INTRODUCTION
The tick Rhipicephalus (Boophilus) microplus infests cattle in many tropical and subtropical regions and causes economic losses by direct parasitism and by transmission of several pathogenic microorganisms (Willadsen, Reference Willadsen2006). Tick control methods are based on the application of chemical acaricides, which induce selection of drug-resistant populations and present potential food and environmental contamination risks (Willadsen, Reference Willadsen2004). Vaccines have been shown to be a feasible tick control method, offering a cost-effective, environmentally friendly alternative to chemical control. However, identifying tick-protective antigens remains a limiting step in the development of a vaccine able to replace chemical acaricides (de la Fuente et al. Reference de la Fuente, Almazan, Canales, Perez de la Lastra, Kocan and Willadsen2007). In this context, more comprehensive knowledge of important tick physiological mechanisms may be helpful in finding new vaccine targets.
Cysteine endopeptidases is a widely distributed group of enzymes, with a broad range of putative functions (Sajid and McKerrow, Reference Sajid and McKerrow2002; Carnevali et al. Reference Carnevali, Cionna, Tosti, Lubzens and Maradonna2006). In mammalian lysosomes, cathepsins play a key role in the intracellular degradation of proteins (McGrath, Reference McGrath1999). In simpler organisms they may have other important roles, such as the cathepsin L-like enzyme responsible for digestion in sponges (Krasko et al. Reference Krasko, Gamulin, Seack, Steffen, Schroder and Muller1997); cruzain from the protozoan Trypanosoma cruzi, which is required for parasite replication (Meirelles et al. Reference Meirelles, Juliano, Carmona, Silva, Costa, Murta and Scharfstein1992); Leishmania cathepsin-L like enzymes that act in macrophage invasion (Frame et al. Reference Frame, Mottram and Coombs2000) and Giardia lamblia cathepsin B-like proteases, which function in excystation or encystation (Yu et al. Reference Yu, Wang and Wang1995). The cathepsin-L-like protease from the tick Ornithodoros moubata plays a role in the digestion of vitellin (Vt), the major reserve protein in arthropod eggs, providing nutrients during embryogenesis (Fagotto, Reference Fagotto1990). In R. microplus, 2 cysteine endopeptidases involved in Vt hydrolysis have been studied: vitellin-degrading cysteine endopeptidase (VTDCE; Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003) and Riphicephalus microplus larval cysteine endopeptidase (RmLCE; Estrela et al. Reference Estrela, Seixas and Termignoni2007). VTDCE was previously purified from R. microplus eggs by a purification protocol in which an autolysis step to hydrolyse Vt is crucial (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). Contrarily to VTDCE, the larval cysteine endopeptidase RmLCE elutes from an anionic resin free of Vt. BmCL1, another cysteine endopeptidase from R. microplus, was localized in probable secretory cells of the gut and may be involved in haemoglobin degradation (Renard et al. Reference Renard, Garcia, Cardoso, Richter, Sakanari, Ozaki, Termignoni and Masuda2000, Reference Renard, Lara, de Cardoso, Miguens, Dansa-Petretski, Termignoni and Masuda2002).
Vitellin, a polydisperse protein, is derived from a maternal protein, vitellogenin (Vg), which is synthesized by the tick fat body and gut after adult females obtain their bloodmeal. In general, the carbohydrate, lipid and amino acid composition of tick Vg is similar to that of insect Vg, except for the fact that tick Vg contains heme from digestion of host haemoglobin (Logullo et al. Reference Logullo, Moraes, Dansa-Petretski, Vaz, Masuda, Sorgine, Braz, Masuda and Oliveira2002). After synthesis, Vg is released into haemolymph and taken up via receptor-mediated endocytosis by the growing oocytes. Vg is partly processed in the endosomal compartment and then stored as Vt in specialized organelles called yolk granules (Raikhel and Dhadialla, Reference Raikhel and Dhadialla1992; Fagotto, Reference Fagotto1995; Mitchell et al. Reference Mitchell, Ross, Osgood, Sonenshine, Donohue, Khalil, Thompson and Michael Roe2007).
