Published online by Cambridge University Press: 19 April 2005
The Py235 merozoite rhoptry protein of the rodent malaria agent Plasmodium (yoelii) yoeli is encoded by the Py235 multigene family whose members are transcribed during the parasite's asexual life-cycle in a fashion where single schizonts subsequently give rise to sets of merozoites containing distinct Py235 transcripts. Homologues of Py235 are found in other malaria species, and antibodies to both Py235 and P. falciparum homologues inhibit merozoite invasion, suggesting a unique survival strategy involving immune evasion and host adaptation. Using a mathematical approach to model this free-living stage of Plasmodium in interaction with specific antibodies and a heterogeneous red blood cell population, we investigate if, and under what conditions, this mechanism of clonal phenotypic variation can play a role in immune evasion and adaptation to a dynamic erythropoietic environment.
During its life-cycle, the malaria parasite must be able to recognize, invade and develop within different cells of different hosts. In the mammalian blood-stream, merozoites are released from the liver after initial infection and must then recognize and penetrate the host's erythrocytes, giving rise to subsequent merozoite populations. Red blood cell invasion is a complex and not fully understood process, but the apical organelles (e.g. rhoptries) are known to play a crucial role in host cell discrimination, binding and invasion (Holder & Freeman, 1981; Bannister & Dluzewski, 1990; Ogun & Holder, 1996). In P. yoelii a multigene family codes for the merozoite rhoptry proteins (Py235) that are thought to determine the subsets of erythrocytes the parasite invades (Keen et al. 1990; Holder et al. 1991; Borre et al. 1995). The mechanism of gene expression by which merozoites originating from a single schizont each express a different member of this multigene family is genetically and functionally distinct from classical antigenic variation (Preiser et al. 1999). Transcription of the Py235 family is initiated in the trophozoite stage of the parasite's intra-erythrocytic life-cycle, where single genes are transcribed. More and more are activated as the parasite matures and RNA replication occurs. Thus, in the early and late schizonts multiple transcripts can be detected and each different Py235 RNA message is then segregated into specific merozoites within the developing schizont. This results in each single merozoite containing a distinct Py235 member, which allows a schizont to produce a set of distinct merozoites.
It has been shown that Py235 is involved in red blood cell discrimination and binding, so variation in this region could have evolved in order to contend with the polymorphism and heterogeneity in host cell ligands (Ogun et al. 2000). There are at least 3 different erythrocyte ligands: glycophorin A, glycophorin B, and another unidentified factor used by different lines of P. falciparum (Handunetti, Mendis & David, 1987; Dolan et al. 1994; Sim et al. 1994) and some parasite clones have been shown to switch the invasion pathway they use when selective pressure is applied due to activation of a different receptor gene in the parasite (Dolan, Miller & Wellems, 1990; Triglia et al 2005). Thus it is conceptually possible that highly specific recognition could account for erythrocyte choice in P. yoelii, although specificity of individual Py235 proteins to defined receptors has yet to be determined.
Additionally, variation in the cysteine rich region of Py235 could allow the parasite to generate antigenic diversity as a form of immune evasion (Khan, Jarra & Preiser, 2001). It has been shown that antibodies to the Py235 protein block erythrocyte binding and therefore inhibit merozoite invasion (Freeman, Trejdosiewicz & Cross, 1980; Ogun et al. 2000). By switching expression of Py235, the parasite may thus evade the specific immune response. Since there is evidence that schizonts preferentially express the same gene as the merozoite they originate from (Khan et al. 2001), it has been assumed that a ‘successful’ member would amplify within the population in terms of a clonal selection process, whilst still producing a whole range of different phenotypes. A change in the host-cell environment or a specific antibody response therefore would not necessarily lead to a complete removal of the population, but result in a subsequent amplification of another Py235 member, thereby prolonging the infection (Snounou & Preiser, 2000).
