INTRODUCTION
Alveolar echinococcosis (AE) is caused by a parasitic tapeworm Echinococcus multilocularis (Deplazes and Eckert, Reference Deplazes and Eckert2001) and is primarily characterized by a tumour-like growth of metacestodes in the liver of rodents or, occasionally, human beings (Zhang et al. Reference Zhang, Wang, Lu, Li, Lu, Mantion, Vuitton, Wen and Lin2012). AE is one of the most dangerous human parasitic zoonosis worldwide (Craig et al. Reference Craig, Hegglin, Lightowlers, Torgerson and Wang2017). Although a high effective governmental measure has been taken, E. multilocularis infection remains wide-spread and cannot be effectively controlled in some mountainous and pastoral areas in China (Wang et al. Reference Wang, Wang and Liu2008). Many Studies on Echinococcus spp., especially the whole genome sequencing of Echinococcus spp. and in vitro culture of E. multilocularis protoscoleces, have provided a possibility for pinpointing miRNA roles in the development and growth and host–parasite interactions, and a potential as diagnostic targets (Tsai et al. Reference Tsai, Zarowiecki, Holroyd, Garciarrubio, Sanchez-Flores, Brooks, Tracey, Bobes, Fragoso, Sciutto, Aslett, Beasley, Bennett, Cai, Camicia, Clark, Cucher, De Silva, Day, Deplazes, Estrada, Fernandez, Holland, Hou, Hu, Huckvale, Hung, Kamenetzky, Keane and Kiss2013; Zheng et al. Reference Zheng, Zhang, Zhang, Zhang, Li, Lu, Zhu, Wang, Huang, Liu, Kang, Chen, Wang, Chen, Yu, Gao, Jin, Gu, Wang, Zhao, Shi, Wen, Lin, Jones, Brejova, Vinar, Zhao, McManus, Chen and Zhou2013a ).
MicroRNAs (miRNAs), a class of small non-coding RNA molecules, mainly execute regulatory functions via repressing translation or triggering degradation of the targeted mRNA (Guo et al. Reference Guo, Ingolia, Weissman and Bartel2010). Increasing evidence showed that most miRNAs are involved in many pathologic processes and diseases (Cai et al. Reference Cai, Gobert and McManus2016). It was shown that host miRNAs were dysregulated in response to pathogen infection (Judice et al. Reference Judice, Bourgard, Kayano, Albrecht and Costa2016). On the other hand, pathogens including parasitic helminths may deliver their proteins and miRNAs into host cells to modulate host cell functions to benefit infection (Carriere et al. Reference Carriere, Barnich and Nguyen2016). Several potential approaches have been proposed for regulation of macrophage functions by E. multilocularis. Previous studies demonstrated that excretory/secretory products of E. multilocularis could downregulate macrophage functions (Rakha et al. Reference Rakha, Dixon, Carter, Craig, Jenkins and Folkard1991). Nono et al. (Reference Nono, Pletinckx, Lutz and Brehm2012) found that excretory/secretory products of E. multilocularis specifically influence dendritic cells to modulate immune responses. Recently, our study found that exosome-like vesicles secreted by E. multilocularis metacestodes could modulate gene expression in macrophages (Zheng et al. Reference Zheng, Guo, Su, Guo, Ding, Yang, Xiang, Cao, Zhang, Ayaz and Luo2017). Above results suggested some active molecules released by E. multilocularis metacestodes probably have important roles in regulating the host response. Macrophages play a crucial role in innate and adaptive immune responses against E. multilocularis infection (Vuitton and Gottstein, Reference Vuitton and Gottstein2010). To date, no studies have examined the effects of E. multilocularis on the RNA-induced silencing complex (RISC) pathway in macrophages. In this study, the expression level of key RISC components and miRNA expression profile in macrophage RAW264·7 cells exposed to E. multilocularis metacestodes were determined. The result will help us further understand the interactions between host and E. multilocularis.
MATERIALS AND METHODS
Ethics statement
Animal experiments were approved by Animal Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All animal experiments in the study were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People's Republic of China.
Parasites and RAW264·7 cell treatment
E chinococcus multilocularis Qinghai isolate was maintained in 6-month-old DBA/2 mice in our laboratory. Larval cysts were collected from infected mice, washed in sterile phosphate buffered saline (PBS), minced and filtered through a 400 µm pore size metal mesh to obtain the protoscoleces. Echinococcus multilocularis protoscoleces were cultured in 25 mL flask contained RPMI 1640 medium and 20% fetal bovine serum (FBS) as previously described (Wang et al. Reference Wang, Li, Guo, Zhao, Zhang, McManus, Wen and Zhang2016).
