INTRODUCTION
Parasite-induced host mortality from macroparasite infections typically occurs in an intensity-dependent manner (Hudson et al. Reference Hudson, Newborn and Dobson1992; Schutgens et al. Reference Schutgens, Cook, Gilbert and Behnke2015). Pathology caused by parasitism, acting in concert with aggregation of parasites (Crofton, Reference Crofton1971; Shaw and Dobson, Reference Shaw and Dobson1995), can have wide-ranging effects on host populations (Anderson and May, Reference Anderson and May1978; May and Anderson, Reference May and Anderson1978; Scott and Dobson, Reference Scott and Dobson1989; Ives and Murray, Reference Ives and Murray1997), depending on the specific mechanisms producing host mortality and on the shape of the intensity–mortality relationship (Stjernman et al. Reference Stjernman, Raberg and Nilsson2008). The effects of parasitism on host mortality can be modified by other stressors on the host, which can act independently but may interact with synergy or antagonism, i.e. with greater or lesser effect than the sum of their independent actions (Rothman, Reference Rothman1974).
Studies on invertebrate hosts show that the effects of parasitism on host mortality can be modified by diverse environmental factors, such as contaminants (Morley, Reference Morley2010; Marcogliese and Pietrock, Reference Marcogliese and Pietrock2011), predation (Coors and De Meester, Reference Coors and De Meester2008) and food shortage (Jokela et al. Reference Jokela, Lively, Taskinen and Peters1999; Schreurs and Janovy, Reference Schreurs and Janovy2008). Synergy between parasitism and environmental stressors is the most common interaction reported (reviewed by Marcogliese and Pietrock (Reference Marcogliese and Pietrock2011)), but antagonisms are known (Heinonen et al. Reference Heinonen, Kukkonen and Holopainen2001; Sanchez et al. Reference Sanchez, Pons, Martinez-Haro, Taggart, Lenormand and Green2016). Yet under natural conditions, simultaneous exposure of a host to severe macroparasite infection and to one or more severe environmental (i.e. non-parasitic) stressors should be uncommon. Few hosts are expected to harbour high-intensity macroparasite infections due to aggregation (Shaw and Dobson, Reference Shaw and Dobson1995), and the likelihood of exposure to high levels or duration of environmental stressors is reduced by their spatial and temporal variation (Mai et al. Reference Mai, Theobald, Lammel and Huehnerfuss2013; Li et al. Reference Li, Niu, Shen, Zhang, Wang and He2014). However, long-lived hosts should have a greater chance of encountering extreme combinations of stressors at some point (Jones et al. Reference Jones, Bull, Brook, Wells, Pollock and Fordham2016). Even if exposed only to the more common, low–moderate levels of those stressors, long-lived hosts should be more likely to experience their chronic effects.
Studies where mortality is an endpoint typically use invertebrate hosts, which are advantageous from both logistical and ethical perspectives (Woolsey et al. Reference Woolsey, Fredensborg, Jensen, Kapel and Meyling2015). Studies of interactions between parasitism and other stressors on the mortality of invertebrate hosts have taken two primary approaches: point estimates of host survival at some arbitrary time (Wedekind, Reference Wedekind1997; Bates et al. Reference Bates, Poulin and Lamare2010), or longitudinal study that monitors survival of a cohort over time (Hurd et al. Reference Hurd, Warr and Polwart2001; Vezilier et al. Reference Vezilier, Nicot, Gandon and Rivero2012; Schutgens et al. Reference Schutgens, Cook, Gilbert and Behnke2015). A longitudinal study is challenging when normal host lifespan is lengthy but has advantages, such as an ability to compare shapes of survival curves. A highly tractable invertebrate laboratory model system for the study of macroparasite infections is the cestode Hymenolepis diminuta in tenebrionid beetles, such as Tribolium confusum, Tribolium castaneum and Tenebrio molitor. In this system, host mortality has been studied at various scales, from physiological, ecological and behavioural (reviewed by Hurd, Reference Hurd1990, Reference Hurd2009; Shostak, Reference Shostak2014a ), to evolutionary (Stevens et al. Reference Stevens, Yan and Pray1997; Zhong et al. Reference Zhong, Pai and Yan2005). Beetles are the intermediate host and ingest the parasite egg, which hatches in the gut to release an oncosphere that penetrates into the haemocoel and develops in about 2 weeks to a cysticercoid stage that can infect a rat definitive host. Under laboratory conditions, T. confusum can be long-lived, >2 years (Schutgens et al. Reference Schutgens, Cook, Gilbert and Behnke2015). The lifespan of H. diminuta in T. confusum is unknown but is at least 12 weeks (Keymer, Reference Keymer1980; Maema, Reference Maema1986).
Parasite-induced mortality due solely to H. diminuta infection has been reported anecdotally for many species of tenebrionid beetles (reviewed by Shostak, Reference Shostak2014a ). In the absence of other stressors, Tribolium spp. are resistant to food shortage and can survive starvation for several weeks (Schneider, Reference Schneider1941; Ducoff et al. Reference Ducoff, Vaughan and Crossland1970). Tribolium confusum can also experience mortality from the pesticide diatomaceous earth (DE), which damages the cuticle and desiccates insects when applied as a dust on stored grains (Korunic, Reference Korunic1998). DE can act acutely, killing beetles within days when exposed to DE dust (Korunic, Reference Korunic1998) or ⩾16% DE mixed with flour (Shostak, Reference Shostak2012, Reference Shostak2014b ). DE can also act chronically at lower concentrations. At 1% DE, T. confusum can live >40 days (Shostak, Reference Shostak2014b ), exhibit reduced egg production (Shostak, Reference Shostak2014b ) and increased surface-seeking behaviour (Shostak et al. Reference Shostak, Van Buuren and Cook2015). Although modest levels of parasitism, food shortage and DE alone have little or no effect on beetle mortality, there is increased short-term mortality when present in combination. The mortality effect of starvation in beetles is enhanced when hosts are infected, particularly with larger numbers of parasites (Keymer, Reference Keymer1980; Robb and Reid, Reference Robb and Reid1996). Exposure to low concentrations of DE prior to infection with H. diminuta, or during parasite development, does not produce parasite-induced mortality (Shostak, Reference Shostak2012), but mortality of beetles increases when beetles with high-intensity H. diminuta infections are exposed to high-concentration DE (Shostak et al. Reference Shostak, Van Buuren and Cook2015). Given the potential lifespan of this host and parasite, the T. confusum–H. diminuta–DE system is a good experimental model to test and compare both acute and chronic mortality effects of multiple stressors on a long-lived host.