We have recently proposed a theoretical framework to account for antigenic variation among the Variant Surface Antigens of Plasmodium falciparum that relies entirely on suppression by transient partially cross-reactive immune responses (Recker et al. 2004). This model shows that within a homogeneous host environment, exclusively variant specific immunity is incapable of promoting immune evasion, but in combination with partially cross-reactive responses is able to produce sequential dominance of antigenic variants. Here we investigate whether clonal phenotypic variation of merozoite rhoptry proteins can lead to a positive selection process within a heterogeneous host (i.e. RBC) environment under conditions where immunity is exclusively variant specific or partially cross-reactive.
The model's underlying principle is a simple multi-dimensional predator-prey interaction between free-living merozoites, mi, and specific antibodies, ai, with one additional equation vector for the population of infected red blood cells, ri. We distinguish infected red blood cells only by the merozoite type that invaded them; red blood cell heterogeneity is thus included implicitly.
Merozoites of type i (i.e. merozoites with the rhoptry protein Py235 variant i) are released from infected red blood cells of all types, but preferentially by red blood cells that were invaded by type i. Antibodies specific to variant i prevent the merozoite from invading red blood cells with ‘efficiency’ κ, and we also consider the case where antibodies are able to cross-protect against a certain (variant specific) subset of other merozoite phenotypes with a lower affinity γ, (0<γ<1). We therefore have for the rate of change in the merozoite population
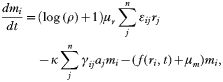
with εij (1/n[les ]εij[les ]1) as a weighing factor for the mixed merozoite subpopulation released from a single schizont, given as
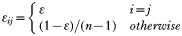
where n is the total number of phenotypes.
Saul (1998) has raised the issue that simply using ρμr as the net production rate of merozoites, with 1/μr as the time to rupture and ρ as the number of merozoites released from one infected red blood cell, results in a substantial over-estimation in parasite growth, and proposed the above formulation instead. One problem with this formulation is that it does not have a clear biological interpretation; but, in effect, neither the elegant alternative proposed by Gravenor & Lloyd (1998), nor the use simply of ρμr as the net production rate, leads to qualitatively different behaviour. The function f(·) describes the removal of free-living merozoites from the blood-stream by invasion of red blood cells, and μm is the removal rate by natural death.
Merozoites are highly vulnerable to immune attack, and therefore have to rapidly find a ‘suitable’ host within a heterogeneous population of cells when they are released into the blood-stream. This heterogeneity can be either due to polymorphism of host cell ligands or simply due to age structure (different species of Plasmodium appear to vary in their ability to parasitize erythrocytes of different phenotype and degree of maturity (Holder et al. 1991)). To implicitly incorporate host cell heterogeneity we can assign fitness differences to the merozoite phenotypes. This way we only have to consider the population of infected red blood cells, defined by the invading merozoite type. Its rate of change can then be given as
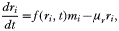
where 1/μr is the average life-span of an infected red blood cell which includes rupture (release of merozoites) and general clearance from the blood-stream.
Antibodies are produced specifically and in proportion to the number of free-living merozoites of type i at rate β, and have an average life-span of 1/μa. So we have:
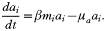
For simplicity, immune memory is not included but the results can easily be extrapolated to the case where a primary stimulation of specific antibodies will lead to the build up of immune memory. In order to investigate how the proposed mechanism could enhance (re-) invasion success when dealing with host cell heterogeneity or other restraints, different invasion profiles of merozoites, f, have been considered: (i) a constant invasion rate, i.e. f=K; (ii) a density dependent invasion rate, i.e. f=f(ri); and (iii) a time-dependent invasion rate, i.e. f=f(t). As the blood picture of the host can vary dramatically during malarial infections (and also differs between hosts) this is of particular interest as it allows the study of adaptation to a dynamic erythropoietic environment. In the following, fitness differences of merozoites reflect host cell heterogeneity. Having a higher fitness correlates with the dominance of a particular type of red blood cell as encounter and invasion is then more likely for phenotypes specialized to this type.