2 × 106 macrophage RAW 264·7 cells were cultured in RPMI 1640 medium supplemented with 10% FBS at 37 °C, 5% CO2, until the confluency reached 70–80%. 6 × 103 E. multilocularis metacestodes were added into each flask. After treatment for 6 and 12 h, the cells were washed for three times in PBS, respectively. Mock-treated cells were used as control by addition of the same volume of RPMI 1640 medium.
Isolation of total RNA and high-throughput sequencing
Total RNA was extracted from the mock- and protoscolex-treated cells using TRIzol reagent (Invitrogen), respectively. The RNA concentration and integrity was determined using Agilent 2100. High-throughput small RNA sequencing was carried out in Novogene (Beijing, China). After RNA sequencing, we performed the following bioinformatics analysis. Briefly, after removing low quality reads and adaptor sequences, only sequences perfectly matching the mouse genome (http://www.ncbi.nlm.nih.gov/genome/genomes/52) were used for the further analysis. Known miRNAs were identified by BLAST searching against the MirGeneDB database (http://www.mirgenedb.org) and the relative miRNA expression levels were analysed using DEGseq as previously described (Wang et al. Reference Wang, Feng, Wang, Wang and Zhang2010). miRNAs with a fold change >2 and a t-test P value < 0·05 were regarded as differentially expressed.
miRNA target gene prediction and bioinformatics analysis
Targets genes of differentially expressed miRNAs were predicted using three databases: TargetScan (http://www.targetscan.org/vert_71) (Agarwal et al. Reference Agarwal, Bell, Nam and Bartel2015), miRanda (http://www.microrna.org/microrna) (Betel et al. Reference Betel, Wilson, Gabow, Marks and Sander2008) and RNAhybrid (Lai and Meyer, Reference Lai and Meyer2016). Gene Ontology (GO) term analysis was performed using GOseq based Wallenius non-central hyper-geometric distribution (Young et al. Reference Young, Wakefield, Smyth and Oshlack2010). KEGG pathway enrichment analysis of DEGSeq was performed by KOBAS software. Hierarchical clustering was performed to distinguish miRNA expression patterns among samples.
Quantitative real-time polymerase chain reaction (qRT–PCR)
Differentially expressed miRNAs were validated using quantitative reverse transcription RT–PCR (qPCR) with SYBR green and specific primers (GeneCopoia). U6 RNA was selected as a reference for normalization. Poly A polymerase was used to added poly A to the 3′ ends of miRNAs. The 20 µL reverse transcription reaction contained 2 µL total RNA (approximate 1 μg), 5 µL 5 × PAP/RT Buffer, 1 µL Polymerase, 1 µL reverse transcriptase mix and 11 µL RNase-free water. The reaction protocol was as following: 60 min at 37 °C and 5 min at 85 °C. RT–PCR was performed using All-in-One™ miRNA qRT–PCR mix containing SYBR Green (GeneCopoia). The 20 µL PCR reaction included 2 µL of cDNA (1:5 dilution), 10 µL 2 × All-in-One qPCR Mix, 2 µL Universal Adaptor PCR Primer, 2 µL 10 × miRNA qPCR Primer, 0·4 µL ROX Dye and 3·6 µL ddH2O. The reactions were incubated in a 96-well plate at 95 °C for 15 min, followed by 40 cycles of 94 °C for 15 s, 55 °C for 30 s and 70 °C for 30 s. Relative fold changes were calculated by 2−ΔΔCt.
Expression levels of nine genes that encode key RISC components were analysed. First-strand cDNA synthesis was conducted using 1 µg total RNA with the ThermoScript™ RT–PCR System (Invitrogen). The reaction mixture was incubated at 42 °C for 1 h and 75 °C for 5 min. The reaction mixture was diluted 10-fold with nuclease-free water. RT–PCR was performed using All-in-One™ qPCR Mix (GeneCopoeia, Washington, USA) with the following thermocycling conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 15 s. Gapdh were screened to identify an appropriate reference gene. Validated primers for all of the above genes were purchased from GeneCopoeia. The relative expression levels of nine genes were calculated using the 2−ΔΔCt formula. Data used in the final analysis were collected from triplicate independent experiments.
