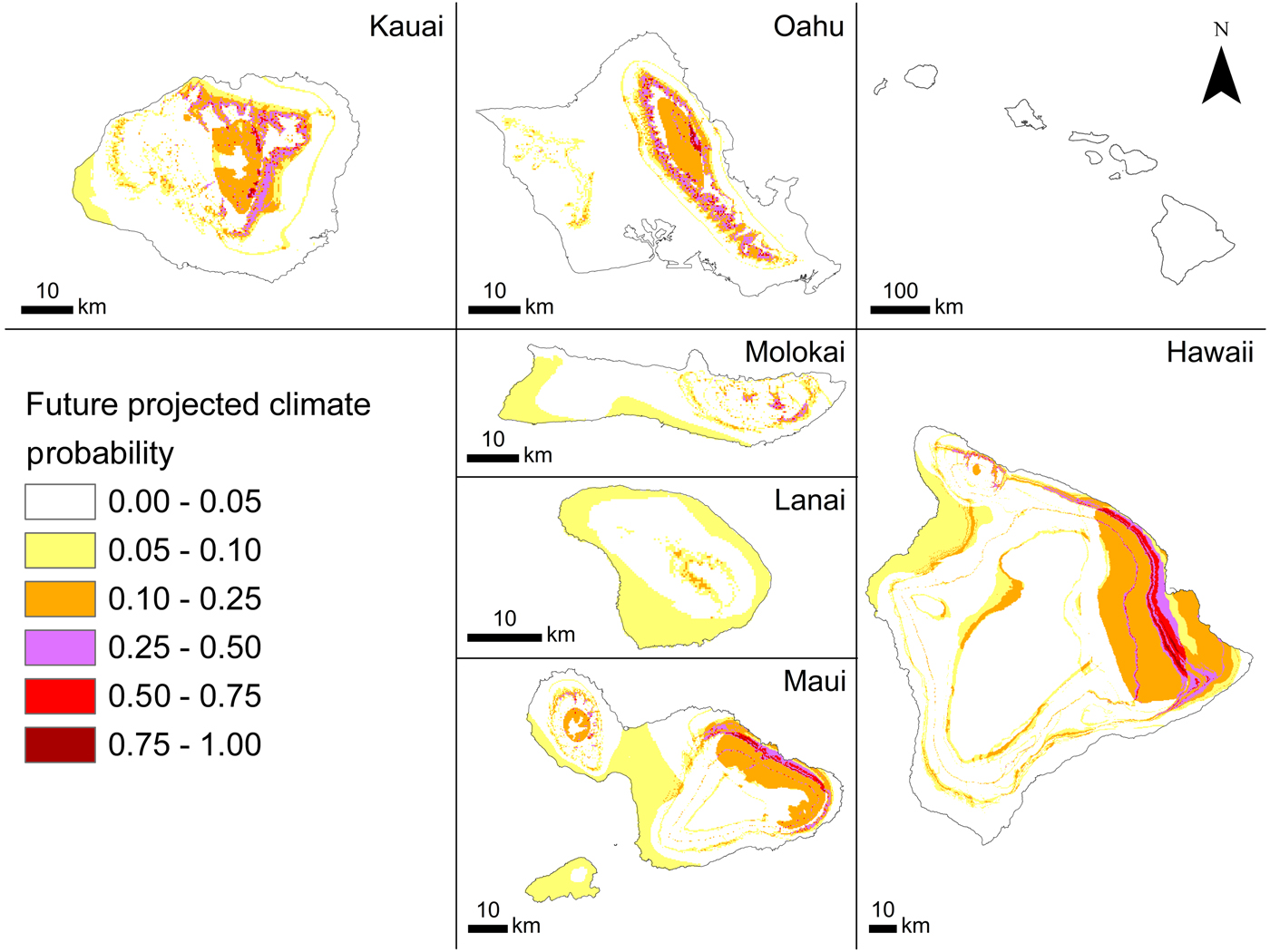
The current probability of occurrence (Fig. 2) ranged between 0.006 and 0.740 (habitat suitability), indicating that although some areas are not suitable for A. cantonensis, many areas are somewhat suitable (Fig. 2). The model predicted higher habitat suitability in windward areas where precipitation is greater than in leeward areas. Higher elevation areas that experience lower temperatures also seem to be less suitable than lower elevation areas, especially on the leeward (western and south-western) sides of the islands. The probability of occurrence for our 2100 climate prediction was forecasted as being between 0.006 and 0.845 (habitat suitability) with range expansion projected for windward regions and slight increases with elevation (Fig. 3).