Seven pathogen phenotypes were used to elucidate whether this mechanism of clonal phenotypic variation could contribute to a positive selection process. In order to examine host adaptation we first consider the case where variants do not inhibit each others reproduction success in terms of cross-inhibition by taking γij=δij, where δij is the Kronecker delta:
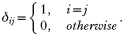
If we assume the completely symmetric case with no fitness differences between variants in a static erythropoietic environment, i.e. fi=f, ∀i and ∂f/∂t=0, we find the dynamics quickly converging towards one of the possible equilibrium states. System (1)–(3) possesses a disease-free equilibrium
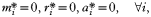
and linear stability analysis shows that this point is unstable as long as
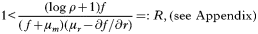
where R can be defined as the pathogen's basic reproductive number. Assuming that (5) holds, we find the system reaching a non-zero state of equilibrium. In the case of a constant invasion profile, i.e. f=K, this is given as
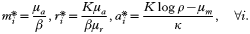
This is interesting as neither the density of infected red blood cells nor the density of free-living merozoites is dependent on the number of merozoites released from an infected red blood cell. If we assume that the invasion profile of free-living merozoites is density dependent, the function f can be defined as
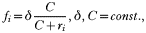
where δ can be taken as the maximum invasion rate in the absence of resource limitation. We assumed that all infected erythrocytes have a negative effect on the invasion success of merozoites of type i. In this case we find two non-trivial fixed-point solutions to system (1)–(3), one of which is density controlled and independent of the immune response:
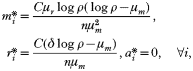
where n is the number of phenotypes. This means that strong resource limitation can lead to self-limited parasitaemia. Incorporating fitness differences by making f variant specific, for example ordered in a hierarchical manner f1<f2< …, does not alter the above results other than leading to structured equilibria in the RBC and antibody population, where phenotype prevalence is in order of their respective fitness. Interestingly, though, free-living merozoites will exhibit no such structure but equilibrate towards the same frequency, despite fitness differences (results not shown here).
Rather than assuming a static composition of the red blood cells, we can incorporate a dynamic erythropoietic environment by making f explicitly time-dependent. By taking
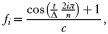
with positive constants Δ and c, we make sure that fi[ges ]0 and that the average availability of red blood cells for invasion stays constant over time, although this is not a necessary requirement to achieve similar results.
For low values of ε the system will quickly converge towards low amplitude oscillations about the fixed point of the equivalent autonomous system (1)–(3), with ∂f/∂t=0. By increasing the frequency at which a merozoite subpopulation is dominated by its originating type, i.e. increasing ε, we can observe sustained oscillations representing sequential selection and amplification of single phenotypes, as shown in Fig. 1. Numerical simulations also reveal a positive correlation between ε and peak heights. That is, clonal selection is more efficient the more clones of a particular variant are produced at each reproduction cycle. In this respect, the clonal phenotypic variation process can enhance the merozoites' survival success by allowing them to adapt to temporal environmental changes. Although the oscillatory behaviour can be attributed to the time-periodic invasion function f, a positive selection process whereby single variants gain an advantage over others is nevertheless apparent. The fluctuating erythropoietic environment thus serves an exogeneous pump to reinforce the natural propensity of the system to oscillate, in a manner analogous to seasonal forcing or demographic stochasticity in simple epidemiological models (as reviewed recently by Levin, Dushoff & Plotkin, 2004).
In a recent theoretical study on antigenically variable pathogens we have shown that immunological interaction between antigenic types or variants can not only prevent the concurrent expression of a large number of possible variants but also create a cascade of sequentially dominant antigenic variants (Recker et al. 2004). In order to see if similar assumptions could produce a positive selection process in this very different system, we include the possibility of specific antibodies to protect against certain other variants. The particular form of non-symmetric cross-inhibition can be represented by the cross-protection matrix:
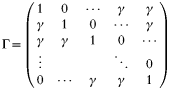
where γ (as previously defined) is a measure of cross-protection.