Statistical analysis
Data analyses were performed using GraphPad Prism 5 software and a one-tailed unpaired t-test was used for comparison of two groups, with a P value <0·05 being considered to be significantly different.
RESULTS
The expression of RISC components in the RAW264·7 cells exposed to E. multilocularis protoscoleces
To detect whether protoscoleces affect miRNA biogenesis and function, the expression levels of nine genes including Ago1, Ago2, Ago4, Tarbp2, Xpo5, DgcR8, Dicer, Drosha and Xrn2 were examined by qPCR in the RAW264·7 cells exposed to E. multilocularis protoscoleces. Compared with untreated RAW264·7 cells, the mRNA levels of Ago2 and Tarbp2 increased up to 1·76-folds and 2·16-folds, whereas Xpo5 and DgcR8 were reduced by 34 and 43%, respectively. The rest were not changed (Fig. 1).
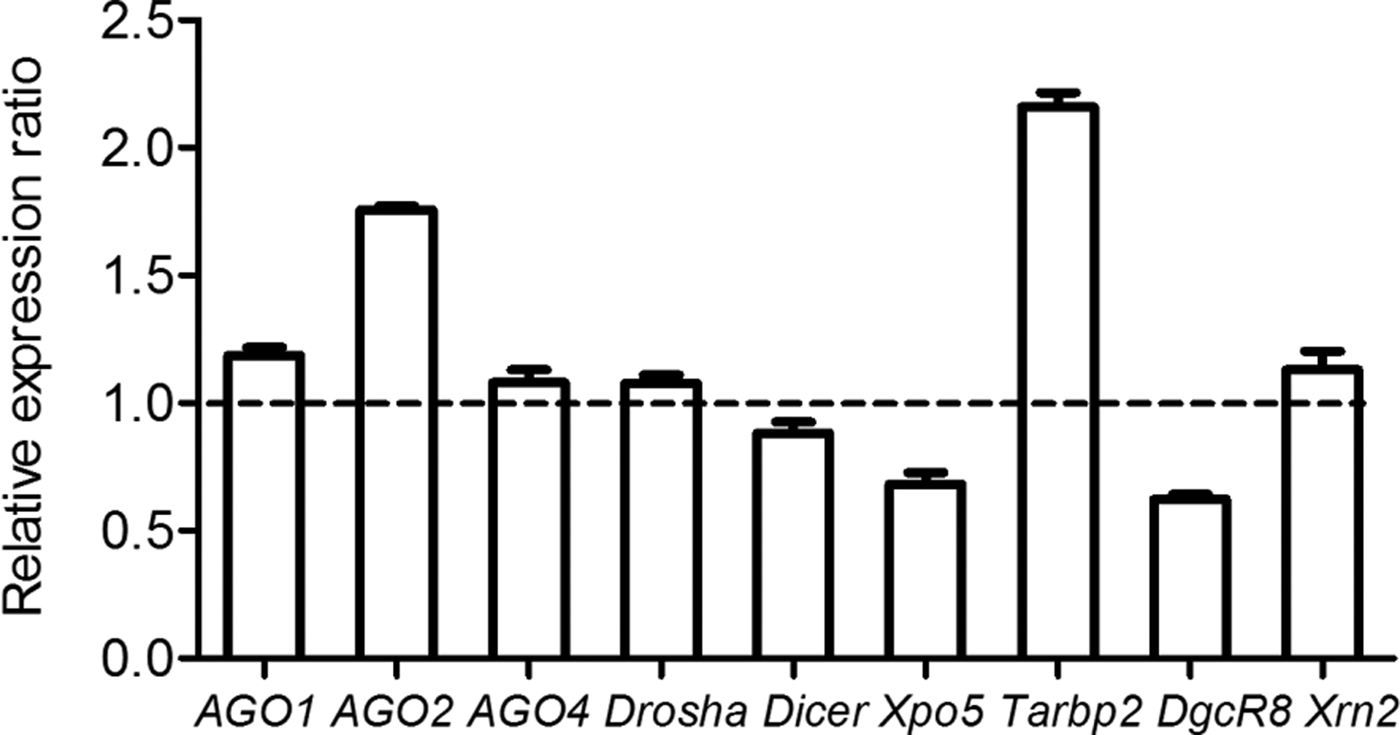
Fig. 1. Effects of E. multilocularis metacestodes on the expression of nine genes involved in miRNA biogenesis in RAW264·7 macrophage cells. The expression levels of nine genes were normalized to that of β-actin and data were expressed as means ± s.e.m.