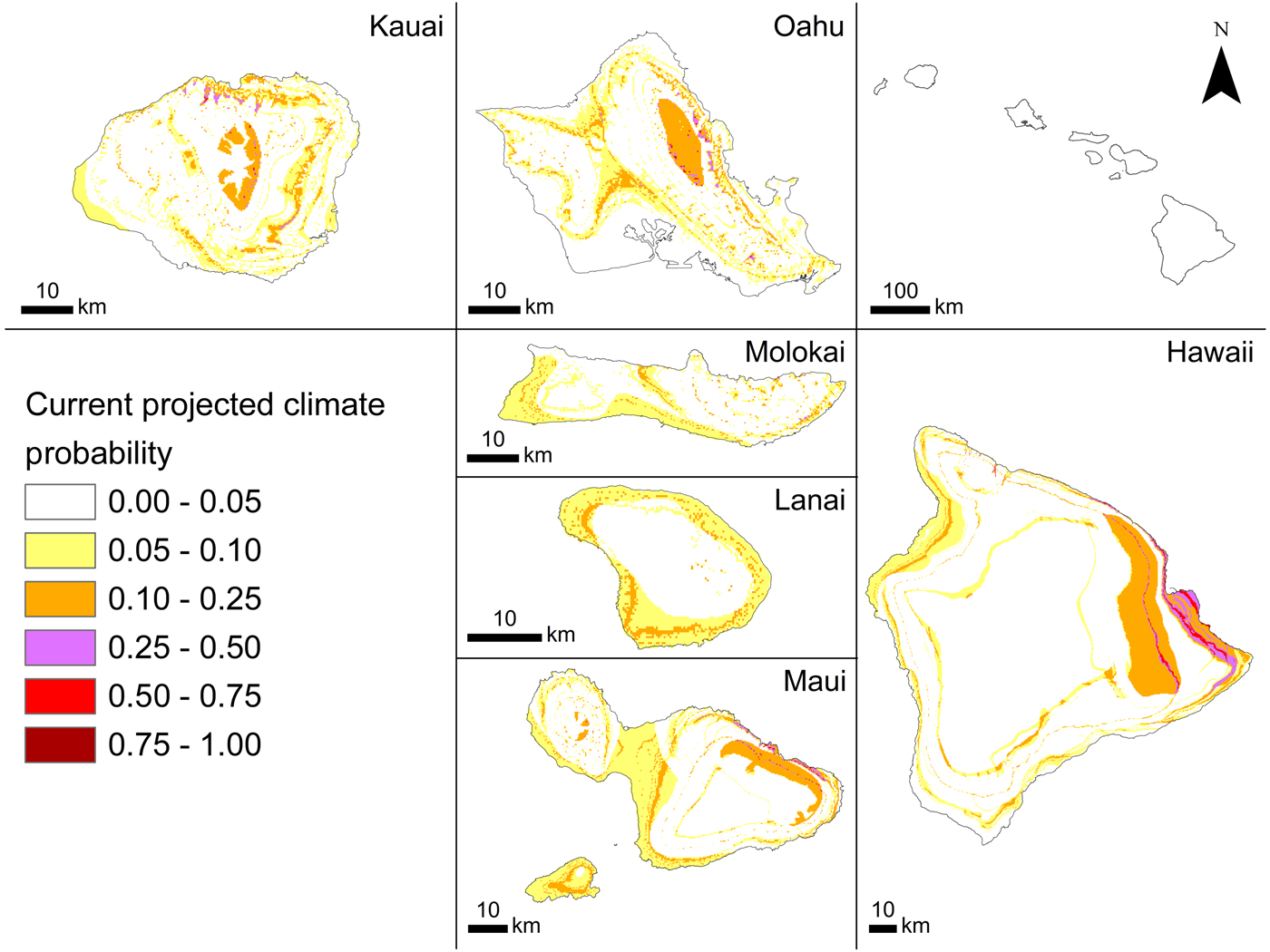
Fig. 2. Angiostrongylus cantonensis (rat lungworm) habitat suitability projected by BRT (tree complexity 5) in current climate conditions in the main Hawaiian Islands.

