Introduction
Haemosporidian parasites are common and widespread in birds and may strongly affect the health of their hosts (Valkiūnas, Reference Valkiūnas2005). They reproduce asexually in birds and sexually in insect vectors. Sporozoites are injected into the birds’ blood when the vectors feed on birds. The sporozoites, after asexual divisions in different bird tissues, produce exoerythrocytic meronts. After exoerythrocytic development, merozoites appear in the blood for gametocyte formation or for merogony and gametocyte formation, depending on parasite genus. Mature gametocytes are transmitted into the vectors when feeding on infected birds; and fertilization, then oocyst formation and sporogony take place in the vectors. During the various stages of the life cycle, these parasites can cause anaemia, block up capillaries in different organs and decrease the oxygen-binding capacity of haemoglobin. The symptoms and the effects of the parasites are more severe during acute infections (Valkiūnas, Reference Valkiūnas2005), however, recent research shows that chronic infections may also have long-term effects on the health of the birds (Asghar et al., Reference Asghar, Hasselquist, Hansson, Zehtindjiev, Westerdahl and Bensch2015). Therefore Haemosporidian parasites, including avian malaria (here Haemoproteus and Plasmodium species) are suitable candidates to study host–pathogen systems and coevolution (Atkinson and van Riper, Reference Atkinson, van Riper, Loye and Zuk1991; Valkiūnas, Reference Valkiūnas2005).
Lot of research focuses on the effects of these parasites on their bird hosts (reviewed in Lapointe et al., Reference Lapointe, Atkinson and Samuel2012), however, the results of experimental infections and field observations are often mixed. The inconsistencies between these studies might be because experimental and field studies investigated the effects of the parasites during different stages of the infection. Under experimental conditions, the negative effects of infections on host physiology were more pronounced under the acute than during the chronic stage (e.g. Atkinson et al., Reference Atkinson, Dusek, Woods and Iko2000; Valkiūnas et al., Reference Valkiūnas, Zickus, Shapoval and Lezhova2006; Palinauskas et al., Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2008; Krams et al., Reference Krams, Suraka, Rantala, Sepp, Mierauskas, Vrublevska and Krama2013). However, the acute stage of infection is difficult to detect in the nature, since during this stage, birds sit and hide, and are rarely caught (e.g. Yorinks and Atkinson, Reference Yorinks and Atkinson2000). Infected individuals sampled under natural conditions are therefore biased towards the active individuals that have already survived the acute stage so that they are in the chronic stage of infection. The effects of malaria parasites on the health of the hosts during chronic infections are smaller and manifested rather on the long-term (Lapointe et al., Reference Lapointe, Atkinson and Samuel2012; Asghar et al., Reference Asghar, Hasselquist, Hansson, Zehtindjiev, Westerdahl and Bensch2015). Moreover, any adverse effect of blood parasite infections on host physiology is more difficult to detect in these ‘good quality individuals’ that have already survived the acute stage of infection.
Moreover, getting an overview about the impacts of the parasites is difficult in the field, because most studies have no information about the local vector fauna that potentially transmits avian haemosporidian parasites. Neither the exact timing nor the exact stage of infection is known at the time of blood sampling of the birds. In addition, little is known about the effects of these parasites on host behaviour, e.g. how they influence the capture-recapture probability of the individuals (Marinov et al., Reference Marinov, Zehtindijev, Dimitrov, Ilieva, Bobeva and Marchetti2017). The other complicating circumstance is that most field studies have focused on the investigation of the effects of these parasites during the breeding period (e.g. Hatchwell et al., Reference Hatchwell, Wood, Anwar and Perrins2000; Schrader et al., Reference Schrader, Walters, James and Greiner2003; Dubiec et al., Reference Dubiec, Podmokła, Zagalska-Neubauer, Drobniak, Arct, Gustafsson and Cichoń2016; Peev et al., Reference Peev, Zehtindjiev, Ilieva, Träff, Briedis and Adamík2016) or the spring migration period of the birds (e.g. Rintamäki et al., Reference Rintamäki, Halonen, Kilpimaa and Lundberg1997; Garvin et al., Reference Garvin, Szell and Moore2006; DeGroote and Rodewald, Reference DeGroote and Rodewald2010). Interestingly, the potential effects of avian malaria parasites beyond the breeding season and spring migration have been less examined and the majority of these studies focused rather on faunistic and basic epidemiological questions (e.g. Deviche et al., Reference Deviche, Greiner and Manteca2001; Waldenström et al., Reference Waldenström, Bensch, Kiboi, Hasselquist and Ottosson2002; Arriero and Møller, Reference Arriero and Møller2008; Pagenkopp et al., Reference Pagenkopp, Klicka, Durrant, Garvin and Fleischer2008; Santiago-Alarcon et al., Reference Santiago-Alarcon, Bloch, Rolshausen, Schaefer and Segelbacher2011).