Data description of three small RNA sequencing libraries
Considering the ectopic expression of Ago2, Tarbp2, Xpo5 and DgcR8, we then examined the miRNA profiles of RAW264·7 cells after protoscolex treatment for 6 and 12 h, respectively. In this study, 13 792 508, 12 090 305 and 14 921 792 total reads were obtained from the mock-infected cells, emu-treated 6 h-cells and emu-treated 12 h-cells, respectively (Table S1). After data cleaning, approximately 95% reads from all the three samples were ultimately obtained and used for downstream analysis, and over 81% of clean reads were mapped to the mouse genome (Table S1). The size distribution of small RNAs was similar in the three libraries, and the majority of these miRNAs were 21–24 nt in length.
Identification of differentially expressed known miRNAs
In total, 661 distinct known miRNAs were non-redundantly identified from three libraries, 82·9% (548/661) of which were commonly shared by all samples. Compared with mock-treated library, 18 and 32 known miRNAs were found to be differentially expressed in the emu-treated 6 h- and emu-treated 12 h-libraries, respectively (Fig. 2; Tables S2 and S3). Of them, 13 known miRNAs were commonly shared in the two emu-treated libraries, with 11 miRNAs being upregulated and two downregulated (Fig. 2).

Fig. 2. Venn diagram showing the unique and overlapping miRNAs (A) and heatmap diagram of differential miRNA expression among mock-treated-, emu-treat 6 h- and emu-treat 12 h-libraries (B). (A) Each row represents a miRNA and each column represents a sample. The colour scale illustrates the relative level of miRNA expression: Red, increased expression; blue, decreased expression; and white, mean value. (B) 18 and 32 known miRNAs were differentially expressed in the emu-treated 6 h- and emu-treated 12 h-libraries compared with the mock-infected library, respectively. 13 commonly shared miRNAs (11 miRNAs upregulated and two miRNAs downregulated) were found.
Validation of miRNA sequencing data by qPCR analysis
To further validate the expression of miRNAs identified, qRT–PCR was carried out for nine selected miRNAs, of which seven were up-regulated and two downregulated (mmu-miR-1981-5p and mmu-let-7c-1-3p). Consistent with the sequencing results, there was an increase in mmu-miR-378d, mmu-miR-155-5p, mmu-miR-146a-5p, mmu-miR-21a-5p and mmu-miR-125a-5p expression, and a decrease in mmu-miR-1981-5p and mmu-let-7c-1-3p expression in two emu-treated libraries compared with the mock-treated cells (Fig. 3).