If we assume that the invasion properties of the merozoite are not density dependent, i.e. f=K, we find that for low values of γ, the system will settle into a non-zero steady state with all strains being present at same equilibrium frequency, given as
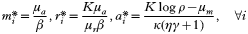
where η is the number of variants that induce cross-reactive antibodies against i (here η=2). Increasing γ, this steady state looses its stability and is replaced by stable limit cycles that represent sustained oscillations of single merozoite variants. These oscillations, shown in Fig. 2, demonstrate the sequential selection and amplification of variants that have a temporal advantage over other variants as their growth is not restricted by antibodies raised against the preceding type(s). That is, because of the cross-inhibitory relationship between variant types defined by Γ, if we would take type i as the peaking variant at any one time it would be followed by one that is not negatively affected by the immune response launched against i or its preceding types. This scenario leads to a traveling wave of single types.

Fig. 1. Time-series of free-living merozoites, mi, showing a pronounced selection process of single variants for increasing ε. ε=0·4, top, and ε=0·8, bottom. Other parameter values: μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, Δ=120, c=0·2, κ=0·1.

Fig. 2. Depending on the degree of immune pressure, in terms of cross-immunity γ, the system will either settle down onto a stable non-zero equilibrium (top, γ=0·5) or exhibit sustained oscillations of single types (bottom, γ=1). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, f=K=10.
The net production rate, ρμr, or rather (log (ρ)+1)μr, does not have a significant impact on the dynamics, whereas the latter is crucially dependent on the composition of the merozoite subpopulation, ε. Decreasing the frequency of a predominantly produced merozoite phenotype (decreasing ε) will lead to a stable equilibrium, whereas an increase will tend to produce much sharper dynamics in terms of period and amplitude. With a more homogeneous subpopulation (high ε) single types cannot be amplified within the population as all antibodies are present at high level at all time. On the other hand, producing only merozoites of the invading type is favourable in a selection process, although very high peaks could be subject to a trade-off in terms of exhausting the host's resources. The parameter space in Fig. 3 shows how an increase in γ and ε will lead to sustained oscillations of single merozoite types that represent the clonal selection process.

Fig. 3. (γ,ε) – parameter-space showing the region of clonal phenotypic selection. The sequential amplification of single types is promoted by a strong immune selection pressure (high γ) and strongly biased transcription (high ε). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, f=K=10.
If we include density-dependent invasion we find that for intermediate or lower degrees of invasion regulation (high values of C), sustained oscillations can still be found in higher regions of cross-protection, whereas low ε and γ will lead to a state of equilibrium. Therefore, provided the invasion success is not too restricted by the number of infected red blood cells, phenotypic variant selection can still be found, as shown in Fig. 4. Adding host-heterogeneity to the density-dependent invasion regulation, in terms of merozoite fitness differences, will lead to similar dynamical behaviour, except that sustained oscillation can occur even in lower regions of γ and peak heights are restricted by a self-regulatory effect.

Fig. 4. A strong dependency on the number of red blood cells already infected will self-limit the disease, independent on the host's immune response (top, C=1E2). For higher values of C phenotypic variation can be observed (bottom, C=1E4). Other parameter values: μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, δ=10, γ=0·8, ε=0·9.
Introducing a dynamical erythropoietic environment we find that the dynamics do not depend that critically on the degree of cross-protection anymore, although an increase in peak heights is still observable with increasing γ. That is, because of the non-autonomous (periodically driven) invasion profile, oscillations are observable even in the very low regions of γ. The weighing factor ε, on the other hand, significantly determines the qualitative behaviour: from low-amplitude oscillations around the associated fixed point to sequential amplification of single types with an increase in ε. In the extreme where ε=1, after an initial peak some of the variants will be driven to extinction by a very strong immune response. Increasing the immune pressure upon the merozoite population (increasing γ) we see more and more variants being driven extinct, and because phenotypes are not being produced by any other types, they are permanently eliminated (see Fig. 5). Under these circumstances the benefit of producing a mixed merozoite subpopulation from a single schizont would be to avoid severe diversity reduction under increased immune pressure.