The R. microplus ovary is classified as panoistic (Saito et al. Reference Saito, Bechara, Nunes, de Oliveira, Denardi and Mathias2005). In this type of ovary, nurse cells are absent and oocytes are attached to the ovarian wall through a cellular pedicel. Balashov (Reference Balashov and Balashov1983) suggested that pedicel cells play the role of nurse cells, normally present in meroistic-type ovaries, incorporating the material that subsequently will be taken up by oocytes. Pedicel cells present a fine layer of cytoplasm in which the presence of vacuoles is observed. The basal lamina that supports the more external cells of the ovary wall is absent at the point of contact between oocyte and pedicel cells, and this contact membrane presents interdigitations that increase the contact surface between these two cell types. The vitellogenesis process in R. microplus occurs by means of the endogenous production of lipids and proteins until oocytes reach developmental stage III, beyond which the incorporation of material from the haemolymph also occurs (Saito et al. Reference Saito, Bechara, Nunes, de Oliveira, Denardi and Mathias2005).
In the present work we sought to investigate the presence of cysteine endopeptidase activity in R. microplus eggs, larvae, adult female haemolymph and tissues. Also, we showed the cellular distribution of the vitellin-degrading cysteine endopeptidase (VTDCE) in engorged female ovary and gut, as well as its association with its natural substrate Vt.
MATERIALS AND METHODS
Ticks
Ticks from the Porto Alegre strain were reared in cattle, which were brought from a tick-free area and maintained in insulated individual boxes protected from any contact with other ticks and insects. Cattle were infested with 15-day-old (from hatching) R. microplus larvae. Partially engorged tick females (20 days of life on the host) were forcibly removed from the host and fully engorged females (spontaneously detached from the host on the 22nd day) were collected. These ticks were used for experiments. Also, fully engorged females were incubated at 28°C and 85% relative humidity, for egg-laying. Eggs were collected on different days after oviposition and stored at −70°C until used, or maintained in glass tubes closed with cotton plugs under the same conditions for larvae hatching. Larvae were separated, 5 or 20 days after hatching, and stored at −70°C until use.
Preparation of tissues, eggs and larval extracts
Fully and partially engorged females were washed with 70% ethanol, immobilized with glue on Petri dishes and flooded in cold phosphate-buffered-saline (PBS; sodium phosphate (10 mm), NaCl (150 mm), pH 7·2). The dorsal cuticle was removed using a microscalpel and gut, salivary glands, ovary and fat body were dissected with forceps. Tissues were homogenized in a tube with a disposable grinder (GE Healthsciences, Uppsala, Sweden) in 300 μl of PBS. Homogenates were centrifuged at 16 000 g/10 min to pellet insoluble material. After removing the soluble fraction, 300 μl of PBS containing 2·5% deoxicolate were added and the insoluble material was again homogenized and centrifuged at 16 000 g/10 min. This supernatant fraction constituted the insoluble protein extract. Tissue extracts were stored at −20°C until use.
Collection of haemolymph and saliva
Haemolymph was collected from immobilized ticks kept chilled for 15 min at 4°C to avoid gut contractions and contamination of haemolymph with blood. A small incision was made in the cuticle, and haemolymph was collected using a micropipette. Tick saliva was collected as described previously (Horn et al. Reference Horn, dos Santos and Termignoni2000). Briefly, adult engorged females recently detached from the host were rinsed, fixed onto glass plates with adhesive tape and induced to salivate by injecting 5 μl of pilocarpine (2% w/v in PBS). Ticks were maintained in a humid chamber and saliva was collected for a period of 2 h with a small vacuum apparatus. Haemolymph and saliva were kept at −20°C until use.
Size exclusion chromatography
Samples (200 μl) were applied onto a size exclusion column Superose 12 (GE Healthcare, Uppsala, Sweden) that had previously been equilibrated in 10 mm sodium phosphate buffer, pH 7·0 in an FPLC System (GE Healthcare, Uppsala, Sweden). Fractions of 1 ml were collected and the protein concentration of the column eluate was monitored by absorbance reading at 280 nm. The column was previously calibrated with the same buffer as used previously employing aprotinin (6·5 kDa), carbonic anhydrase (29 kDa), conalbumin (75 kDa), alcohol dehydrogenase (150 kDa), catalase (232 kDa), and ferritin (440 kDa) as standards.