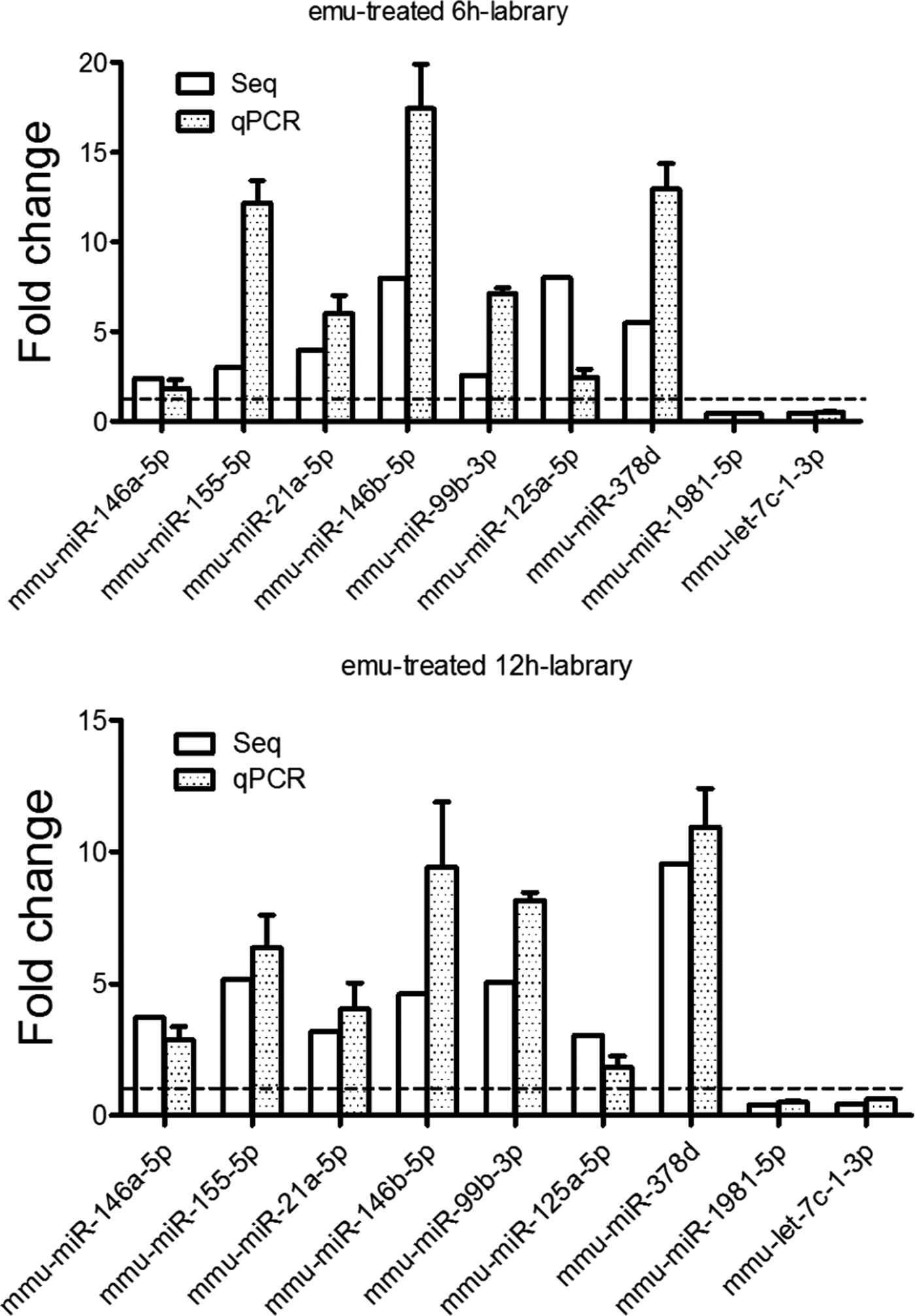
Fig. 3. Validation of miRNA expression in the RAW264·7 cells exposed to E. multilocularis protoscoleces for 6 h (A) and 12 h (B) by qPCR. qPCR data were expressed as means ± s.e.m. Every sample was tested in triplicate and data used for the final analysis were from three independent experiments.
Analysis of potential targets of differentially expressed miRNAs
A total of 2719 putative target genes were obtained for these differentially expressed miRNAs. According to P values of the enrichment analysis of target genes, the top 15 GO terms of biological process, molecular function and cellular component were shown in Fig. 4, respectively. Most of target genes were highly enriched in biological process (e.g. response to stimulus, immune response, signal transduction), molecular function (e.g. protein binding, cytokine receptor binding and cytokine activity) and cellular component (e.g. cell part, organelle). In contrast, Inflammatory response genes were highly enriched in inflammatory response in emu-treated 6 h- library, but apoptotic and cell death genes were highly enriched in emu-treated 12 h- library. KEGG pathway analyses showed that significantly enriched pathways included tumour necrosis factor (TNF) signalling pathway, F-kappa B signalling pathway, apoptosis (Table S4). However, Toll-like receptor signalling pathway was only enriched in emu-treated 6 h- library.

Fig. 4. GO molecular function annotations of the target genes of differentially expressed miRNAs. According to P value, top15 GO terms of biological process, molecular function and cellular component were shown in the emu-treated 6 h- (A) and 12 h-libraries (B), respectively.
Correlation of expression between microRNAs and their potential targets
It was interesting to note that among the target genes, several belonged to cytokine families. In order to check if these cytokines were correlated with the corresponding miRNAs in expression, we measured their expression levels using qRT–PCR. Fig. 5 showed the relative expression levels of mmu-miR-125a-5p, mmu-miR-21a-5p, mmu-miR-374d-5p, mmu-miR-125a-3p, mmu-miR-378d, mmu-miR-1981-5p and mmu-let-7c-1-3p and of seven cytokine genes (TNF-α, IL1α, IL6, IL12α, IL12β, CCL22 and CCL18,) of their predicted targets at 24 h (Fig. 5). These results indicate that there is a statistically significant correlation (r = −0·802; P = 0·049) between the expression levels of seven selected miRNAs and their corresponding cytokine targets (Fig. S1).