In the present study, we conducted several longitudinal experiments to explore the effects of parasitism and environmental stressors on mortality of T. confusum over the lifespan of the host, and particularly on the influence of environmental stressors on the ability to detect intensity-dependent parasite-induced host mortality. As environmental stressors, we used host starvation and acutely or chronically lethal levels of DE, alone and in various combinations. Given the unknown fate of H. diminuta beyond 12 weeks of age, we also evaluated the longevity of this parasite in this host as a first step in assessing potential temporal changes in parasite-induced harm.
MATERIALS AND METHODS
General procedures
Details of sources and maintenance of beetles (T. confusum) and parasites (H. diminuta), and methods for recovery of parasite eggs and infection of adult beetles (exposure to oatmeal flakes containing an aqueous suspension of eggs), are described in detail in Shostak (Reference Shostak2009), and beetle necropsy procedures in Chin et al. (Reference Chin, Luong and Shostak2017). All procedures complied with the ethical standards set by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and the Canadian Council on Animal Care (Ottawa, Canada) on the care and use of laboratory animals, using the minimum number of animals judged necessary to achieve statistically significant results, and were conducted under University of Alberta Animal Use Protocol AUP00000078. Briefly, fresh beetles were killed by crushing the head, then dissected in saline, whereas beetles found dead during experiments were stored in 1·5 mL micro-centrifuge tubes, rehydrated in 5% KOH for 24 h, then necropsied as for fresh beetles. We could not reliably sex all rehydrated beetles, so we did not use host sex data. Our beetle colony typically has an even sex ratio for younger beetles (Shostak, Reference Shostak2008), and sex differences in survival are minimal (Pearl et al. Reference Pearl, Park and Miner1941; Shostak et al. Reference Shostak, Van Buuren and Cook2015). We used random selection of uninfected beetles and assignment of treatments to control for any sex effects. We conducted three experiments between November 2014 and May 2016 to assess survival under different combinations of various stressors. Sample sizes, beetle age and exposure conditions varied according to quantities of beetles and parasite eggs available at the time of each experiment, but were standardized within each experiment. All beetle infection and handling was done under ambient room conditions, but otherwise beetles were stored in an incubator (dark, 28 °C, 10–25% relative humidity). Randomization procedures used a random number generator (Excel 2010 spreadsheet software, Microsoft Co., Redmond, Washington, USA).
We chose to introduce the environmental stressors after parasites had completed development into cysticercoids, to reduce confounding by short-term carryover effects from the infection procedure (Shostak, Reference Shostak2009, Reference Shostak2012), potential dose-dependent (Shostak et al. Reference Shostak, Van Buuren and Cook2015) mortality from initial penetration of the beetle gut by oncospheres (Keymer, Reference Keymer1980) or the variable demands on the host during the brief period of parasite development (Shostak et al. Reference Shostak, Walsh and Wong2008). Moreover, the 2-week parasite developmental period represents a small portion of the host's expected lifespan and, in practice, is associated with little or no beetle mortality in our system (Shostak, Reference Shostak2008; Shostak et al. Reference Shostak, Walsh and Wong2008, Reference Shostak, Van Buuren and Cook2015).
Parasitism and starvation (experiment 1)
This experiment was intended to test for effects of starvation on survival across a range of infection intensities. The general design was based on Keymer (Reference Keymer1980), except that we incorporated the determination of infection intensity of individual hosts at the time of death.
An initial group of ~1100 mixed-sex beetles, 2–3 months old, was treated with four rounds of fasting followed by infection, then feeding on flour–yeast medium (Shostak, Reference Shostak2009). Fasting was for 7 days prior to the first infection, then for 3 days prior to the next three infections, at the exposure rate 500 eggs/beetle. Beetles were fed for 2 days following the first three infections. After the last round of infections, hosts were haphazardly divided into five groups, and each group was placed on 20 g medium for 2 weeks to allow all parasites to complete development. We did not include an explicit unexposed control group in this experiment because we expected, based on typical exposure success in our laboratory, that this protocol would still produce a group of uninfected beetles.
Following parasite development, the surviving hosts (n = 840, now 3·5–4·5 months old, ~85% survived four rounds of infection) were passed over a 250 µm mesh sieve to remove medium, then allocated at random to one of five arenas (100 mm diameter glass dishes) and stored in the incubator without food to commence starvation treatment. Arenas were checked for dead hosts twice daily (06:00–09:00 h; 15:00–20:00 h). Carcasses were removed and stored in micro-centrifuge tubes until necropsy. Arenas were wiped with 70% ethanol, surviving hosts were returned to the arenas, and the arenas were returned to the incubator. The experiment continued until all hosts died.
Parasitism and chronic pesticide exposure (experiment 2)
This experiment examined the interaction between infection and exposure to a chronic, low-level pesticide on beetle survival over their entire lifespan. To simulate chronic pesticide exposure, we used DE (Insect Stop©, Aerokure International Inc., Courcelles, Quebec, Canada; 91·1% SiO2) mixed with medium.
An initial group of ~400 mixed-sex beetles (⩽1 month old) was fasted for 7 days. Survivors were randomly assigned to parasite exposure (2500 eggs/beetle) or sham exposure (water and oatmeal flakes) treatments. Parasite-exposed and sham-exposed hosts were stored separately in 20 g medium for 2 weeks to allow parasites to develop.
Following parasite development, the surviving parasite-exposed hosts (~85% survival) were assigned at random to either immediate necropsy to determine baseline parasite numbers (n = 37) or to treatment groups of 10 hosts per 16 mL vial containing 2 g medium with 0, 1 or 4% DE prepared as in Shostak (Reference Shostak2012). DE concentrations ⩽4% are not expected to cause significant mortality for at least 1 month (Shostak, Reference Shostak2012, Reference Shostak2014b ). Sham-exposed hosts (~98% survival) were prepared similarly, but kept separate. We randomly allocated five vials as replicates to each combination of parasite exposure and DE concentration. Hosts were censused weekly until all hosts died. For each census, the medium in each vial was passed through a 250 µm mesh sieve, carcasses of dead hosts were removed and stored in micro-centrifuge tubes, eggs or larvae were discarded, and surviving adults were returned to the same vial with 2 g fresh medium.
Parasitism, acute pesticide exposure and starvation (experiment 3)
This experiment was intended to test the combined effects of a range of infection abundances and the presence of multiple severe stressors on beetle survival. We used combinations of food availability and DE exposure intended to be rapidly lethal.
An initial group of ~2500 mixed-sex beetles (⩽7 months old) was fasted for 7 days. We allocated them at random into groups of ~100, and assigned each group to one of the following parasite exposure rates: low (40 eggs/beetle), medium (150 eggs/beetle), high (600 eggs/beetle) or sham exposure (water and oatmeal flakes).