Cysteine endopeptidase activity assay
Cysteine endopeptidase activity was tested as previously described (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). Briefly, 10 μl aliquots of column fractions, tissues extracts, haemolymph and saliva, or other samples, were incubated with 50 mm sodium citrate/sodium phosphate buffer, pH 3·5, and 10 mm DTT at 37°C in the presence or absence of cysteine endopeptidase inhibitor E-64 (10 μ m; L-trans-epoxysuccinyl-leucylamide-(4-guanido)-butane). After 10 min, a fluorogenic substrate, N-Cbz-Phe-Arg-MCA (Cbz, carboxibenzoyl, MCA amido-4-methyl coumarin) was added to a final concentration of 1·4 μ m. Hydrolysis was monitored at 11 sec intervals by fluorimetry in an M2e Microplate Reader (Molecular Devices Corporation, Sunnyvale, USA). The wavelength pair for excitation and emission was 370 nm/460 nm (Oliveira et al. Reference Oliveira, Hirata, Chagas, Boschcov, Gomes, Figueiredo and Juliano1992). Enzyme activity is given by the enzyme initial rate obtained from kinetic measurements, where 1 enzyme unit (U) corresponds to an increase by 1 relative fluorescence unit (RFU) per sec.
Protein determination
Protein concentration was determined by the bicinchoninic acid (BCA) method, according to the method reported by Smith et al. (Reference Smith, Krohn, Hermanson, Mallia, Gartner, Provenzano, Fujimoto, Goeke, Olson and Klenk1985), using bovine serum albumin (BSA) as standard.
VTDCE purification
VTDCE was purified from R. microplus eggs as described by Seixas et al. (Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). Briefly, an homogenate was prepared with eggs from the 1st to the 12th day after oviposition in 10 mm phosphate buffer, pH 7·2 (0·1 g of eggs/ml). The sample was then applied onto a 0·5×5·0 cm Mono Q HR 5/5 (Pharmacia, Uppsala, Sweden) column previously equilibrated with 10 mm sodium phosphate buffer, pH 7·2, and eluted with a 0–0·8 m NaCl gradient in the same buffer system at room temperature with a flow rate of 0·5 ml/min. Fractions of 1·0 ml were collected. Fractions containing activity (6–7 ml) were pooled and submitted to autolysis. In this step, the pooled fractions were acidified to pH 3·5 with 1·0 m citric acid and incubated at 37°C for approximately 3 h, after which the sample was centrifuged at 3000 g for 15 min.
After centrifugation, the supernatant was concentrated in a Centricon-10 and applied onto a 1·0 cm×30 cm Superdex 75 HR (Pharmacia, Uppsala, Sweden) column equilibrated with 10 mm acetate buffer, pH 4·0, using a Fast-Purification-Liquid Chromatography system (FPLC) at room temperature with a flow rate of 0·3 ml/min. The pool of active fractions was then applied onto a second Mono Q HR 5/5 column (0·5 cm×5·0 cm), previously equilibrated with acetate buffer 10 mm, pH 4·0. The enzyme was eluted with a 0–0·8 m NaCl gradient in the same buffer. Enzyme activity in the fractions was monitored with N-Cbz-Phe-Arg-MCA as substrate.
Polyclonal antibodies against VTDCE
Anti-VTDCE antiserum was obtained as described by Seixas et al. (Reference Seixas, Leal, Nascimento-Silva, Masuda, Termignoni and da Silva Vaz2008). Briefly, rabbit serum was inoculated subcutaneously with 100 μg of purified VTDCE emulsified in Freund's complete adjuvant followed by 3 boosters of VTDCE (100 μg) in Freund's incomplete adjuvant at 15-day intervals between each dose.
Immunoblotting
Extracts were separated by SDS-PAGE using a Bio-Rad Mini-Protean Cell II unit. The resolving and stacking gels contained 12·5% and 5% polyacrylamide, respectively. Proteins were transferred to a nitrocellulose membrane (0·45 μm, Schleicher & Schuell, Dassel, Germany) in a semi-dry system (GE-Healthcare, Uppsala, Sweden) using 25 mm Tris, 192 mm aminoacetic acid, 30% methanol, pH 8·4. Nitrocellulose membranes were blocked with ‘Blotto’ (5% cow non-fat dry milk in sodium phosphate (10 mm), NaCl (150 mm), pH 7·2) and probed with rabbit polyclonal antibodies against VTDCE (1:100). After 3 washes with Blotto, anti-rabbit IgG alkaline phosphatase conjugate was used as a secondary antibody. Development was performed with NBT/BCIP (nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate p-toluidine salt; USB Corporation, Cleveland, USA). Dot blot was performed under the same conditions. Anti-VT monoclonal antibodies were kindly supplied by Sandra E. Farias, Centro de Biotecnologia do Estado do Rio Grande do Sul, Brazil.