However, the migration itself has long been studied in birds, since during this complex, directional and energetically highly demanding movement, birds have to optimize their arrival and departure time to achieve the best environmental and weather conditions for flying, refuelling and moulting (Alerstam et al., Reference Alerstam, Hedenström and Åkesson2003; Newton, Reference Newton2008). Beyond the unpredictability of weather and habitat quality, birds have to face different predators, they have to compete with resident bird species and face an increased risk of getting new parasitic infections [including avian malaria (Clark et al., Reference Clark, Clegg and Klaassen2015)] on their migratory routes and wintering sites. As avian malaria may impose strong negative effects on the physiology of the birds also during migration (Valkiūnas, Reference Valkiūnas2005), it is expected that migration speed/timing of the individuals is negatively affected by these parasites.
Some of the studies indeed found that malaria infected individuals or individuals with higher parasitaemia arrived later and in some species more infected individuals were in worse energetic condition after spring migration (Møller et al., Reference Møller, de Lope and Saino2004; Garvin et al., Reference Garvin, Szell and Moore2006; DeGroote and Rodewald, Reference DeGroote and Rodewald2010; Emmenegger et al., Reference Emmenegger, Bauer, Hahn, Müller, Spina and Jenni2018). On the other hand, others found no detectable negative effects of parasite infections during the preparation for autumn migration (Hahn et al., Reference Hahn, Bauer, Dimitrov, Emmenegger, Ivanova, Zehtindjiev and Buttemer2018; Hegemann et al., Reference Hegemann, Abril, Sjöberg, Muheim, Alerstam, Nilsson and Hasselquist2018a), though the effects of malaria infections in the context of autumn migration are generally understudied. Therefore, to gain more insight in the potential relationship between migration phenology and avian malaria infection and to understand the seasonal role of these parasites in host life history, more studies are clearly needed.
Here we study avian malaria infections in European Robins (Erithacus rubecula; later Robins) during autumn migration, because this bird species is one of the most abundant autumn migrants in Europe and other populations of the species were found to be infected with avian malaria (MalAvi; Bensch et al., Reference Bensch, Hellgren and Pérez-Tris2009). The migratory behaviour, orientation, important stopover and wintering areas of this species are well studied (for details see Harnos et al., Reference Harnos, Ágh, Fehérvári, Karcza, Ócsai and Csörgő2018), however until now there is no data on the effects of blood parasites during migration.
During autumn migration, a mixed population of Robins crosses our stopover site in the Carpathian basin, which consists mainly of birds originated from Eastern-Central Europe (Hromádko, Reference Hromádko, Cepak, Klvăna, Škopek, Schröpfer, Jelínek, Hořák, Formánek and Zárybnický2008; Gyurácz and Csörgő, Reference Gyurácz, Csörgő, Csörgő, Karcza, Halmos, Magyar, Gyurácz, Szép, Bankovics, Schmidt and Schmidt2009; Bairlein et al., Reference Bairlein, Dierschke, Salewski, Geiter, Hüppop, Köppen, Fiedler, Bairlein, Dierschke, Salewski, Geiter, Hüppop, Köppen and Fiedler2014) and in a smaller proportion of birds from the Baltic coast (Ściborska and Busse, Reference Ściborska and Busse2004; Adamska and Filar, Reference Adamska and Filar2005). Populations originated from Scandinavia seem to avoid the Carpathian basin (reviewed in Harnos et al., Reference Harnos, Ágh, Fehérvári, Karcza, Ócsai and Csörgő2018). The breeding populations in Hungary start the migration already in the middle of August, leave the Carpathian Basin by the middle of September and are continuously replaced by migrating birds that arrive from geographically closer regions. Birds from geographically more distant populations arrive at the stopover site in the second part of September, show a peak in number in the middle of October (Gyurácz and Csörgő, Reference Gyurácz, Csörgő, Csörgő, Karcza, Halmos, Magyar, Gyurácz, Szép, Bankovics, Schmidt and Schmidt2009; Gyimóthy et al., Reference Gyimóthy, Gyurácz, Bank, Bánhidi, Farkas, Németh and Csörgő2011) and leave our study site by the beginning of November.
Here we examined (1) whether the overall prevalence of avian malaria parasites differs between age and sex groups; (2) if there is a relationship between malaria infection status and biometrical traits (wing feather length, body mass) and condition (fat scores) of the individuals and (3) whether the timing of arrival during autumn migration differs between infected and not infected individuals.
Materials and methods
Field methods
We captured Robins at the Ócsa Bird Ringing Station (Central Hungary: 47°17′N, 19°12′E) using mist nests and following the Actio Hungarica capturing methods (for details see Csörgő et al., Reference Csörgő, Harnos, Rózsa, Karcza and Fehérvári2016). This area is a post-glacial peatbog with mosaic, heterogeneous vegetation ranging from reed beds to mature forests and is under international protection (Ramsar Convention, Natura 2000). Robins are common breeders at this site and during spring and autumn they are regular and common passage migrants.
In 2016 we took blood samples from 406 Robins from 13th August till 5th November. However, to include only birds that are most likely under migration we narrowed this time period so that we analysed samples collected from late August (20th August) to early November (5th November) (N = 403). Sampling was conducted periodically so that four sampling days were followed by a 3-day-break period. The birds included in our study arrived from different areas of Europe, however, based on morphological characteristics their exact breeding habitat cannot be determined (reviewed in Harnos et al., Reference Harnos, Ágh, Fehérvári, Karcza, Ócsai and Csörgő2018).