Our experimental design addressed the potentially confounding effect of presenting hosts both DE and a food source. The presence of food may modify the exposure of hosts to DE or modify the habitat of the beetle in a way that might alter the desiccating action of DE. We tested six treatments using arenas (80 mm diameter glass dish) in a 3 × 2 factorial design with food at three depths, and DE treatment that had a comparable absolute amount or concentration of DE across food depths. Treatment A had 20 g food + 0 g DE (‘deep food’, no DE). Treatment B had 16·8 g food + 3·2 g DE (‘deep food’, 16% DE). Treatment C had 0·105 g food + 0 g DE (‘shallow food’, no DE). Treatment D had 0·105 g food + 0·02 g DE (‘shallow food’, 16% DE). Treatment E had 0 g food + 0 g DE (‘no food’, no DE). Treatment F had 0 g food + 0·02 g DE (‘no food’, 100% DE). ‘Deep food’ (~15 mm deep) treatments allow beetles to burrow, whereas the ‘shallow food’ (<1 mm deep) treatments provide food but no ability to burrow. The 100% DE level (DE dust alone) used as treatment F represents the manufacturer's recommended surface application rate to kill within days (Korunic, Reference Korunic1998). We added a single sheet of filter paper (70 mm diameter, no. 1 qualitative, Whatman International Ltd., Maidstone, England) to treatments C–F prior to the addition of any food or DE to provide traction for beetles in the glass arena.
Following parasite exposure, and 2 weeks storage for parasites to develop (each sham or parasite exposure group was stored separately in 50 g medium), 94–96% survived in all groups and we prepared 18 arenas by adding, to each, 30 randomly selected sham-exposed hosts, 20 hosts from low, 30 hosts from medium and 30 hosts from high exposure rates. We then randomly assigned three arenas as replicates for each of treatments A–F. Remaining beetles (n = 99) were necropsied immediately to determine baseline parasite numbers prior to treatment. Arenas were stored in the incubator but removed daily for census. The contents of each arena were passed through a 250 µm mesh sieve, carcasses of dead hosts removed and stored in micro-centrifuge tubes, the dishes cleaned with 70% ethanol, fresh medium and/or DE reapplied, and surviving adults returned. Any hosts surviving to 6 weeks were killed by chloroform (Chin et al. Reference Chin, Luong and Shostak2017) and stored in micro-centrifuge tubes for later necropsy.
Parasite persistence (experiment 4)
This experiment was intended to identify changes in morphology and viability of parasites present in the host long term. We started with a haphazard group of mixed-age, mixed-sex beetles pooled from miscellaneous parasite exposures over a 13-week period and stored together in medium in the incubator. Because of the limited number of beetles available in this pool, we used all beetles and all undamaged cysticercoids recovered from them in this experiment. We randomly selected a subset of beetles (n = 37) for initial morphological study of their cysticercoids, which were 0–13 weeks old, and stored the remainder for an additional 16 weeks. At that time, we randomly selected some of the beetles for morphological study or a viability assay of their cysticercoids (now 16–29 weeks old), and stored the remainder for an additional 14 weeks. Then we randomly allocated the remaining beetles to morphological study or a viability assay of their cysticercoids (now 30–43 weeks old). Thus, the age range of cysticercoids within a group varied, but there was no overlap among groups. We did not have available any uninfected, age-matched beetles, so we infected young (<4 weeks old) beetles 2 weeks prior to each viability assay to produce newly differentiated cysticercoids as positive controls for the assay procedure. The excystment properties of cysticercoids with respect to beetle age are unknown, but we examined photographs of 2-week-old cysticercoids (n = 361 cysticercoids from n = 170 beetles infected 1–105 weeks post-eclosion) that were taken during a previous study (Shostak, Reference Shostak2008) and found them to be morphologically indistinguishable with respect to host age.
Morphological examination was done on cysticercoids dissected in insect saline from freshly killed beetles and photographed as in Chin et al. (Reference Chin, Luong and Shostak2017). We used beetles from the baseline group in experiment 3 to represent 0-week-old cysticercoids (i.e. 2 weeks post-infection (PI), having just completed differentiation into cysticercoids), and from experiment 4 to represent older categories. We categorized cysticercoids in the photographs for presence/absence of a cercomer, capsule damage (absence of the distinct outer membrane) and abnormalities in the scolex (fragmentation of granules, presence of inclusions) that might indicate degeneration.
We assayed parasite viability using a cysticercoid activation procedure (Behnke, Reference Behnke, Halton, Behnke and Marshall2001) with modifications. All chemicals unless otherwise noted were from Fisher Scientific (Ottawa, Ontario, Canada). Briefly, each beetle was dissected in insect saline (Chin et al. Reference Chin, Luong and Shostak2017), and its cysticercoids were counted and then placed in a 1·5 mL micro-centrifuge tube with 1 mL insect saline. The saline was replaced with 1 mL acid–pepsin solution [1 g pepsin (Ward's, Rochester, New York, USA), 0·5 mL concentrated HCl, 100 mL 0·85% NaCl] for 10 min, washed 3× with Tyrode's saline, stored in 1 mL Tyrode's bile–trypsin solution [0·5 g trypsin, 0·5 g porcine bile extract (Sigma, St. Louis, Missouri, USA), 100 mL Tyrode's saline] for 20 min, and then examined for the presence of liberated scolices. All solutions were prepared within 1 h of the start of each assay, were pre-warmed to 37 °C and micro-centrifuge tubes were stored in a 37 °C water bath during all incubation steps.
Data analysis
We use the terms prevalence, abundance and intensity in accordance with Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, North Carolina, USA). Comparison of parasite recovery after treatment to baseline values used a Kruskal–Wallis test (PROC NPAIR1WAY). Survival curves were compared using the Kaplan–Meier method (PROC LIFETEST) with the Wilcoxon test, and pointwise 95% confidence limits (CLs) for survivor functions were calculated following log–log transformation. Hosts lost during handling, or which survived to the end of survival monitoring, were treated as censored observations. Exposed but uninfected hosts were pooled with sham-exposed hosts for analysis. Pairwise comparison of survival functions used a Tukey adjustment for multiple comparisons. Parasite viability was analysed using logistic regression (PROC LOGISTIC) to compare the proportion of old cysticercoids that excysted relative to the positive control for each assay.
RESULTS
Parasitism and starvation (experiment 1)
The multiple exposure protocol used in experiment 1 produced 100% prevalence in the beetles at the start of starvation treatment, and infection intensities of 2–132 parasites, with large sample sizes in all infection categories (Fig. 1A). All hosts died 3–22 days after removal of food. Survival differed among parasite abundance classes (Wilcoxon test, χ 2 4 = 84·7, P < 0·001). There were no uninfected hosts. The survival curves for hosts with 1–20 and 21–40 parasites overlapped and were indistinguishable statistically (Fig. 1A, B), and only for hosts with >40 parasites did survival decline linearly with increasing numbers of parasites (Fig. 1B). The survival of hosts with >80 parasites was reduced only by ~25% compared with hosts with 1–20 parasites. No beetles were lost or damaged during treatment.