Transmission electron microscopy immunohistochemistry
Fully engorged tick ovaries were dissected as described above and fixed at 4°C for 12 h in 0·25% glutaraldehyde, 4% paraformaldehyde, 10 mm calcium chloride, 0·1 m cacodylate buffer, pH 7·3. Following that, the material was dehydrated in an ascending series of ethanol concentrations and embedded in LR-White resin (Electron Microscopy Sciences, Hatfield, PA, USA) at 4°C. Ultrathin sections (70 nm) were caught in Formvar cover copper grids, blocked by incubation with Blotto for 15 min, and then incubated with antiserum (anti-VTDCE) in the same buffer for 1 h. Anti-rabbit IgG conjugated to 10 nm colloidal gold was used as secondary antibody. Finally, grids were stained with saturated uranyl acetate and lead citrate (Glauert, Reference Glauert1974) and observed through a transmission electron microscope 900 Zeiss (Zeiss, Oberkochen, Germany) at 80 kV.
Immunofluorescence
Fully engorged females were dissected on the 3rd day after a bloodmeal. Tissues were fixed as described above, embedded with PBS-sucrose 20% for 12 h/4°C, O.C.T. polymer (TissueTek, Minnetonka, USA) for 2 h and then frozen in liquid nitrogen. Thin sections measuring 5 μm were cut, exposed to anti-VTDCE rabbit polyclonal antibody (1:100) or pre-immune serum and then to a goat anti-rabbit antibody conjugated with fluorescein (1:500) (DAKO, Glostrup, Denmark). Samples were visualized under an Axioplan 2 fluorescence microscope (Zeiss, Oberkochen, Germany).
Binding assay
Purified VTDCE (260 μg) was iodinated with 125I-sodium iodide (17·4 Ci/mg, GE Healthcare, Little Chalfont, UK) using Iodogen (Pierce, Rockford, USA) as described elsewhere (Gondim and Wells, Reference Gondim and Wells2000). To remove free iodide, the reaction mixture was extensively dialysed against PBS, pH 7·4. A 125I-VTDCE-specific activity around 150 000 cpm/μg of protein was obtained. For the binding assay, 20 μg of sample were fixed in pieces of nitrocellulose membrane that were placed in the wells of a 96-well plate. Membranes were blocked with BSA (25 mg/ml), washed twice with PBS, incubated with 1 μg of 125I-VTDCE in 100 μl of 2·5 mg/ml BSA in PBS for 2 h in the presence or absence of leupeptin, and then washed thoroughly with the same buffer. Assays were performed in triplicate. Radioactivity associated with the filters was determined in a γ-counter. Radioactivity in control membranes was subtracted from values of experimental membranes, containing vitellin. Results were expressed as mean±s.d. Comparisons among groups were performed by one-way analysis of variance (ANOVA) followed by the Tukey's multiple comparison test. The significance level was 0·05, and P-values are indicated in the legend of Fig. 4.
RESULTS
Cysteine endopeptidase activity in R. microplus female tissues, saliva and haemolymph
Cysteine endopeptidase activity was present in fat body, gut, salivary glands, and ovary extracts from partially and fully engorged females (Table 1). In partially engorged females, the gut was the richest source of enzyme, when compared to other tissues (904±100 U/mg tissue). In contrast, after female full engorgement, gut cysteine endopeptidase specific activity reduces almost 500 times (1·9±0·1 U/mg tissue). Other female tissue extracts like ovary (1063±69 U/mg tissue), fatty body (27±0·8 U/mg tissue), and salivary gland (21±0·2 U/mg tissue) showed an increase in cysteine endopeptidase-specific activity after engorgement. At this life stage, the ovary was the richest source of this enzyme. Significantly, all activity was inhibited by E-64, confirming that all endopeptidase activity detected is due to cysteine endopeptidases.
Table 1. Cysteine endopeptidase activity in partially and fully engorged female tissues, saliva and haemolymph of Rhipicephalus microplus

U, Relative fluorescence units/sec.
a U/mg of tissue.
b U/ml.
Numbers 1, 2 and 3 in haemolymph indicate days after female engorgement.