Blood samples were collected into 96% alcohol and stored at −20 °C until analysis. We also prepared blood smears that were air dried and fixed in absolute methanol. We determined the age of the birds based on plumage characteristics and upper mandible colour (Svensson, Reference Svensson1992; Demongin, Reference Demongin2016) and measured each individual based on the standard methodology of bird ringing. We measured wing feather length (the flattened maximum wing chord), from the carpal to the tip of the longest primary (to the nearest millimetre using a ruler) (Svensson, Reference Svensson1992) and body mass (to the nearest 0.1 g using digital balance) too. We generally estimate the subcutaneous fat deposit by fat scores developed by Kaiser (Reference Kaiser1993); ranges from 0 (no visible fat) to 8 (fully covered with fat), however, the birds included in this study had fat scores between 0 and 3. We defined arrival time as the ‘day of the year’ when the given bird was first caught at the study site (Harnos et al., Reference Harnos, Fehérvári and Csörgő2015). In our sample, there were 345 juvenile (hatched in the year of capture) and 58 adult (older) individuals.
Laboratory methods
We stained blood smears with Giemsa stain solution (1:10) for 45 min according to the manufacturer's protocol. Blood smears were visualized under 1000× magnification by G.M. to identify samples positive for either Haemoproteus or Plasmodium. Blood samples corresponding to malaria positive blood smears were served as ‘positive controls’ during the molecular detection of parasites. DNA was extracted using a GeneAid Genomic DNA Mini Kit (Tissue) following the manufacturer's protocol (Thermo Scientific™). Molecular sexing was performed using the primer pairs CHD1-i9F and CHD1-i9R (Suh et al., Reference Suh, Kriegs, Brosius and Schmitz2011) to determine the sex of the birds and to check the quality of the DNA.
For the molecular detection of avian malaria parasites, we used a highly efficient nested polymerase chain reaction (PCR) method (Waldenström et al., Reference Waldenström, Bensch, Hasselquist and Ostman2004). The thermal profile of the PCRs and the concentration of the reagents used in this study were the same as described in Waldenström et al. (Reference Waldenström, Bensch, Hasselquist and Ostman2004). In all PCRs both negative (ddH2O) and positive controls (samples that were confirmed to be infected based on blood smear investigations) were included to control for possible contaminations and amplification failures during PCRs, respectively. However, neither negative controls nor positive controls ever showed contamination or amplification failures (respectively) during PCR. To reduce the risk of losing infections because of sampling error we screened negative blood samples twice for blood parasites. Positive infection was indicated by the presence of a fragment of an approximately 520 bp after gel electrophoresis.
Statistical analysis
We calculated the prevalence in all age and sex categories with Sterne methods (Sterne, Reference Sterne1954) and compared them with the Fisher test. To test for differences in the average wing feather length and body mass of infected and non-infected individuals we used two-way analysis of variance with multiple comparison tests. We compared the means of wing feather length and body mass of infected and non-infecting individuals in each sex and age group combination.
Due to the small sample size in the infected adult group (N infected females = 7, N infected males = 3), we discarded all adult birds from the latter models. We assessed the relationship between infection status and fat scores using a generalized linear model (GLM) with binomial distribution and logit link function with multiple comparison tests (Venables and Ripley, Reference Venables, Ripley, Venables and Ripley2002). As sex did not correlate with fat scores, in this model infection status was the dependent variable while only fat score was included as an independent variable. We used a GLM also to study the changes in an infected/non-infected ratio during autumn migration in relation to arrival time and sex. The model was fitted with binomial error distribution and logit function, where infection status was the response variable and day of the year, sex and their interaction were the independent variables. To study the possible differences in the average arrival time of the individuals we used general linear models. Independent variables were sex and infection status and their interaction. Prevalence was calculated and compared between and within age and between sex categories with Quantitative Parasitology 3.0 (Reiczigel and Rózsa, Reference Reiczigel and Rózsa2005). We selected the best model in all case using Akaike's information criterion accomplished by the ‘stepAIC’ function from the package MASS (Ripley et al., Reference Ripley, Hornik, Gebhardt and Firth2012). All other analyses were performed using R version 3.4.2 (R Development Core Team, 2017). For GLM we used the ‘stats’ package, for multiple comparisons we used the ‘multcomp’ package, which contains P value correction too (Hothorn et al., Reference Hothorn, Bretz and Westfall2008).
Results
The overall prevalence of avian malaria parasites was 14.9% [confidence interval (CI) 0.1163–0.1871]. The prevalence did not differ significantly between age [odds ratio (OR) = 0.81, P value = 0.552] or sex categories (OR = 1.50, P value = 0.164). The prevalence in the adult age group was 17.2% [N infected = 10 (three males and seven females), CI: 0.0919–0.2918] and 14.4% in the juvenile age group [N infected = 50 (22 males and 28 females), CI: 0.1104–0.1850].
We found no significant difference in mean wing feather length and body mass between parasitized and non-parasitized individuals (Table 1). There was no significant relationship between infection status and fat scores (χ 2 = 2.7304, P value = 0.4351) either. Multiple comparisons between fat scores show that the odds to have a higher fat category did not differ between infected and non-infected group (Table 2).