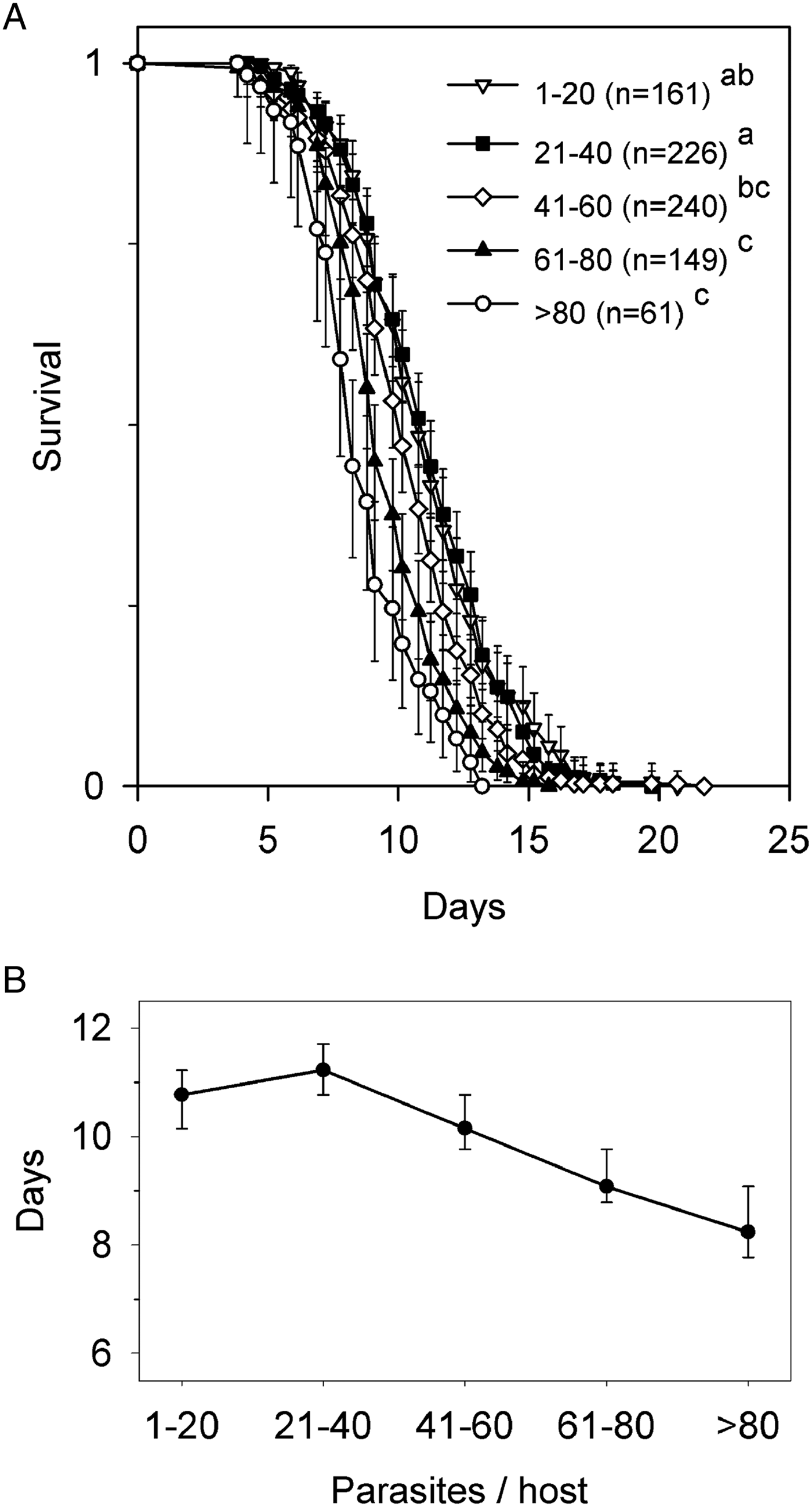
Fig. 1. Survival of Hymenolepis diminuta-infected Tribolium confusum following removal of food (experiment 1). (A) Survival curves segregated by parasite abundance. Values are proportion live ±95% confidence limits (CL). Initial number of hosts is indicated by n. Survival functions of abundance classes sharing the same lowercase letter do not differ (Kaplan–Meyer analysis, Tukey multiple comparison adjustment). (B) Median time to death ±95% CL for each abundance class.
Parasitism and chronic pesticide exposure (experiment 2)
The exposure protocol produced 98% prevalence for exposed beetles at the start of DE exposure. Most (n = 87) infected hosts had 1–20 parasites, fewer (n = 43) had 21–40 parasites and there were only sporadic infections (n = 17) with 41–76 parasites. The sham-exposed hosts in experiment 2 plus the 3 exposed non-infected hosts, gave n = 152 uninfected hosts. There was no difference in parasite abundance between baseline (mean ± s.d. = 18 ± 11·4; range = 1–46), and hosts in 0, 1 or 4% DE treatments (Kruskal–Wallis test, χ 2 3 = 0·94, P = 0·82). Only 5 (1·6%) beetles were lost or damaged during treatment.
Survival in the absence of DE (Fig. 2A) differed among parasite abundance classes (Wilcoxon test, χ 2 2 = 26·1, P < 0·001). Pairwise comparisons indicated that survival of hosts with 1–20 parasites was statistically indistinguishable from uninfected hosts, but that both groups had greater survival than hosts with >20 parasites. Survival in the presence of 1% DE (Fig. 2B) differed among parasite abundance classes (Wilcoxon test, χ 2 2 = 10·3, P = 0·006). Pairwise comparisons indicated that survival of uninfected hosts was greater than hosts with >20 parasites, but that hosts with 1–20 parasites were indistinguishable from either group. Survival in the presence of 4% DE (Fig. 2C) differed among parasite abundance classes (Wilcoxon test, χ 2 2 = 7·6, P = 0·023), but the source of this difference could not be identified statistically after adjustment for multiple comparisons.