Cysteine endopeptidase activity was also detected in haemolymph of partially and fully engorged R. microplus females (Table 1). In partially engorged females this activity was 278±12 U/ml, increasing to 589±73 U/ml after full engorgement (host detachment day; Table 1). During the days preceding oviposition, activity in haemolymph gradually decreased (Table 1). Variation in total haemolymph protein content was also observed. Protein content in partially engorged female haemolymph was 7·97 mg/ml, increasing to 10·93 mg/ml in fully engorged females (1st day after detachment). At the 2nd day after detachment, the highest protein level was achieved (31·03 mg/ml), remaining almost constant until the 3rd day (29·76 mg/ml). In contrast to all other samples tested, no activity was detected in saliva (Table 1).
Cysteine endopeptidase activity of partially and fully engorged female tissues
Figure 1 shows the filtration profile of soluble proteins extracted from partially and fully engorged female fat body (A, B), gut (C, D), salivary gland (E, F), ovary (G, H) and purified VTDCE (I). Protein patterns differed, depending on the tissue studied, although a major common peak (at 9 ml) was observed in all tissues. Cysteine endopeptidase patterns were more consistent. In the fat body, salivary gland, and ovary, cysteine endopeptidase activity eluted at ca. 11 ml, while in gut it eluted at 13·5 ml.

Fig. 1. Gel filtration fractioning profile of soluble proteins from partially (upper panels A, C, E, G) and fully (lower panels B, D, F, H) engorged female tissues. Panel I shows the gel filtration profile of VTDCE purified from eggs (100 μg). Protein loaded from each tissue was: (A) partially engorged female fat body, 800 μg; (B) fully engorged female fat body, 390 μg; (C) partially engorged female gut, 800 μg; (D) fully engorged female gut, 800 μg; (E) partially engorged female salivary gland, 460 μg; (F) fully engorged female salivary gland, 526 μg; (G) partially engorged female ovary, 800 μg; (H) fully engorged female ovary, 800 μg. Protein elution (A 280 nm –––); proteolytic activity (U - - -•- - -).
Major differences between protein profiles from fully and partially engorged female tissues were observed in gut (Fig. 1C and D) and ovary (Fig. 1G and H). In fully engorged gut, 3 major groups of proteins elute at 9, 11 and 16 ml and only the 1st peak co-eluted with one from the earlier life stage (partially engorged, Fig. 1C and D). Partially engorged female ovary proteins that eluted between 14 and 18 ml (Fig. 1G) were almost non-existent in the fully engorged female (Fig. 1H). The fully engorged gut cysteine endopeptidase activity eluted in 2 distinct peaks, one at the same volume observed in partially engorged females (13·5 ml) and a new one at 11·5 ml, suggesting stage-specific changes (Fig. 1D). In fully engorged female ovaries, in addition to the same peak observed in partially engorged females (eluting at 10·5 ml), 2 new small activity peaks were observed, eluting at 18·5 and 24·5 ml (Fig. 1H). Similarity among the purified VTDCE and tissue extract peptidase activity-elution profiles (Fig. 1I) indicates VTDCE is present in all tick tissues investigated.
Enzyme activity profile in egg and larva
Egg cysteine endopeptidase eluted from gel filtration chromatography as a single major peak (at 9·5 ml) and a minor peak detectable in a single fraction at 12·5 ml (Fig. 2A). Larval cysteine endopeptidase activity also eluted with 1 major and 1 minor peak, but the major peak eluted at 12·5 ml and the minor peak eluted at 9·5 ml (Fig. 2B). This shows that enzymatic profiles were once again different across life stages.