Fig. 5. Increasing the immune pressure will lead to diversity reduction within the merozoite population, (γ,ε)=(0·2,1), (0·2,1), ( 0·6,1) and (0·8,1) (top left to bottom right). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, Δ=120, c=0·2.
The sequential dominance of single merozoite phenotypes observed seems to follow a particular order. So far, no variant order has been observed within real merozoite populations (Preiser et al. 1999), as the sequential amplification of certain types is still only a hypothesis. In our model, the order of phenotypes that are amplified over time is determined by the non-reciprocal form of cross-protection matrix Γ, and, in the case of a dynamic environment by the invasion function f. Looking at Γ we see that antibodies against type i cross-protect against types i+1 and i+2. Growth of type i+3, for example, is thus not constrained by an immune response against i and can therefore be amplified within the population. (Note, in case i=n, i+1=n+1=:1.) Introducing a time-dependent composition of the red blood cell population promotes oscillations of single variants in order of the distribution defined by f. Growth of variants that are fittest at any one point will thus be promoted. Interestingly though, the order in which variants peak is changed when immune pressure is increased (Fig. 6). Two different selection phenomena are displayed by the system: one which can be attributed to immune evasion (variant order determined by Γ), and the other one to host/environment adaptation (variant order determined by f). We therefore note two driving forces for positive selection that could contribute to a successful evasion and adaptation strategy.

Fig. 6. Low degree of immune pressure will preserve the variant order (arbitrarily chosen 1→2→ … →8→1 … ) imposed by f, γ=0·3 (top). An increase results in an order such that succeeding variants do not interfere with each others growth, determined by cross-protection matrix Γ (γ=0·8, bottom). ε=0·9, μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, Δ=120, c=0·2.
We studied the dynamics of free-living merozoite populations in order to elucidate the conditions necessary for successful clonal phenotypic variation under different constraints upon the population. System (1)–(3) describes the interaction of n merozoite phenotypes with a specific and cross-protective immune response, incorporating details of their erythrocytic life-cycle (invasion of and release from infected red blood cells). For a solely variant-specific immune response (no cross-protection between specific antibodies) numerical simulations show three possible behaviours: disease-free equilibrium, a non-zero steady state and sustained oscillations of single types. The condition for a disease-free state, R<1, is completely independent of any immune action and represents a fundamental property of the pathogen's life-cycle to establish disease. Furthermore, if we assume that invasion of red blood cells by merozoites is impeded by the number of red cells already infected, then there exists another state of equilibrium independent of the immune response but resource dependent. In reality, equilibria are very unlikely to be observed and might be an artifact of the model itself, especially as no stochastic effects are taken into account.
Assuming a dynamic red blood cell population, in which the composition of subtypes changes over time, results in low-amplitude oscillations in the merozoite population. These are due to the non-autonomous (i.e. periodically driven) form of the resulting system, although peak heights are positively correlated with the propensity of schizonts to produce specific merozoite variants, ε. That is, high values of ε allow successful variants to be rapidly amplified within the population before the immune system reacts. The more type-specific the transcription process of Py235 is, the faster specific phenotypes can be produced, enabling rapid clonal expansion. Thus, by assuming a dynamic erythropoietic environment, we can observe a positive selection process.
When assuming cross-inhibition between variants, we can observe sustained oscillations of single variants in a sequential manner even within a static erythropoietic environment. That is, allowing antibodies to cross-protect against certain other phenotypes will increase the immune selection pressure upon the merozoite population, resulting in subsequent amplification of single variants within the population. Provided that the transcription process of single merozoites, as described for P. yoelii, predominantly produces merozoites of the original type, the most successful type is allowed to grow in number as the result of clonal selection. In reality, this process would continue until either the immune system is overwhelmed or sufficient memory is built up to control the disease.