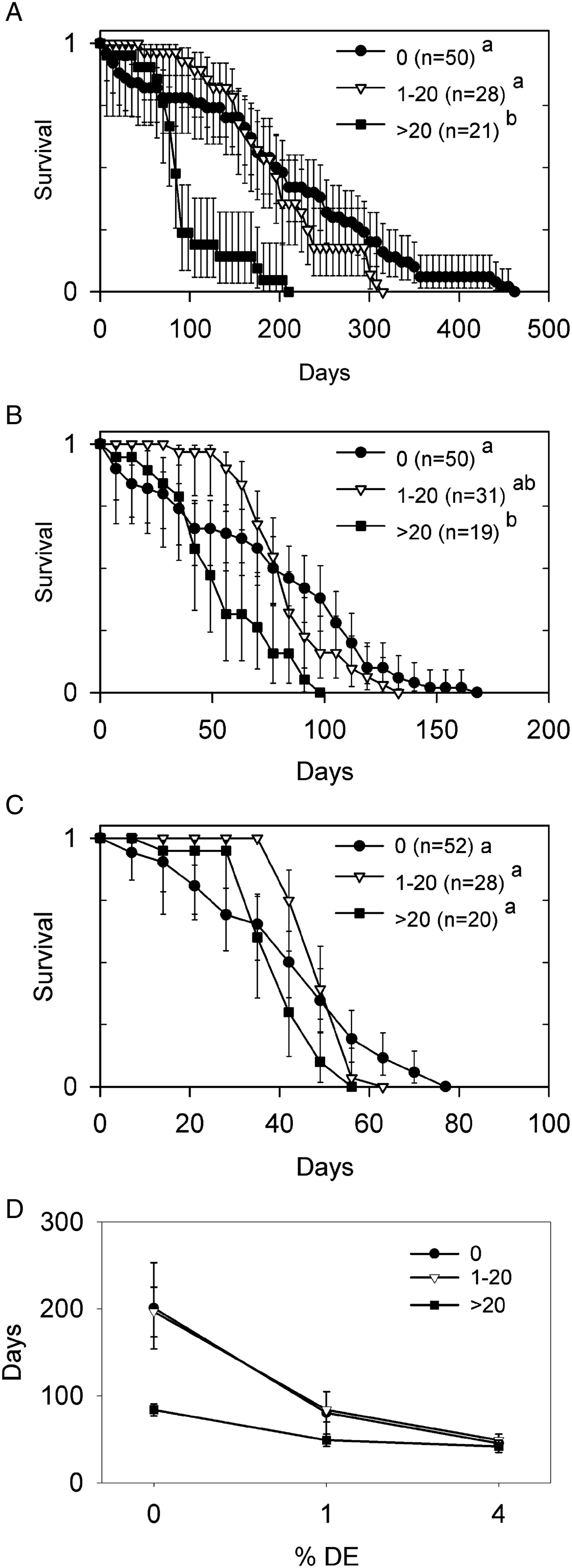
Fig. 2. Survival of Hymenolepis diminuta-infected Tribolium confusum following storage in different concentrations of diatomaceous earth (DE) (experiment 2). Survival curves are segregated by parasite abundance following storage in (A) 0%, (B) 1% or (C) 4% DE. Values are proportion live ±95% confidence limits (CL). Initial number of hosts is indicated by n. Survival functions of abundance classes sharing the same lowercase letter do not differ (Kaplan–Meyer analysis, Tukey multiple comparison adjustment). (D) Median time to death ±95% CL in each DE–parasite abundance combination.
Median survival of uninfected hosts and those with ⩽20 parasites overlapped, and both declined steeply with increasing DE (Fig. 2D). By contrast, median survival of hosts with >20 parasites was reduced by ~60% in the absence of DE, but converged with hosts having ⩽20 parasites as DE concentrations increased (Fig. 2D).
An interesting recurring pattern was noted in the shape of the survival curves. Hosts with ⩽20 parasites, and to a lesser extent those with >20 parasites, had greater survival initially than uninfected hosts, but this was followed by a steep decline in survival. This produced a crossing over of survival curves for all levels of DE, such that infected host ended up with consistently shorter maximum lifespans than uninfected beetles (Fig. 2A–C).
Parasitism, acute pesticide exposure and starvation (experiment 3)
The exposure protocol produced ~50% prevalence in parasite-exposed beetles, which along with sham-exposed hosts resulted in many (n = 1014) uninfected hosts distributed among treatments. Most (n = 864) infected hosts had 1–20 parasites, and there were only sporadic infections (n = 80) with 21–50 parasites. There was no difference in parasite abundance between baseline (mean ± s.d. = 2·8 ± 6·7; range = 0–33), and hosts in treatments A–F (Kruskal–Wallis test, χ 2 6 = 4·71, P = 0·58). Only 5 (0·3%) beetles were lost or damaged during treatment.
The presence of deep food without DE (treatment A, Fig. 3A) produced similar survival among parasite abundance classes (Wilcoxon test, χ 2 2 = 0·7, P = 0·708). Survival of all infection categories was still >90% when the experiment was terminated after 41 days. By comparison, deep food containing 16% DE (treatment B, Fig. 3B) killed all hosts within 13 days, but produced no survival differences among parasite abundance classes (Wilcoxon test, χ 2 2 = 3·4, P = 0·186). In contrast to the crossing of survival curves noted under the milder conditions of experiment 2 (Fig. 2), survival of infected hosts in experiment 3 was similar to uninfected hosts treatment (Fig. 3B).

Fig. 3. Survival of Hymenolepis diminuta-infected Tribolium confusum following storage in different amounts of food and diatomaceous earth (DE) (experiment 3). Panels (A–F) correspond to treatments A–F described in detail in the text. Values are proportion live ±95% confidence limits. Initial number of hosts is indicated by n. Survival functions of abundance classes sharing the same lowercase letter do not differ (Kaplan–Meyer analysis, Tukey multiple comparison adjustment).
The presence of shallow food without DE (treatment C, Fig. 3C) produced similar survival among parasite abundance classes (Wilcoxon test, χ 2 2 = 2·3, P = 0·313). Survival of all infection categories was >95% when the experiment was terminated after 41 days. This was significantly higher than in treatment A (data pooled across infection categories within a treatment; Wilcoxon test, χ 2 1 = 6·9, P = 0·009). The presence of shallow food containing 16% DE (treatment D, Fig. 3D) produced survival differences among parasite abundance classes (Wilcoxon test, χ 2 2 = 18·2, P < 0·001). Pairwise comparisons indicated that survival of uninfected hosts and hosts with >20 parasites differed statistically, but hosts harbouring 1–20 parasites were indistinguishable from the other groups. Even though treatment D had the same absolute amount of DE as treatment B, host survival was significantly greater than treatment B in all parasite abundance classes (uninfected: Wilcoxon test, χ 2 1 = 243, P < 0·001; 1–20 parasites: Wilcoxon test, χ 2 1 = 220, P < 0·001; >20 parasites: Wilcoxon test, χ 2 1 = 23·6, P < 0·001).
The absence of food and DE (treatment E, Fig. 3E) produced survival differences among parasite abundance classes (Wilcoxon test, χ 2 2 = 10·9, P = 0·004). Pairwise comparisons indicated that survival of hosts with 1–20 parasites and >20 parasites was statistically indistinguishable, but both were significantly lower than uninfected hosts. The absence of food but presence of DE dust (treatment F, Fig. 3F) also produced survival differences among parasite abundance classes (Wilcoxon test, χ 2 2 = 31·7, P < 0·001). Pairwise comparisons indicated that survival of uninfected hosts and hosts with >20 parasites differed statistically, with hosts harbouring 1–20 parasites indistinguishable from the other groups.