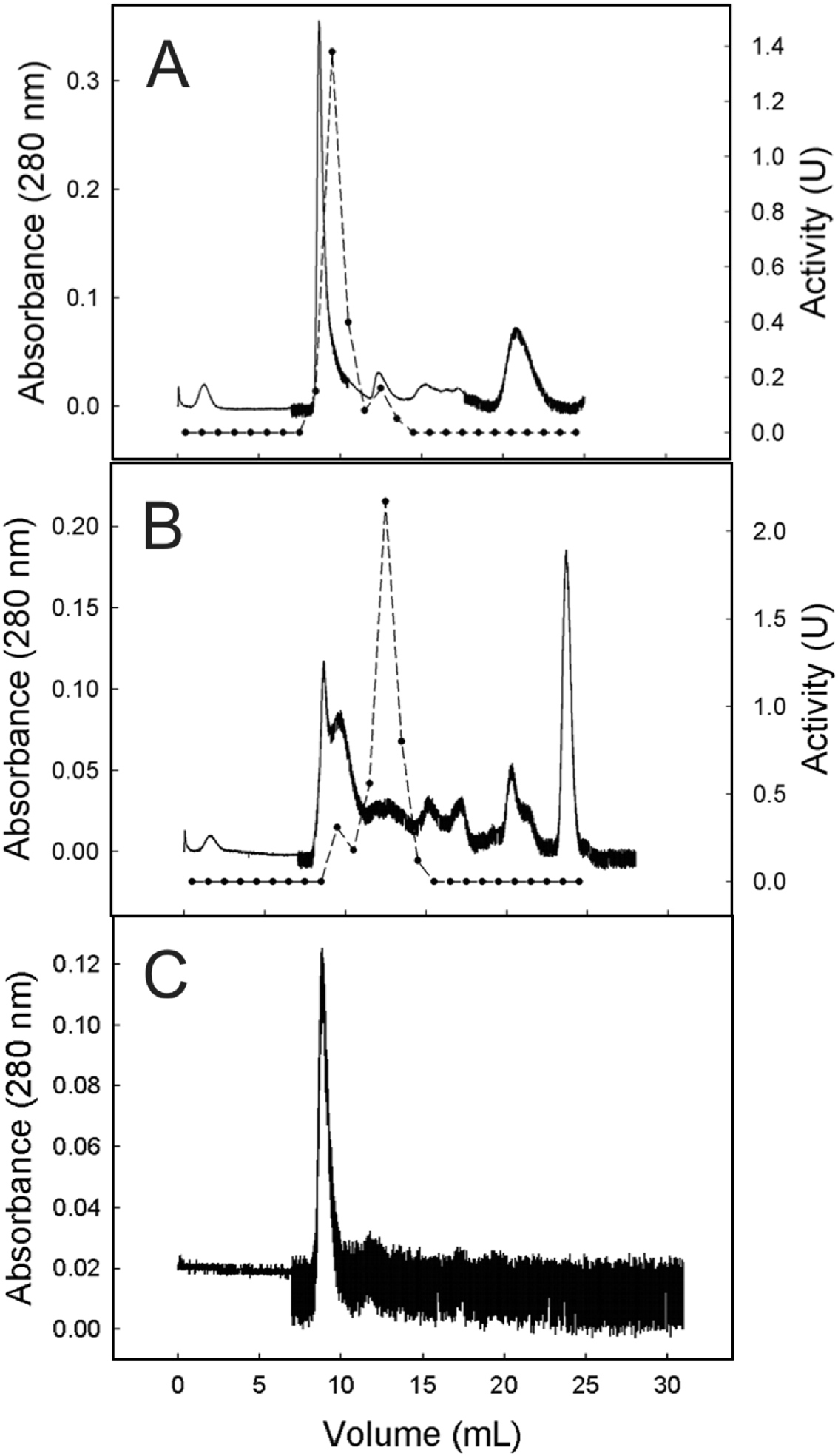
Fig. 2. Gel-filtration analysis of egg and larva protein extracts and purified vitellin. Samples were applied onto a Superose 12 column in sodium phosphate buffer (10 mm, pH 7·0) at 0·5 ml/min in an FPLC system. Protein loaded from each sample was: (A) egg extract, 3·6 mg; (B) larva extract, 4·1 mg; (C) purified Vt, 750 μg. For A and B, proteolytic activity of fractions was tested on a fluorogenic substrate (N-CBz-Phe-Arg-MCA) at 37°C in sodium citrate/sodium phosphate buffer (pH 3·5) and 10 mm DTT. Protein elution (A 280 nm –––); proteolytic activity (U - - -•- - -).
Gel filtration analysis of egg and larval extracts showed Vt eluting at the major peak of protein (9·5 ml; Fig. 2). However, in the larva, Vt seems to be degraded and proteins of lower molecular weight, or Vt fragments, were detected. Here, Vt identification was done based on (i) tick Vt property of the contained heme (Sonenshine, Reference Sonenshine1991), which confers a brownish colour to the sample; (ii) similarity to the standard of purified Vt (Fig. 2C) and (iii) qualitative dot-blot using anti-Vt antibodies.