Table 1. Differences in the mean wing feather length and body mass between the sexes and age groups

The estimates and standard errors were calculated from multiple comparisons. The estimates mean the calculated differences between non-infected and infected individuals.
Table 2. Odds ratios expressing (OR with 95% CI) how much more probable to have a certain fat score for infected birds compared to non-infected birds in the previous fat category

OFS0 means the odds of having fat score 0, OFS1 means the odds of having fat score 1, etc.
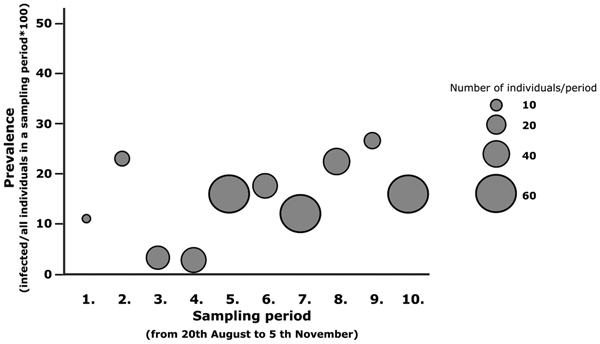
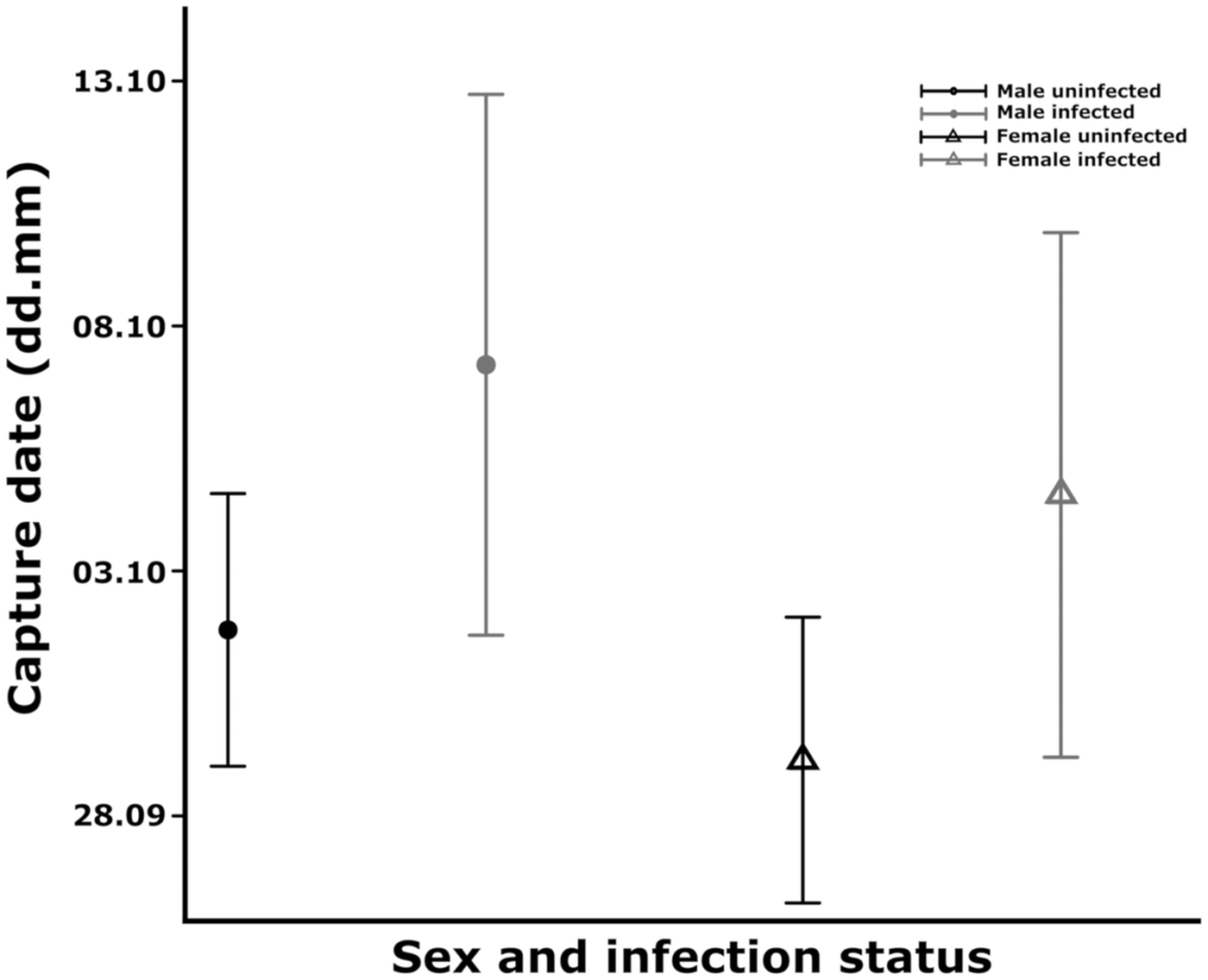
We found a marginal increase in the odds of catching infected individuals (ORper day = 1.016, s.e. = 0.009, P value = 0.066) (Fig. 1) in relation to arrival date but the sex (ORfemale/male = 1.454, s.e. = 0.310, P value = 0.227) and arrival time×sex interaction (OR = 1.000, s.e. = 0.018, P value = 0.986) were not significant. The mean arrival time of infected and non-infected juveniles differed marginally so that infected juveniles arrived on average 5 days later compared to uninfected ones (estimateinfected ± s.e.: 5.411 ± 2.796, P value: 0.054) (Fig. 2). Timing of the two sexes did not differ significantly (estimatefemale ± s.e.: − 2.657 ± 1.969, P value: 0.178), the non-significant interaction between sex and infection status (estimate ± s.e.: 2.139 ± 5.624, P value: 0.704) was dropped out from the model.