Parasite viability
Immediately following completion of development, the cysticercoid possessed a cercomer, was surrounded by a defined outer membrane, had suckers clearly visible in the scolex and had two clusters of dense granules present anterior to the suckers (Fig. 4A). Morphology of older cysticercoids was variable, but was characterized by a gradual loss of defined structure. The outer membrane was sometimes absent, exposing its underlying layer of radial fibres (Fig. 4B). Some older cysticercoids had large spheroidal inclusions in the posterior portion of the scolex (Fig. 4B), different from the individual granules forming the anterior clusters. Patches of debris or cells adhered to some older cysticercoids (Fig. 4C), but we saw no evidence of complete encapsulation. The discrete clusters of dense granules present in young cysticercoids (Fig. 4A) remained intact in some older cysticercoids (Fig. 4B), but were fragmented in others (Fig. 4C). The oldest cysticercoids (Fig. 4C) retained the layered structure of younger cysticercoids (Fig. 4A, B) but lacked the transparency and crisp definition of internal tissues, and were generally darker.

Fig. 4. Hymenolepis diminuta from freshly killed Tribolium confusum. (A) Cysticercoid recovered 2 weeks post-infection (PI), possessing a distinct outer membrane (arrow) and discrete scolex granules anterior to the suckers. (B) Cysticercoid recovered 16 weeks PI, possessing distinct granules, but lacking a distinct outer membrane or scolex suckers and possessing inclusions (arrows) in the scolex region. (C) Cysticercoids recovered 30–43 weeks PI, typically with fragmented scolex granules (arrows) but varying damage to the outer membrane.
We quantified morphological observations (Table 1) after grouping beetles into intensity categories similar to those used in our other analyses. In general, signs of damage to the cercomer, capsule and scolex became more common in older parasites. Damage appeared earliest for the cercomer, later for the capsule and last for the scolex. At a given age, the proportion of cysticercoids having a damaged cercomer > damaged capsule ⩾ damaged scolex. Intensity of parasite infection had little or no effect on damage to the cercomer or scolex. Interestingly, the proportion of cysticercoids with capsule damage was significantly lower when they came from high-intensity (21–40 parasites) infections (Table 1).
Table 1. Temporal changes in morphological features of cysticercoids of Hymenolepis diminuta recovered from Tribolium confusum with different infection intensities (experiment 4)

*Significant difference (Fisher's exact test, P < 0·05) of the 21–40 parasites intensity group compared with the 1–20 group for the same morphological feature and time.
We initiated viability assays with 815 of the 819 cysticercoids recovered from 45 beetles, and found 761 (93%) capsules (with or without their scolex) after the assays; analyses were based on the number of capsules found. Excysted scolices moved actively. No signs of movement were observed in non-excysted scolices. The first round of assays used 13 control beetles (3–51 cysticercoids each, 384 total cysticercoids tested, 57% excysted) and 13 beetles with 16–29-week-old infections (8–27 cysticercoids each, 171 cysticercoids tested, 19% excysted). The second round of assays used eight control beetles (1–32 cysticercoids each, 63 total cysticercoids tested, 78% excysted) and 11 beetles with 30–43-week-old infections (6–24 cysticercoids each, 143 cysticercoids tested, 4% excysted). Logistic regression indicated a small decrease in likelihood of excysting with increasing intensity in the source beetle (Wald χ 2 1 = 4·7, P = 0·030; odds ratio = 0·983, 95% Wald CLs =0·968–0·998). Adjusting for differences in intensity, logistic regression also indicated a strong interaction between assay round and parasite age (control vs older) on ability to excyst (Wald χ 2 1 = 25·8, P < 0·001). This interaction was evident by the small reduction in ability to excyst relative to controls for cysticercoids from 16–29-week-old infections (odds ratio = 0·26, 95% Wald CLs =0·15–0·44) compared with the large reduction for cysticercoids from 30–43-week-old infections (odds ratio = 0·013, 95% Wald CLs =0·005–0·037).
Patterns across experiments
From the perspective of median host survival time, the various combinations of parasite, food and DE treatment that we used produced survival that varied among treatments by almost two orders of magnitude, from just 3 days for the ‘harsh’ condition of hosts with >20 parasites exposed to DE dust and no food (Fig. 3F), to 197 days for the ‘mild’ condition experienced by hosts with 1–20 parasites in deep food with 0% DE, which did not differ from unstressed hosts (Fig. 2A). In most cases, median survival of parasitized hosts was lower than uninfected hosts in an intensity-dependent manner (Figs 1–3). The two cases where no intensity dependence in median survival was detected (Figs 2C and 3B) were intermediate on this ‘harsh’–‘mild’ continuum.
From the perspective of host survival at comparable time points, all infected hosts had survival equal to or higher than uninfected beetles for at least 42 days (Fig. 3A, C) and as long as 70 days (Fig. 2A) when parasites were the only stressor. During this time when there was no mortality from parasitism alone, parasitism in the presence of starvation (Figs 1 and 3E), 4% DE (Fig. 2C), 16% DE (Fig. 3B, D) and starvation plus DE (Fig. 3F) all resulted in significant intensity-dependent mortality at one or more time points.
DISCUSSION
Our longitudinal observations on non-stressed (i.e. uninfected, no DE, food ad libitum) hosts produced typical survival curves for T. confusum (Pearl et al. Reference Pearl, Park and Miner1941). Our results for young, low-intensity infections corroborated the observations of previous studies that failed to demonstrate parasite-induced mortality due solely to H. diminuta infection (Maema, Reference Maema1986; Shostak et al. Reference Shostak, Walsh and Wong2008, Reference Shostak, Van Buuren and Cook2015; Shostak, Reference Shostak2012). However, we found that infections with >20 parasites can, after several months, substantially reduce median and maximum lifespan of T. confusum in the absence of other stressors. Moreover, we found that environmental stressors modified the effects of parasitism on host survivorship. These findings are particularly interesting in light of the changes that occurred in the parasites themselves over the timespan that was needed to cause host mortality.
We showed for the first time that H. diminuta cysticercoids acquired by young T. confusum persist throughout the host's life. All cysticercoids are infective to rats for 4–6 weeks PI, and a declining proportion up to 12 weeks PI (Keymer, Reference Keymer1981). We extended the age to which at least some cysticercoids show signs of viability (ability to excyst) to the 29–43 weeks PI range. The declining ability to excyst was associated with increasing morphological damage to cysticercoids and suggests gradual senescence of the parasite after 4 weeks. Early infections elicit no strong host reaction (Heyneman and Voge, Reference Heyneman and Voge1971), perhaps because the parasites damage host haemocytes (Lackie, Reference Lackie1976) or modify expression of host defence genes (Hitchen et al. Reference Hitchen, Shostak and Belosevic2009). The reduced proportion of damaged cysticercoids in higher intensity infections may indicate an overload of host defences, because intraspecific crowding is normally associated with various increased negative effects on the parasite (Shostak et al. Reference Shostak, Walsh and Wong2008). Whether degradation of older cysticercoids was due to a late-developing host response, or to an internal ageing mechanism in the parasite, we found no evidence that any cysticercoids completely disappeared, and conclude that all cysticercoids acquired early in life persist until host death.