VTDCE and Vt distribution during tick development
VTDCE and Vt distribution across tick development stages were investigated by Western blot (Fig. 3). Analysis with anti-Vt showed different Vt polypeptides present in all samples tested: ovaries, eggs from different days, young and old larvae (Fig. 3B). The presence of VTDCE was verified (i) in ovaries of females recently detached from the host (Fig. 3B, ovary 1); (ii) in ovaries of females 3 days after detachment (Fig. 3B, ovary 3), (iii) during the embryonic development (1-day-old eggs, 3-day-old eggs, 7-day-old eggs, 12-day-old eggs, 20-day-old eggs); (iv) in young larvae (5-day-old larvae), and (v) in old unfed larvae (20-day-old larvae). In addition to the enzyme, proteins of high molecular weight, similar to those recognized by anti-Vt, were also recognized by anti-VTDCE antibodies prepared from a checked pure VTDCE preparation (Fig. 3C). This suggests that VTDCE binds to different Vt polypeptides. Therefore, this protein interaction was further investigated.

Fig. 3. VTDCE and Vt immunolocalization in different developmental stages of Rhipicephalus microplus. Extracts of 1-day-old eggs (1), 3-day-old eggs (3), 7-day-old eggs (7), 12-day-old eggs (12), 20-day-old eggs (20), 5-day-old larvae (5), 20-day-old larvae (20), ovary of fully engorged female 1 day after detachment (1) and 3 days after detachment (3) were separated by SDS-PAGE (A), transferred to nitrocellulose and probed with anti-Vt (B) and anti-VTDCE (C). Molecular weights in kDa are shown. Replicate membranes probed with pre-immune serum showed no reactivity.
VTDCE-Vt association
The capacity of VTDCE to bind to Vt was verified using 125I-VTDCE and membrane-fixed Vt in a radio-binding assay. Figure 4 shows that VTDCE binds to Vt in a dose-dependent manner (Fig. 4A). The enzyme was able to bind to Vt also in the presence of leupeptin (a cysteine endopeptidase inhibitor) (Fig. 4B). This association was shown to be specific, since the binding to immobilized Vt was reduced by adding an excess of soluble Vt (in the presence or absence of leupeptin; Fig. 4B).

Fig. 4. VTDCE-vitellin binding. The association of soluble 125I-VTDCE with Vt (20 μg), fixed on nitrocellulose membrane, was tested (see Materials and Methods section). (A) Binding assay performed in the presence of different amounts of 125I-VTDCE. (B) Vt (20 μg, fixed on membrane) was incubated with: (1) 1 μg 125I-VTDCE; (2) 1 μg 125I-VTDCE + 100 μ m leupeptin; (3) 1 μg 125I-VTDCE + 1 mg Vt (in solution); (4) 1 μg 125I-VTDCE + 1 mg Vt (in solution) + 100 μ m leupeptin. Results are expressed as total bound 125I-VTDCE, and are means±s.d. Different letters above bars denote statistically significant differences for P<0·05.
VTDCE localization in fully engorged female guts and ovary
Immunofluorescence analysis of R. microplus female gut sections showed the presence of VTDCE in basophilic cells. Additionally, an intense signal was observed in the basal lamina (Fig. 5, panel I). In the ovary, labelling was observed in the pedicel cells, oocyte cytosol, chorium, and basal lamina (Fig. 5 panel II). The region close to the germinal vesicle was also labelled (Fig. 5, panel II A, B). No signal was observed in yolk granules and in controls with non-immune serum (Fig. 5, panel I-D, panel II-D).

Fig. 5. VTDCE localization by immunofluorescence in Rhipicephalus microplus gut and ovary. Fully engorged females were dissected on the 3rd day after a bloodmeal and tissues reacted with anti-VTDCE as described in the Materials and Methods section. Gut tissue showed a stronger signal in the basal lamina (BL; Panel I, A and B) contrasting with the signal in the basophilic cells (asterisk; Panel I, C). Scale bar = 10 μm. Panel II, ovary thin sections presenting a strong labelling at the oocyte cytosol and basal lamina/chorium (CO) (Panel II, A and B), and also at the laminal region of the pedicel cells (PC) (Panel II, C); yolk granules (asterisk) do not present a significant VTDCE signal. Scale bar = 20 μm. No labelling was observed in controls with non-immune serum (Panel I and II letter D). I, II, III, IV – oocyte developmental stage; gv – germinal vesicle.
Using gold-immunohistochemistry in electron microscopy, we observed that VTDCE is clearly located in typical material-exchanging areas in the ovary (Fig. 6). The enzyme was immunolocalized at the membrane of vesicles present in the pedicel cells, which appear highly decorated with gold particles (Fig. 6A), and in the ovary basal region in close contact with haemocoel (Fig. 6B).