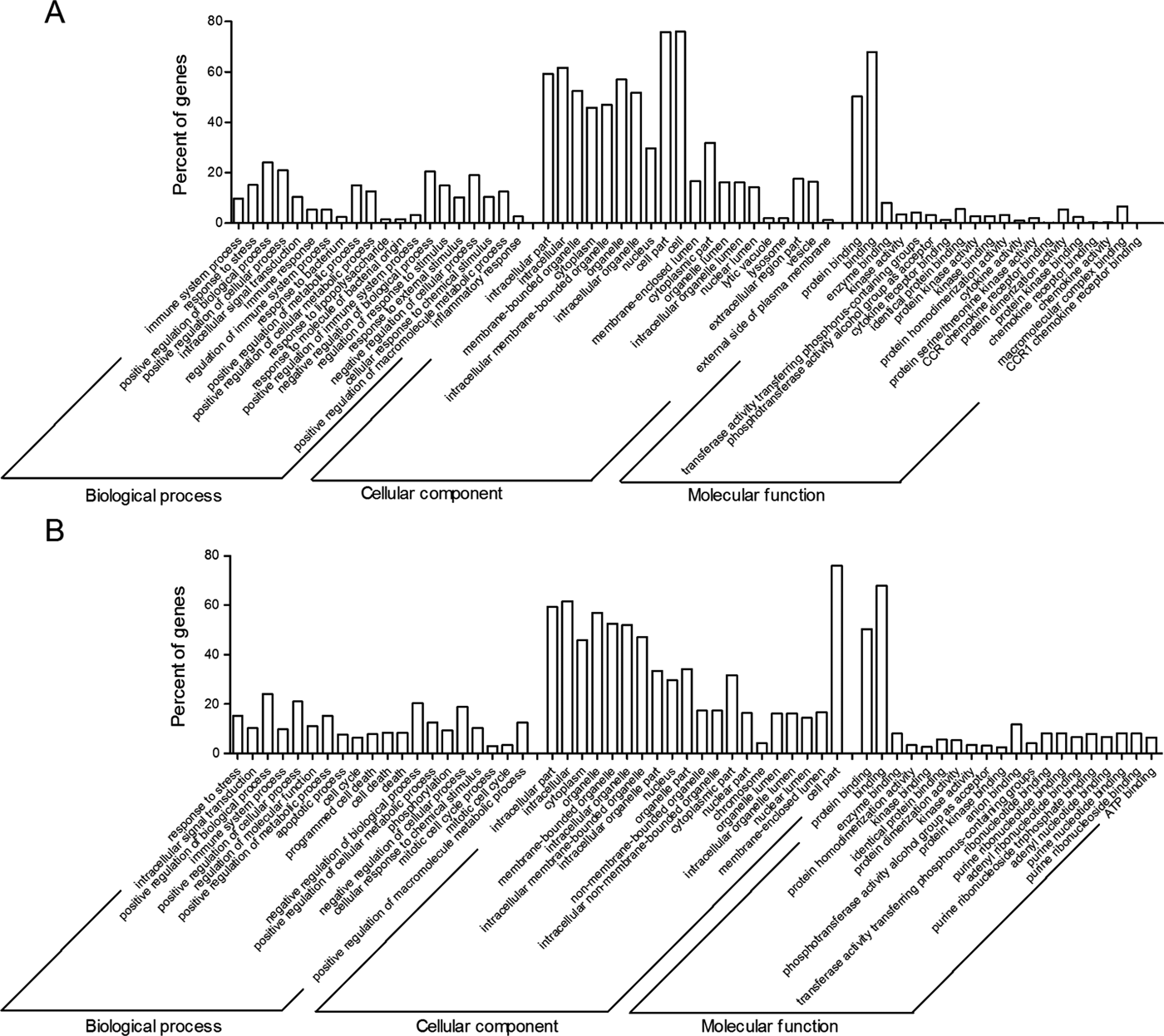
Fig. 5. Correlation between the miRNA expression and the corresponding targeting mRNA of cytokines and chemokines. (A) Shown is the predicted interaction between miRNAs and its corresponding target genes. (Band C) Expression means of mmu-miR-125a-5p, mmu-miR-125a-3p, mmu-miR-374d-5p, mmu-miR-21a-5p, mmu-let-7c-1-3p, mmu-miR-378d and mmu-miR-1981-5p (B) is negatively correlated with TNF-α, IL1α, IL6, ILl2α, CXCL22 and CCL18 (C) in the emu-treated cells at 12 h post infection.