Fig. 3. Angiostrongylus cantonensis (rat lungworm) habitat suitability projected by BRT (tree complexity 5) in future projected climate conditions for 2100 in the main Hawaiian Islands based on HRCM (from Zhang et al., Reference Zhang, Wang, Lauer and Hamilton2012).
Discussion
Infected snails were found on five of the six largest Hawaiian Islands. They were not found on Lanai, although a human case of infection has been recorded on that island (Hochberg et al., Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007). It is possible that produce regularly shipped from Maui to Lanai was contaminated and the victim was infected through consumption of such produce, or that the victim became infected while on another island.
The parasite was found predominantly at lower elevation sites with warmer temperatures and higher precipitation, which supports the original hypotheses and predictions of the current model. These patterns are reasonable considering the parasite's generally tropical and subtropical range (York et al., Reference York, Butler and Lord2014), which is related to the threshold temperature for larval development in its intermediate mollusc hosts (Lv et al., Reference Lv, Zho, Zhang, Liu, Zhu, Yin, Steinmann, Wang and Jia2006), and its susceptibility to dry conditions (Richards and Merritt, Reference Richards and Merritt1967). Higher precipitation may also facilitate transfer of the parasite to hosts by keeping rat faeces hydrated and able to support the first-stage larvae, as well as providing suitable conditions for greater numbers and diversity of intermediate hosts.
Kauai and Hawaii are the two islands with the highest proportions of positive sites. Most positive leeward sites on Kauai are clustered around a landfill, where there may be higher rat densities that could facilitate higher rates of infection in nearby areas. The island of Hawaii, the largest island, has the greatest area of highly suitable current habitat (Fig. 2), which is reflected not only in the high proportion of positive sites but also in the high incidence of human infection on that island (Hochberg et al., Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007; Cowie, Reference Cowie2017; Howe and Jarvi, Reference Howe and Jarvi2017). Oahu had the lowest proportion of positive sites, possibly because of the higher proportions of freshwater species and native species sampled from this island. Freshwater snails are less likely to encounter rat faeces and are less likely to be eaten by rats than terrestrial snails, and native snails are generally found at higher elevations than non-native species. Overall, freshwater snails in the Hawaiian Islands have lower infection rates than terrestrial gastropods, as do native compared with non-native gastropods (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014).
The south-eastern part of the island of Hawaii has been the area with most human infections (Howe and Jarvi, Reference Howe and Jarvi2017). The optimal current model (BRT, tree complexity 5, learning rate 0.025, bag fraction 0.75) predicted that this area is highly suitable for A. cantonensis, perhaps because of its particularly wet conditions. In contrast, current habitat suitability is generally low across the island of Lanai, which has relatively low precipitation.
Habitat suitability by 2100 was projected under a single climate scenario, a regional downscaled HRCM based on CMIP3 SRES A1B emission scenario. This scenario probably underestimates future conditions as the current trajectory has far surpassed the defining estimates (Schröder and Schmidt, Reference Schröder and Schmidt2014). However, GCM projections may have limited value for interpolated applications in the Hawaiian Islands.
The range of A. cantonensis is constrained within the range of its hosts. High elevation sites (e.g. the summits of Mauna Kea and Mauna Loa on the island of Hawaii, ~4200 m) are cold, dry and barren and support no intermediate gastropod hosts. In addition, other factors such as soil type, soil moisture, vegetation type, aspect and habitat connectivity may be involved in habitat suitability for the gastropod hosts (some of which were investigated but the data available were unsatisfactory for the analysis; see Materials and methods section). Factors such as proximity to human settlements or agricultural land may have higher densities of definitive rat hosts and consistent levels of moisture from artificial irrigation.