Fig. 1. Changes in prevalence during the sampling periods. Sampling was conducted periodically, four sampling days were followed by a three-day-break period. Prevalence means infected individuals/all individuals×100 in total sample per sampling period. The area of the circle relates to the sample size per periods.
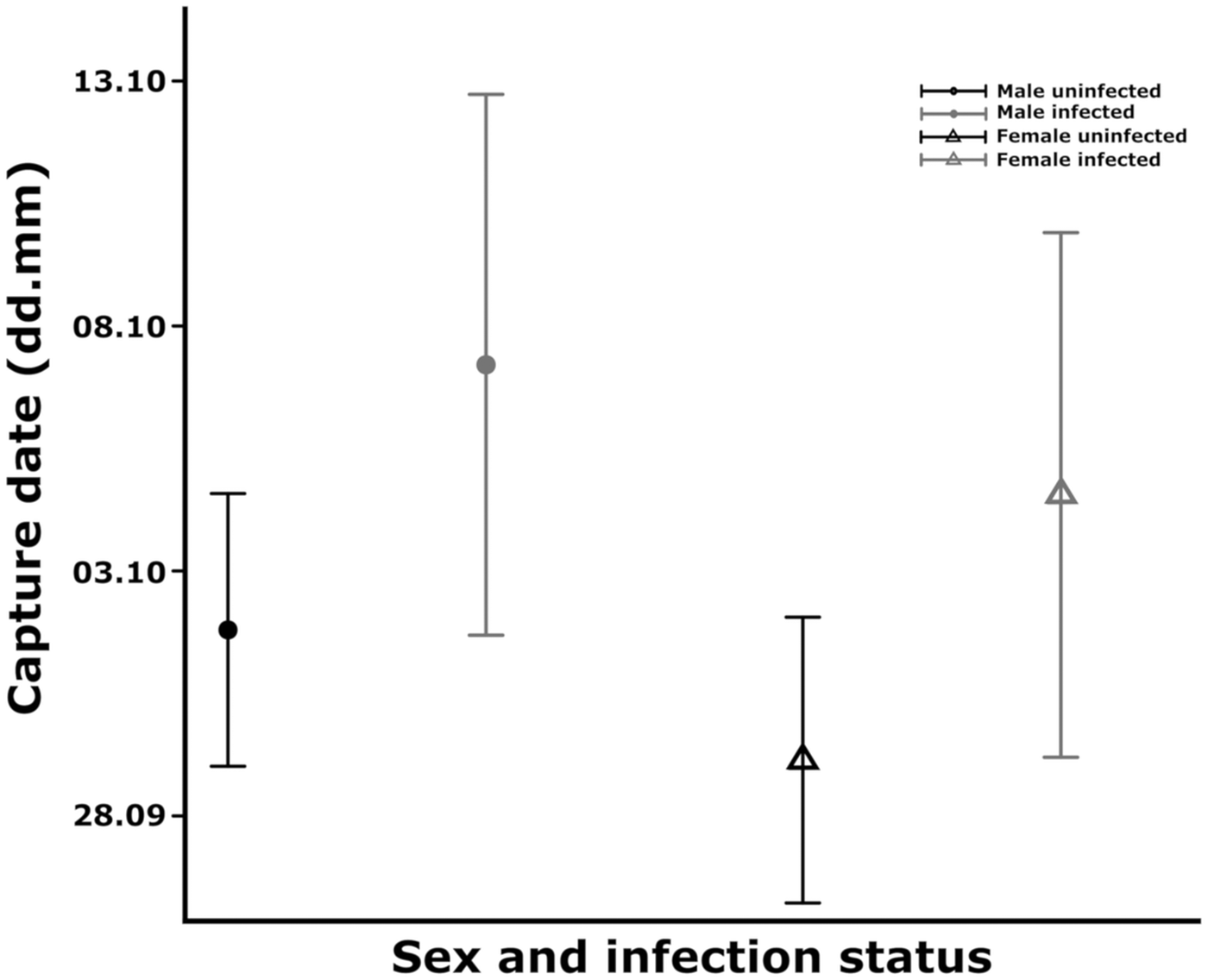
Fig. 2. Comparison of the mean arrival dates between uninfected (black colour) and infected (grey colour) male (circle) and female (triangle) juvenile Robins. The mean values were estimated from the linear model; the bars represent the 95% CI.
Discussion
Based on screening a large number of European Robins, we showed that there were no age or sex related differences in the prevalence of avian malaria parasites during autumn migration. Infected individuals did not differ either in their biometrical traits or in their fat accumulation scores, however, infected juveniles tended to arrive later at the stopover site.