DISCUSSION
As the important post-transcriptional regulators of gene expression, miRNAs play important roles in intricate host–pathogen interactions (Zheng et al. Reference Zheng, Cai and Bradley2013b ). Through the use of high-throughput methods (e.g. deep sequencing and miRNA microarray), changes of host miRNA profiles have been explored during parasite infection (Britton et al. Reference Britton, Winter, Marks, Gu, McNeilly, Gillan and Devaney2015). Increasing evidence shows that host miRNAs in the relevant cells or tissues are dysregulated during helminthic infection, hinting that miRNAs are crucial mediators in regulating parasite–host interplay (Cai et al. Reference Cai, Gobert and McManus2016). For instance, our previous study found that E. multilocularis infection disturbed miRNA expression in mouse liver (Jin et al. Reference Jin, Guo, Zhu, Ayaz and Zheng2017). In recently, we found that 58 host circulating miRNAs were dysregulated in the sera of E. multilocularis-infected mouse (Guo and Zheng, Reference Guo and Zheng2017). To date, no studies have examined the expression profile of miRNAs in immune cells in response to E. multilocularis exposure. Therefore, we herein investigated miRNA expression profiles in the macrophage cells exposed to E. multilocularis protoscoleces, providing clues to further pinpoint a role of miRNAs in regulation of immune responses during infection.
During host responses to infection, different active molecules released by Echinococcus species at distinct infectious phages modulate expression of a variety of host genes (Lin et al. Reference Lin, Lu, Wang, Zhang, Xie, Lu, Mantion, Martin, Richert, Vuitton and Wen2011). Our recent study demonstrated that exosome-like vesicles secreted by E. multilocularis metacestodes could convey protein and miRNA cargoes into host cells and modulate host gene expressions (Zheng et al. Reference Zheng, Guo, Su, Guo, Ding, Yang, Xiang, Cao, Zhang, Ayaz and Luo2017). Moreover, it was also found that E. multilocularis infection could disturb the expression of four key genes involved in miRNA biogenesis in the mouse liver (Jin et al. Reference Jin, Guo, Zhu, Ayaz and Zheng2017). In this study, we found the transcript levels of Ago2, Tarbp2, Xpo5 and DgcR8 were significantly altered in the RAW264·7 cells exposed to E. multilocularis protoscoleces compared with the control. Downregulation expression of Ago2 or Dicer1 could decrease the expression of selected apoptosis- and development-related miRNAs in mouse preimplantation embryos (Shen et al. Reference Shen, Han, Cui and Kim2010). Further study found Ago2 and Dicer1 may regulate GW182 (glycine-tryptophan protein of 182 kDa) protein expression to affected miRNA biogenesis (Shen et al. Reference Shen, Han, Cui and Kim2010). Therefore, although the detailed regulation mechanisms are not yet clear, the abnormal expression of these key genes may affect the action of RISC, subsequently altering the miRNA biogenesis and lifespan.