Laevicaulis alte exhibits a high frequency of infection and the highest individual parasite load (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014). It is currently found on all six of the largest Hawaiian Islands. However, Parmarion martensi, recorded on the islands of Oahu and Hawaii (Cowie et al., Reference Cowie, Hayes, Tran and Meyer2008), and more recently on Maui (Cowie et al., Reference Cowie, Hayes, Kim, Bustamente and Yeung2018), exhibited the highest frequency of infection as well as a high parasite load (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014). Parmarion martensi was first recorded in the Hawaiian Islands in the 1990s but quickly became widespread and abundant in the south-eastern part of the island of Hawaii. It has been suggested that this species may be connected with many of the recent cases of angiostrongyliasis (Howe and Jarvi, Reference Howe and Jarvi2017). These and other species may well have been introduced and spread via the horticulture industry, which plays a major role in the spread of snails throughout the Hawaiian Islands (Cowie et al., Reference Cowie, Hayes, Tran and Meyer2008) and globally (Cowie and Robinson, Reference Cowie, Robinson, Ruiz and Carlton2003; Bergey et al., Reference Bergey, Figueroa, Mather, Martin, Ray, Kurien, Westrop and Suriyawong2014) and may contribute to the continued emergence of this disease.
Significant advances in preventing the spread of pathogenic diseases to human populations can be achieved by integrating rapid molecular genetic methods with field epidemiology and habitat suitability models to estimate the pathogenic range and its potential expansion under climate change (Kim et al., Reference Kim, Hayes, Yeung and Cowie2014; York et al., Reference York, Butler and Lord2014, Reference York, Creecy, Lord and Caire2015; Stockdale Walden et al., Reference Stockdale Walden, Slapcinsky, Roff, Calle, Goodwin, Stern, Corlett, Conway and McIntosh2017). Globally, York et al. (Reference York, Butler and Lord2014) suggested that the centre of the range of A. cantonensis will move northwards approximately 100 km per decade during the 21st century. The analogous change of temperature with both elevation and latitude means that a 1000 m change in elevation is roughly equivalent to a 1000 km change in latitude, or a 1 °C change in temperature occurs over roughly 150 m elevation or 150 km of latitude (Jump et al., Reference Jump, Mátyás and Peñuelas2009). Thus, in Hawaii, an elevational microcosm of wide latitudinal change, we have shown that in general suitable habitat will occur increasingly at higher elevations.
Habitat suitability models may provide valuable information for the application of prevention and management strategies. Across the main Hawaiian Islands, our forecasted habitat suitability maps indicate an increase in both range and probability of A. cantonensis occurrence by 2100. If the definitive and intermediate hosts are present in such areas, spread of A. cantonensis appears likely and may have negative impacts on public health. Using a framework for infectious disease modelling coupled with ecological and social network models (e.g. Haak, Reference Haak2015) may facilitate a better understanding of disease transmission and further inform resource management and policy.
Supplementary material
Data for each snail specimen screened (species, island, collection site geocoordinates and elevation, collection date, whether infected with A. cantonensis or not, and whether A. cantonensis was detected in any snails at the site) are available from https://doi.org/10.1017/S0031182018001026
Acknowledgements
We thank Tom Giambelluca for providing access to GIS layers, Chris Lepczyk and Kenton Kramer for constructive comments on early drafts of this manuscript, and all the people who participated in the collection, identification and curation of the specimens. Contribution number 10399 of the University of Hawaii School of Ocean and Earth Science and Technology.
Financial support
This work was supported by the United States Department of Agriculture Cooperative Agricultural Pest Survey program (04-8510-0796-CA, 05-8510-0796-CA, 06-8510-0796-CA, 07-8510-0796-CA, 08-8510-0796-CA, 09-8510-0796-CA), the National Science Foundation (DEB-1120906), the Watson T. Yoshimoto Foundation through the Ecology, Evolutionary and Conservation Biology program at the University of Hawaii, the American Malacological Society, the Hawaiian Malacological Society and the National Socio-Environmental Synthesis Center (SESYNC) (NSF DBI-1052875).
Conflicts of interest
None.
Ethical standards
Not applicable.