The overall prevalence of blood parasites was 14.9% in our sample, which is higher than the prevalence that was previously detected in a wintering population of the species on the Iberian-Peninsula (6.7%; Rivera et al., Reference Rivera, Barba, Mestre, Schaefer and Segelbacher2013), and the prevalence detected right after the breeding period of the birds in Germany (8.19%; Santiago-Alarcon et al., Reference Santiago-Alarcon, MacGregor-Fors, Kühnert, Segelbacher and Schaefer2016). The prevalence in our study was similar to that was found previously during the spring migration period of the species in Finland (18.5%; Rintamäki et al., Reference Rintamäki, Halonen, Kilpimaa and Lundberg1997). We have to note, however, that the Scandinavian populations use different migratory routes (Bairlein et al., Reference Bairlein, Dierschke, Salewski, Geiter, Hüppop, Köppen, Fiedler, Bairlein, Dierschke, Salewski, Geiter, Hüppop, Köppen and Fiedler2014; Valkama et al., Reference Valkama, Saurola, Lehikoinen, Piha, Sola, Velmala, Valkama, Saurola, Lehikoinen, Piha, Sola and Velmala2014; Harnos et al., Reference Harnos, Ágh, Fehérvári, Karcza, Ócsai and Csörgő2018) than the populations cross the Carpathian basin (i.e. populations from Eastern-Central Europe and from the Baltic coast), therefore birds from the Finnish population were certainly not sampled in this study.
Previous studies showed that the prevalence of avian malaria parasites is the highest during the breeding season (e.g. Schrader et al., Reference Schrader, Walters, James and Greiner2003; Valkiūnas, Reference Valkiūnas2005). On the other hand Cosgrove et al. (Reference Cosgrove, Wood, Day and Sheldon2008) found that prevalence shows two peaks; one in the breeding season and one during early autumn in a European resident species, the Blue Tit (Cyanistes caeruleus). Another study also showed two prevalence peaks in Yellow Wagtails (Motacilla flava) during spring and autumn migration, however, during spring, the prevalence was two times higher (Shurulinkov et al., Reference Shurulinkov, Chakarov and Daskalova2012).
The peak in prevalence during spring is probably partly caused by the fact that the immune system of the birds is suppressed during courtship and chick rearing due to the increasing level of stress and sexual hormones and because of the high workload of the parents (Hasselquist, Reference Hasselquist2007). In addition, the parasites synchronize their presence in the blood with the availability of vectors and host individuals in order to maximize their transmission success (Valkiūnas, Reference Valkiūnas2005; Cornet et al., Reference Cornet, Nicot, Rivero and Gandon2014). The second peak in prevalence during early autumn might be because after the nestling stage, newly infected juveniles enter into the populations (Cosgrove et al., Reference Cosgrove, Wood, Day and Sheldon2008). After the end of autumn, prevalence detected from the blood decreases (Deviche et al., Reference Deviche, Greiner and Manteca2001; Garvin et al., Reference Garvin, Szell and Moore2006; Santiago-Alarcon et al., Reference Santiago-Alarcon, Bloch, Rolshausen, Schaefer and Segelbacher2011) because from late autumn malaria parasites persist in internal organs (Deviche et al., Reference Deviche, Greiner and Manteca2001; Jarvi et al., Reference Jarvi, Atkinson and Fleischer2001; Valkiūnas, Reference Valkiūnas2005). Though we have no information about how malaria prevalence varies in our birds in other seasons, based on the above argument, the patterns detected during autumn migration suggest approximately the same or even higher prevalence pattern for spring migration.
Different malaria prevalence in males and females was previously detected in another bird species during the breeding season. Some studies found female-biased (e.g. McCurdy et al., Reference McCurdy, Shutler, Mulie and Forbes1988), while others found male-biased (e.g. Wood et al., Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007) or approximately equal (e.g. Dubiec et al., Reference Dubiec, Podmokła, Zagalska-Neubauer, Drobniak, Arct, Gustafsson and Cichoń2016; Peev et al., Reference Peev, Zehtindjiev, Ilieva, Träff, Briedis and Adamík2016) prevalence. The sex related differences in blood parasite prevalence during breeding might result from the fact that males and females of various species follow different strategies during spring migration and/or during breeding. In general, males of migratory bird species are in a hurry to arrive at the breeding site as early as possible to occupy the best territories (Coppack and Pulido, Reference Coppack and Pulido2009), they often get injured during male–male competition (McCurdy et al., Reference McCurdy, Shutler, Mulie and Forbes1998) and the level of the immunosuppressive male sexual hormone also increases (Hasselquist, Reference Hasselquist2007). All of these factors may limit the energy available for parasite defence. Males are also more likely to be exposed to the vectors of avian malaria when singing in the canopy (e.g. Deviche et al., Reference Deviche, Greiner and Manteca2001). On the other hand, females are less protected against the bites of the vectors in species where incubation of the brood is the exclusive task of the mothers, especially in open-cup nester species (e.g. Garvin and Remsen, Reference Garvin and Remsen1997). In addition, in species where mothers proportionally invest more into chick rearing than fathers, the resulting worse body condition and worse immune defence may result in an increased probability of infections in females. Interestingly, during autumn migration or overwintering, the differences in the blood parasite prevalence between the sexes are less studied. The few studies that have investigated this question found no sex related infection patterns but the lack of the relationship is not examined in details (Neto et al., Reference Neto, Pérez-Rodríguez, Haase, Flade and Bensch2015; Sorensen et al., Reference Sorensen, Asghar, Bensch, Fairhurst, Jenni-Eiermann and Spottiswoode2016, but see Deviche et al., Reference Deviche, Greiner and Manteca2001). In line with these studies, we did not find sex differences in blood parasite prevalence in Robins crossing our Hungarian stopover site during autumn migration.