Exposure to any pathogen leads to significant changes in the expression of specific miRNAs in immune cells (Buck et al. Reference Buck, Coakley, Simbari, McSorley, Quintana, Le Bihan, Kumar, Abreu-Goodger, Lear, Harcus, Ceroni, Babayan, Blaxter, Ivens and Maizels2014; Carriere et al. Reference Carriere, Barnich and Nguyen2016). In agreement with this idea, significant alterations in miRNA expression profile were observed in the RAW264·7 cells exposed to E. multilocularis protoscoleces. It was found that 18 and 32 known miRNAs were differently expressed in emu-treated 6 h-library and 12 h-library, respectively. The number of downregulated miRNAs was increased with increasing treatment time. It is well known that Echinococcus species dynamically secrete active molecules and induce distinct immune response profiles at different infectious stages. The rapid changes of miRNAs at different time points are in agreement with this idea. From this point, these differentially expressed miRNAs can be used as a potential target to decipher the pathogenesis. Interestingly, some immune-related miRNAs (e.g. miR-146a/b, miR-155a and miR-125a-5p) were predominantly upregulated in the treated RAW264·7 cells. It was shown that the expression levels of miR-146a and miR-155 were increased in response to Toxoplasma Gondii infection (Cannella et al. Reference Cannella, Brenier-Pinchart, Braun, van Rooyen, Bougdour, Bastien, Behnke, Curt, Curt, Saeij, Sibley, Pelloux and Hakimi2014). Several studies revealed that mmu-miR-146a regulated immune and inflammatory response genes via target IRAK1, IRAK2 and TRAF6 (Taganov et al. Reference Taganov, Boldin, Chang and Baltimore2006). Early studies showed that miR-155 was upregulated following lipopolysaccharide-stimulation in macrophages (Hung et al. Reference Hung, Liu, Chou, Kao, Yang, Chang, Chiu and Lin2013). miR-155 promotes inflammation via suppression of inhibitors of inflammation, such as the suppressor of cytokine signaling 1 (SOCS-1) and Src homology-2 domain-containing inositol 5-phosphatase 1 (SHIP1) (O'Connell et al. Reference O'Connell, Chaudhuri, Rao and Baltimore2009; Zhao et al. Reference Zhao, Dong, Yang, Wang, Ma, Wang, Li and Zheng2017). These data imply that miR-146a-5p and miR-155a might play important roles in the regulation of host immune response modulation during E. multilocularis infection. The regulatory roles of differentially expressed miRNAs were explored by predicting the potential target genes of those miRNAs. The most enriched GOs were involved in cell communication, signal transduction, signalling receptor activity, cytokine activity and immune response. The expression levels of cytokine mRNAs such as TNF-α, IL1α, IL6, IL12α, IL12β, CCL22 and CCL18 were alerted in the macrophages exposed to E. multilocularis protoscoleces. Cytokines are involved in many aspects of inflammation and immunity, especially to parasite infection (Scales et al. Reference Scales, Ierna and Lawrence2007; Amri et al. Reference Amri, Mezioug and Touil-Boukoffa2009). Previous studies showed that IL-12a, IFN-γ and IL-4 were significantly expressed in the hepatic parasitic lesion at the early stage of E. multilocularis infection, CXCL9, IL-4, IL-5, CCL17 upregulated at the middle stage, and IL-10 and TGF-b permanently expressed at the late stage (Wang et al. Reference Wang, Lin, Zhang, Li, Gottstein, Blagosklonov, Lü, Zhang, Lu, Vuitton and Wen2014). There were statistically significant correlations between the expression levels of cytokines and their corresponding miRNAs, implying a role for these miRNAs in regulating host immune responses against E. multilocularis. Further studies will be needed to verify whether the miRNA changes lead to altered expression of cytokine gene, either directly (by acting as miRNA targets) or indirectly (e.g. by affecting transcription factors).
Concluding remarks
Previous study showed that RAW 264·7 cells most closely mimic primary bone marrow-derived macrophages in terms of cell surface receptors and responses to microbial ligands that initiate cellular activation via Toll-like receptors 3 and 4 (Berghaus et al. Reference Berghaus, Moore, Hurley, Vandenplas, Fortes, Wolfert and Boons2010). However, caution must be taken when extrapolating the findings obtained with RAW 264·7 cells to other primary macrophage-lineage cells, primarily because phenotype and function of the former cells may change with continuous culture. In conclusion, we report for the first time that the miRNA expression profile of macrophages exposed to E. multilocularis metacestodes is significantly and rapidly altered. It is suggested that the alterations in macrophage miRNA levels likely reflect the remarkable capacity of parasites in modulation of host immune responses.
ACKNOWLEDGEMENTS
The author would like to thank Miss Jing Yang for help in qRT-PCR and also thank the reviewer and editor for their constructive suggestions.
FINANCIAL SUPPORT
The study was financially supported by grants from the National Key Basic Research Program (973 program) of China (No. 2015CB150300), the National Natural Science Foundation of China (grant numbers 256 31201900 and 31472185) and State Public-interest Institution Basal Research Fund (grant no. 1610312017017). The funders had no role in study design, data collection and analysis, decision to publish and preparation of the manuscript.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001652.