The simulations show that the more heterogenous the progeny descending from one merozoite (i.e. low ε), the easier it is for the immune system to gain control over the pathogen. On the other hand, if the progeny is too homogenous (i.e. high ε), rapid clonal expansion could potentially lead to exhaustion of resources (specific RBC populations), showing a trade-off between merozoites producing subpopulations that are too heterogenous and subpopulations that are too uniform. From these results we would postulate a medium to high degree of preference in gene transcription in order to achieve the evolutionary advantage of clonal phenotypic variation under the pressure of a network of cross-protective antibodies.
In summary, we find that, in the absence of immune selection, sustained oscillations representing sequential selection and amplification of single phenotypes can only occur under fluctuating erythropoetic environment. Within our framework, this is encoded as a deterministic process, but we can safely assume that stochastic fluctuations will have the same pumping effect. We find, not surprisingly, that a network of cross-reactive responses can result in the sequential emergence of phenotypic variants, but additionally that a fluctuating RBC environment extends the region of parameter space over which this can occur. It appears therefore that this phenomenon is mainly of consequence within a fluctuating erythropoetic environment, both in the absence of cross-immunity and where cross-immunity promotes chronicity. The phenomenon described here for P. yoelii could also apply to other Plasmodium species. Py235 homologues have been identified in P. berghei (Owen et al. 1999), P. vivax and P. chabaudi (Galinski et al. 1992; Keen et al. 1994; Galinski & Barnwell, 1996), and P. falciparum (Rayner et al. 2000). Although a comparable mechanism of phenotypic variation has so far not been found in other Plasmodium species, it is conceivable that it might also operate in other protein families.
We would like to thank the MRC and the Wellcome Trust for financial support.
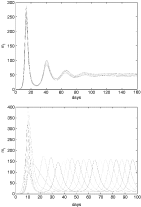
Fig. 1. Time-series of free-living merozoites, mi, showing a pronounced selection process of single variants for increasing ε. ε=0·4, top, and ε=0·8, bottom. Other parameter values: μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, Δ=120, c=0·2, κ=0·1.

Fig. 2. Depending on the degree of immune pressure, in terms of cross-immunity γ, the system will either settle down onto a stable non-zero equilibrium (top, γ=0·5) or exhibit sustained oscillations of single types (bottom, γ=1). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, f=K=10.
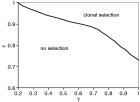
Fig. 3. (γ,ε) – parameter-space showing the region of clonal phenotypic selection. The sequential amplification of single types is promoted by a strong immune selection pressure (high γ) and strongly biased transcription (high ε). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, f=K=10.

Fig. 4. A strong dependency on the number of red blood cells already infected will self-limit the disease, independent on the host's immune response (top, C=1E2). For higher values of C phenotypic variation can be observed (bottom, C=1E4). Other parameter values: μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, δ=10, γ=0·8, ε=0·9.

Fig. 5. Increasing the immune pressure will lead to diversity reduction within the merozoite population, (γ,ε)=(0·2,1), (0·2,1), ( 0·6,1) and (0·8,1) (top left to bottom right). μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, Δ=120, c=0·2.

Fig. 6. Low degree of immune pressure will preserve the variant order (arbitrarily chosen 1→2→ … →8→1 … ) imposed by f, γ=0·3 (top). An increase results in an order such that succeeding variants do not interfere with each others growth, determined by cross-protection matrix Γ (γ=0·8, bottom). ε=0·9, μm=1 [h−1], μr=1/30 [h−1], μa=5E-3 [h−1], β=1E-4, ρ=20, κ=0·1, Δ=120, c=0·2.