In other passerines, older individuals were frequently found to be more infected than 1-year-old ones (e.g. Wojczulanis-Jakubas et al., Reference Wojczulanis-Jakubas, Jakubas, Czujkowska, Kulaszewicz and Kruszewicz2012; Dubiec et al., Reference Dubiec, Podmokła, Zagalska-Neubauer, Drobniak, Arct, Gustafsson and Cichoń2016; Szöllősi et al., Reference Szöllősi, Garamszegi, Hegyi, Laczi, Rosivall and Török2016; but see Merilä et al., Reference Merilä, Björklund and Bennett1995; Peev et al., Reference Peev, Zehtindjiev, Ilieva, Träff, Briedis and Adamík2016). This is because vectors had more time and opportunities to infect those individuals that have already lived longer (Valkiūnas, Reference Valkiūnas2005; Knowles et al., Reference Knowles, Wood, Alves, Bensch and Sheldon2011). Contrary to the expectations, in Robins, the prevalence of avian malaria parasites during autumn migration did not differ between the two age groups, though the sample size was small in the adult group. We have to note, however, that we compared the prevalence patterns of juveniles (i.e. hatched in the year of capture) and older individuals and not that of 1-year-old and adult individuals. By definition, juvenile birds certainly got infected in their first year (i.e. when they were still in the nest or after fledging) while most adult birds probably got infected earlier, thus parasitaemia in juveniles and adults might show different dynamics. According to this scenario, the majority of the juveniles probably still carried infections with relatively high parasitaemia, on the other hand, the parasites had already started to disappear from the blood of adult birds and therefore a part of the infections went undetected in adults at the time of blood sampling. If this is the case, then juveniles and adults might show similar parasite prevalence in their blood during autumn migration. It is also possible that the first migration is energetically more demanding for juveniles than for adults [e.g. because juveniles are less successful in foraging (Heise and Moore, Reference Heise and Moore2003)] and thus the level of parasitaemia remained high or increased over the detection limit in more juvenile individuals than adult birds. If the first migration is indeed more stressful for juveniles, then proportionally more infected juveniles (i.e. those in worse general condition) will perish during their first migration and proportionally more uninfected individuals from this group will return to breed in the following year. If this stands then higher malaria prevalence in adult than 1-year-old birds is going to be detected in the following year after spring migration.
We found no significant differences in body size, body mass and fat accumulation in relation to infection status, which would suggest little effects of malaria parasites on the condition of the individuals during autumn migration. Nevertheless, it is important to note that experimental infections with Haemosporidian parasites in naïve host individuals showed negative effects of parasite infections on body condition only in the acute stage. Therefore, the negative effects of parasites on body condition might have no longer been detectable among birds that are in the chronic stage of infection (Valkiūnas et al., Reference Valkiūnas, Zickus, Shapoval and Lezhova2006).
On the other hand, our result suggests that malaria infections may still impose negative effects during autumn migration, since infected juveniles tended to arrive later at our Hungarian stopover site. This is in line with previous studies conducted during the spring migration period of the birds. These studies found that individuals infected with blood parasites (Møller et al., Reference Møller, de Lope and Saino2004; Emmenegger et al., Reference Emmenegger, Bauer, Hahn, Müller, Spina and Jenni2018) or individuals more infected with blood parasites arrived later at their breeding grounds (DeGroote and Rodewald, Reference DeGroote and Rodewald2010). A recent study conducted during autumn migration arrived at similar conclusion. In this study, blood parasite infected individuals of different short-distance migrant species had a longer stopover duration than non-infected individuals (Hegemann et al., Reference Hegemann, Abril, Muheim, Sjöberg, Alerstam, Nilsson and Hasselquist2018b). These findings suggest that parasite infections may indeed impose a negative effect on the birds and affect the timing and length of migration in different bird species.
It was previously shown in many species, that blood parasite prevalence differs in populations sampled at different geographic regions (e.g. Bensch and Åkesson, Reference Bensch and Åkesson2003; Pagenkopp et al., Reference Pagenkopp, Klicka, Durrant, Garvin and Fleischer2008; Marzal et al., Reference Marzal, Ricklefs, Valkiūnas, Albayrak, Arriero, Bonneaud, Czirják, Ewen, Hellgren, Hořáková, Iezhova, Jensen, Križanauskienė, Lima, de Lope, Magnussen, Martin, Møller, Palinauskas, Pap, Pérez-Tris, Sehgal, Soler, Szöllősi, Westerdahl, Zetindjiev and Bensch2011; Szöllősi et al., Reference Szöllősi, Cichoń, Eens, Hasselquist, Kempenaers, Merino, Nilsson, Rosivall, Rytkönen, Török, Wood and Garamszegi2011). Therefore, the differences detected in the arrival time of infected and uninfected juveniles could have also been attributed to differences in the blood parasite prevalence patterns in the breeding sites from where host populations depart for migration. Ringing recovery data showed that by the middle of September the Hungarian breeding populations leave the Carpathian basin and the proportion of birds arriving from the northern regions is gradually increasing; containing more and more birds from the northern populations as the time elapsed (Gyurácz and Csörgő, Reference Gyurácz, Csörgő, Csörgő, Karcza, Halmos, Magyar, Gyurácz, Szép, Bankovics, Schmidt and Schmidt2009).