The long life of T. confusum, and of H. diminuta within it, requires consideration of an ageing parasitic infection in the context of an ageing host. From the perspective of an ageing parasite, our results and an absence of literature reports of damage to young cysticercoids, suggest that a shorter lived host, such as T. molitor (4–6 weeks, Hurd et al. Reference Hurd, Warr and Polwart2001), should harbour ‘healthy’ parasites throughout its life. A longer lived host, such as T. confusum, may harbour ‘healthy’ parasites for only a fraction of its life, and senescent or damaged parasites for the remainder of its life. We do not know whether the cysticercoid damage we observed in T. confusum can be generalized to other long-lived Tenebrionidae. We note that T. confusum also seems unable to clear cysts of a different helminth, the nematode Protospirura muricola, which remains present up to 602 days PI (Schutgens et al. Reference Schutgens, Cook, Gilbert and Behnke2015). We expect that cysticercoid impact on the host will change over time, and that the host–parasite relationship of this generalist parasite will differ depending on the lifespan of the particular intermediate host species that acquires it.
From the perspective of an ageing host, survival of newly emerged adult T. confusum typically remains >80% for 4–7 months before a final sigmoid decline (Pearl et al. Reference Pearl, Park and Miner1941; Ducoff et al. Reference Ducoff, Vaughan and Crossland1970; Shostak, Reference Shostak2008). Fecundity of females increases until 1–2 months of age, and then declines (Mertz, Reference Mertz1975; Maema, Reference Maema1986; Shostak, Reference Shostak2008), but may remain high for up to 24 months (Shostak, Reference Shostak2014b ). Susceptibility to starvation increases with age (Ducoff et al. Reference Ducoff, Vaughan and Crossland1970; Shostak, Reference Shostak2008), but beetle strain can have a greater effect on starvation tolerance than age (Ducoff et al. Reference Ducoff, Vaughan and Crossland1970). Beetles are not killed by ⩽4% DE exposure until >12 weeks post-emergence (Shostak, Reference Shostak2014b ). Studies on T. confusum for age-related changes in defence mechanisms against pathogens appear lacking, although T. castaneum has increased phenoloxidase activity from 17 to 77 days post-pupation (Khan et al. Reference Khan, Prakash and Agashe2016). However, T. confusum remains susceptible to infection with H. diminuta for >50 weeks and produces normal-appearing cysticercoids throughout (Kelly et al. Reference Kelly, O'brian and Katz1967; Shostak, Reference Shostak2008). With the exception of the 0% DE treatment in experiment 2, all of our experiments were completed, while beetles were in the first 1/3 of their normal lifespan. This is a period for which we would characterize the host qualitatively as most ‘vigorous’, with low mortality, high reproduction and greatest resistance to environmental stressors, yet we still observed intensity-dependent mortality and mortality from environmental stressors, as well as interactions among stressors.
Our experiments incorporated the continual application of non-parasitic stressors to relatively young, recently infected beetles, with host survival reflecting the cumulative effect of those stressors. Chronic exposure to parasites and other stressors creates a possibility of covariation of their effects with host age. There are certainly examples of age-dependent mortality of parasitized hosts (Hawlena et al. Reference Hawlena, Abramsky and Krasnov2006), and age-dependent mortality of beetles from DE (Vayias and Athanassiou, Reference Vayias and Athanassiou2004). However, we have found no examples where multiple combinations of parasitism and other stressors have been explicitly examined in an ageing host.
It is common to observe non-overlapping survival curves, where infected hosts have consistently lower survival relative to uninfected individuals (Robb and Reid, Reference Robb and Reid1996; Jokela et al. Reference Jokela, Lively, Taskinen and Peters1999; Heinonen et al. Reference Heinonen, Kukkonen and Holopainen2001; Fredensborg et al. Reference Fredensborg, Mouritsen and Poulin2004; Benesh and Valtonen, Reference Benesh and Valtonen2007; Vezilier et al. Reference Vezilier, Nicot, Gandon and Rivero2012). Cross-over of survival curves, whereby infected hosts have higher initial survival than uninfected hosts, but reduced survival in older infections leading to a shorter lifespan overall, is much less common (Ashworth et al. Reference Ashworth, Kennedy and Blanc1996; Fredensborg et al. Reference Fredensborg, Mouritsen and Poulin2005; Schutgens et al. Reference Schutgens, Cook, Gilbert and Behnke2015). When parasitism by T. confusum was combined with mild environmental stressors that allowed the host to live for several months, there were statistically significant crossing over events among the three levels of infection indicating that H. diminuta-infected T. confusum also have extended early survival, but cross-overs were not observed in the presence of more severe stressors.
One hypothesis for the cross-overs, given the higher mortality of exposed beetles during the parasite developmental period, is that we selected for ‘robust’ beetles in the parasite-exposed group, which then had higher initial survival after parasite development. However, if there was a ‘robust’ subpopulation of beetles in our colony, we would expect to observe host mortality during parasite development routinely, and we have not (Shostak, Reference Shostak2008; Shostak et al. Reference Shostak, Walsh and Wong2008, Reference Shostak, Van Buuren and Cook2015; experiments 1 and 3). We suspect that the mortality in experiment 2 was due simply to some beetles ingesting an unusually large number of eggs, or to unusual handling deaths in one of the exposure arenas.
An alternative hypothesis for the cross-overs is a direct effect of the parasite on the host. Hurd et al. (Reference Hurd, Warr and Polwart2001) demonstrated that H. diminuta increases lifespan of the normally short-lived T. molitor, and suggested that parasite-produced molecules inhibit host reproduction and increase resource availability for the parasite. No parasite-produced molecules with reproductive effect have been identified yet from H. diminuta in T. confusum, but fecundity of recently emerged T. confusum is unaffected by infection with ⩽7 parasites for up to 5 weeks PI. Fecundity does decline in an intensity-dependent manner in heavier infections (Shostak, Reference Shostak2009). While we cannot assess whether the specific mechanism proposed by Hurd et al. (Reference Hurd, Warr and Polwart2001) is operating, the same principle may apply. The expression of other molecules by T. confusum infected with H. diminuta is modified by infection in a way speculated to favour parasite persistence (Hitchen et al. Reference Hitchen, Shostak and Belosevic2009). If the production of these molecules by young cysticercoids does indeed promote extended early host survival, then declining production with parasite senescence should return infected beetles not only to a state of increased mortality normal for their age, but also the resumption of parasite-induced mortality, leading to the cross-over of survival curves that we observed for this long-lived host. This parasite and DE also alter host behaviour such as the tendency of beetles to remain on the surface of their medium. Some studies have reported increased surface seeking by H. diminuta-infected T. molitor or T. confusum (Hurd and Fogo, Reference Hurd and Fogo1991; Yan et al. Reference Yan, Stevens and Schall1994) or by uninfected T. confusum in the presence of DE (Shostak, Reference Shostak2012; Shostak et al. Reference Shostak, Van Buuren and Cook2015). This combination could contribute to a cross-over by reducing the contact of infected beetles with DE while tunnelling, and contribute to higher survival of infected beetles. Yet, infection reduces surface seeking by T. castaneum (Yan et al. Reference Yan, Stevens and Schall1994), and in our own system infection by H. diminuta either does not affect, or reduces, surface seeking by T. confusum even in the presence of DE (Shostak, Reference Shostak2012; Shostak et al. Reference Shostak, Van Buuren and Cook2015).