Most previous studies showed no clear relationship between latitude and parasite prevalence (e.g. Merilä et al., Reference Merilä, Björklund and Bennett1995; Merino et al., Reference Merino, Moreno, Vásquez, Martínez, Sánchez- Monsálvez, Estades, Ippi, Sabat, Rozzi and MCGehee2008; Svoboda et al., Reference Svoboda, Marthinsen, Pavel, Chutný, Turčoková, Lifjeld and Johnsen2015). However, Nunn et al. (Reference Nunn, Altizer, Sechrest and Cunningham2005) previously showed that both the abundance and/or the diversity of vector species usually decrease towards higher latitudes, which would cause rather lower than higher prevalence of blood parasites in the northern regions. In addition, the generally lower temperature in the northern regions results in a more fluctuating vector population and a suboptimal temperature for the development of parasites (Valkiūnas, Reference Valkiūnas2005). Therefore it is not surprising that another study screening a fairly large sample size of House Sparrows (Passer domesticus) found that both prevalence and lineage diversity decreased towards the north (Marzal et al., Reference Marzal, Ricklefs, Valkiūnas, Albayrak, Arriero, Bonneaud, Czirják, Ewen, Hellgren, Hořáková, Iezhova, Jensen, Križanauskienė, Lima, de Lope, Magnussen, Martin, Møller, Palinauskas, Pap, Pérez-Tris, Sehgal, Soler, Szöllősi, Westerdahl, Zetindjiev and Bensch2011). However, we have to underline that in general prevalence is probably more affected by local factors than by latitudinal patterns. This is because small scale geographic and climatic differences may occur within a habitat and may affect the composition and distribution of vector fauna and as a result prevalence of vector-borne parasites (Pérez-Tris and Bensch, Reference Pérez–Tris and Bensch2005; Wood et al., Reference Wood, Cosgrove, Wilkin, Knowles, Day and Sheldon2007; Ferrer et al., Reference Ferrer, García-Navas, Sanz and Ortego2012; van Rooyen et al., Reference van Rooyen, Lalubin, Glaizot and Christe2013; Ferraguti et al., Reference Ferraguti, Martínez-de la Puente, Bensch, Roiz, Ruiz, Viana, Soriguer and Figuerola2018). This means that in our case probably not latitude per se might be responsible for the fact that proportionally more infected individuals were detected with the progress of migration. We rather suggest that birds arriving at the same time might come from farther locations with the same parasite prevalence.
To summarize, we showed that during autumn migration arrival time of juvenile Robins tended to correlate with their infection status independent of their actual condition. Infected individuals arrived later at the stopover site in Hungary. This is either because later arriving individuals came from populations with similarly higher prevalence of avian malaria; or because of the adverse effects of parasites which manifest in a delay in the arrival time of infected individuals. However, to distinguish between these two scenarios a Europe-wide analysis of the genetic structure of Robin populations would be needed both in the breeding season and during migration. To improve our understanding of the impacts of these parasites on the migratory phenology of their hosts, further studies are clearly needed. More inter- and intra-populational comparative studies, especially the simultaneous sampling of the host populations along their migratory routes, on their breeding and wintering sites would be needed to understand the annual dynamics and impacts of infection.
Acknowledgements
The authors express their gratitude for the work of the volunteers at the Ócsa Bird Ringing Station. We thank Ármin Csipak and Krisztián Barna for their voluntary contribution in the sampling on the field. We are grateful for the members of Conservation Genetics Research Group of the University of Veterinary Medicine who helped in the laboratory work and Alexandra Juhász, Lajos Rózsa and Károly Erdélyi for helpful comments in the study and methods development.
Author ORCIDs
Nóra Ágh, 0000-0001-9184-912X.
Financial support
This work was supported by the Hungarian Ministry of Human Capacities (National Talent Program, grant numbers NTP-NFTÖ–16-0493) to N.Á., the National Scientific Research Fund of Hungary (OTKA under Grant No. 108571), the National Research, Development and Innovation Office (grant no. PD124043, FK127917) and the János Bolyai research scholarship from the Hungarian Academy of Sciences (bo_50_17) to E.S.
Conflict of interest
None.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Research was permitted by the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management (under Registration Number KTF: 27251-1/2014). Blood sampling was performed by N.Á. (Certificate Registration Number 6/2015, issued by the Institutional Animal Welfare Committee of the University of Veterinary Medicine Budapest) and I.S.P. (Certificate Registration Number 19/2016, issued by the Institutional Animal Welfare Committee of the University of Veterinary Medicine Budapest).