However generated, the presence of cross-overs limits the ability to generalize about the effects of stressors. For example, our conclusions on the effects of parasitism (or of parasite intensity) based on statistical testing of median host survival often differed from patterns evident in early or late survival. This caution applies particularly to studies where host survival is evaluated only at a single point in time.
Many studies support the broad notion that negative effects of parasitism are enhanced by the presence of other stressors on the host (Marcogliese and Pietrock, Reference Marcogliese and Pietrock2011), i.e. a synergistic outcome (Greco et al. Reference Greco, Bravo and Parsons1995). Our observation of increased mortality of parasitized hosts when exposed to environmental stressors, at a time when the parasites were present but not causing mortality on their own, is consistent with an interpretation of synergy. However, few studies incorporate multiple levels of environmental stressors and levels of parasitic infection beyond ‘uninfected’ vs ‘infected’, so there is less evidence available to identify scenarios where parasitism becomes a more or less important mortality factor relative to other stressors on the host. These studies show that while host mortality due to parasitism is always greater in the presence of other stressors, it may (i) progressively increase with increases in level of other stressors (Moles, Reference Moles1980; Fredensborg et al. Reference Fredensborg, Mouritsen and Poulin2005; Gheorgiu et al. Reference Gheorgiu, Marcogliese and Scott2006), (ii) exhibit a consistent proportional increase over a range of stressor values (Pascoe and Cram, Reference Pascoe and Cram1977; Granath and Esch, Reference Granath and Esch1983), or (iii) be highest at low levels of the other stressors but less prominent or even masked at higher levels (Brown and Pascoe, Reference Brown and Pascoe1989). The stressors we used, although acting on different timescales, affected parasite-induced host mortality separately and in combination. Some combinations of stressors masked intensity dependence, at least over the range of intensities we generated. However, some level of intensity-dependent mortality generally occurred throughout the host's lifespan, whether long or short, and the type of response did not appear readily predictable simply by the ‘harshness’ of the stressor.
The mechanisms of action of the stressors we chose, which might explain a synergistic effect on host mortality, are not well understood. Each stressor has multiple, possibly overlapping, direct and indirect effects on the host, but nutrition and water appear to be common threads. Infection with H. diminuta changes metabolite concentrations and reproduction of T. confusum (reviewed by Shostak, Reference Shostak2014a ), and also host water content (Granath, Reference Granath1980; Nishina et al. Reference Nishina, Matsushita, Kato, Takahashi and Kono1998). Starvation drastically shortens the lifespan of T. confusum (Ducoff et al. Reference Ducoff, Vaughan and Crossland1970) with associated changes in both dry mass and water content (Saleem and Shakoori, Reference Saleem and Shakoori1994). DE clearly desiccates insects (Korunic, Reference Korunic1998), but may also affect their reproduction (Shostak, Reference Shostak2014b ) or behaviour (Shostak et al. Reference Shostak, Van Buuren and Cook2015) in ways that could alter energetics. Furthermore, water acquired during feeding may counteract the desiccating effects of DE (Vayias and Athanassiou, Reference Vayias and Athanassiou2004). The examples above suggest a mix of positive and negative effects of parasitism and the other stressors, which on balance moderated but did not eliminate the negative effects of parasitism.
Our results suggest that parasites should retain some regulatory capability on the host population via intensity-dependent mortality (Anderson and May, Reference Anderson and May1978; May and Anderson, Reference May and Anderson1978) even when their hosts are severely stressed by environmental factors. However, intensity-dependent reduction of fecundity is strong on young Tribolium spp. (Keymer, Reference Keymer1980; Shostak, Reference Shostak2009), which are at their greatest fecundity (Mertz, Reference Mertz1975; Shostak, Reference Shostak2008) and least susceptible to parasite-induced mortality in the absence of other stressors. In view of this, we speculate that in a natural setting without environmental stressors, parasite effects on fecundity would have a greater influence on regulation than mortality effects, since mortality effects would primarily impact older beetles already reduced in number and fecundity. On the other hand, in the presence of environmental stressors, intensity-dependent parasite-induced mortality would also act on younger beetles, in conjunction with intensity-dependent fecundity reduction, to enhance regulation.
Previous studies on H. diminuta in beetles typically used infections within 4 weeks, and not beyond 12 weeks, of cysticercoid differentiation (Shostak, Reference Shostak2014a ). This has provided a good picture of the host–parasite relationship in young infections, but represents a small fraction of what we now know is the potential length of the infection in T. confusum. We have identified a new temporal component to the host–parasite relationship by showing that much older, degenerating cysticercoids remain in the host, are an ongoing mortality factor, and also seem capable of interacting with other stressors. However, the extent to which mechanistic explanations derived from young parasites can be applied to older infections is unknown. Regardless of mechanism, our results suggest that while T. confusum may, in the absence of other stressors, outlive this parasite, it cannot use its age to escape mortality effects associated with the persistence of the degenerating parasite.
Point estimates of host mortality may be the only practical choice in studies of host–parasite interactions when numerous contributing factors are being considered. However, we have clearly shown using longitudinal studies with varied levels of parasitism and environmental stressors that point estimates have the potential to produce misleading conclusions. Even within the limited range of conditions we tested, the use of just one time point at which to assess host mortality could have affected conclusions on: (i) whether parasites enhanced, decreased or had no effect on host mortality; (ii) whether or not effects of parasitism were modified by environmental stressors; and (iii) the intensity at which intensity-dependent effects of parasitism on host mortality become evident. Although logistics may necessitate the occasional use of point estimates of parasite-induced mortality, longitudinal studies conducted under various conditions can provide not only a more complete picture, but also reveal unexpected processes.
ACKNOWLEDGEMENTS
The authors thank Dr Keith Tierney, and two anonymous reviewers, for helpful comments on earlier versions of the manuscript.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.