Fig. 6. VTDCE distribution in Rhipicephalus microplus ovary by immunoelectron micrograph. Fully engorged females were dissected on the 3rd day after a bloodmeal and ovary tissue reacted with anti-VTDCE as described in the Materials and Methods section. The enzyme was immunolocalized in the pedicel cell vesicle membrane (A) and in ovary basal region (B). Scale bar=2 μm. V – pedicel cell vesicle; Hc – haemocoel; BL – basal lamina.
A possible endogenous VTDCE inhibitor
Surprisingly, a 90-fold increase in haemolymph cysteine endopeptidase activity was observed after gel filtration fractionation (Table 2 and Fig. 7). This finding suggests that tick haemolymph contains a cysteine endopeptidase inhibitor. The presence of an inhibitor was investigated by incubating 10 μl of each gel filtration fraction with purified egg VTDCE, before substrate addition. A cysteine endopeptidase inhibitory activity eluted from gel filtration column between 13 and 15 ml (Fig. 7A). These pooled fractions (MW between 34 and 11 kDa) inhibited VTDCE in a dose-dependent manner (Fig. 7B).

Fig. 7. (A) Gel-filtration fractioning profile of engorged female haemolymph. Haemolymph (1:5 in PBS; 200 μl) was applied onto a Superose 12 column in sodium phosphate buffer (10 mm, pH 7·0) at 0·5 ml/min in an FPLC system. Proteolytic activity of fractions tested upon the fluorogenic substrate N-CBz-Phe-Arg-MCA is shown as U - - -•- - -; the black bar shows the cysteine endopeptidase inhibitor activity. Protein elution (A 280 nm –––). (B) Dose-dependent inhibition of VTDCE (1 μg) by the partly purified haemolymph cysteine endopeptidase inhibitor (gel filtration pool). Small letters indicate volumes of inhibitor fraction used: (a) control, (b) 2 μl, (c) 5 μl, (d) 8 μl.
Table 2. Fractionation of Rhipicephalus microplus engorged female haemolymph by gel filtration chromatography and analysis of cysteine endopeptidase activity profile

U, Relative fluorescence units/sec.
DISCUSSION
Previous studies demonstrated the presence of acidic peptidase activity in R. microplus gut, ovary, egg and larva (Renard et al. Reference Renard, Lara, de Cardoso, Miguens, Dansa-Petretski, Termignoni and Masuda2002; Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003; Estrela et al. Reference Estrela, Seixas and Termignoni2007). The egg peptidase (vitellin-degrading cysteine endopeptidase; VTDCE) was purified and characterized as a cathepsin-L-like enzyme, active at acidic pH and totally inhibited by E-64 (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). Here, we investigated the distribution of VTDCE in tissues and haemolymph of the cattle tick R. microplus female and the association of VTDCE with its natural substrate vitellin.
Data presented here show that cysteine endopeptidase activity is widely distributed in ticks, being found in engorged female fat body, ovary, gut, salivary glands and haemolymph. Tissues differ as regards stage-specific protein patterns. Yet, the enzyme profile has a constant pattern in all tissues, with a cysteine endopeptidase activity peak eluting at 11·5 ml. Peculiar profiles were observed in the egg and larva. Most egg peptidase activity elutes at 9·5 ml (≅244 kDa) followed by a small activity peak eluting at 12·5 ml (≅45 kDa). In the larva, on the other hand, this 9·5 ml activity peak is reduced and most cysteine endopeptidase activity elutes at 12·5 ml, which indicates stage-specific changes in the enzyme profile. Active egg extract fractions reacted positively to anti-VTDCE antibodies and co-eluted with Vt. Thus, variations in VTDCE gel filtration elution profile can be correlated with its association with multiple Vt subunits. A Vt-VTDCE association has been previously suggested (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003), and is well observed in Western blot analysis localizing VTDCE in the ovary, eggs and larvae. During embryogenesis, VTDCE is observed in a low MW form corresponding to a free enzyme, and several high molecular weight forms, which are also recognized by anti-Vt antibodies and correspond to VTDCE associated with different Vt subunits and/or Vt partial digestion products. This kind of association that alters the apparent molecular weight was previously described for other arthropod enzymes and could be related to Vt-degradation control (Giorgi et al. Reference Giorgi, Bradley and Nordin1999).
The VTDCE purification protocol includes an autolysis step at acidic pH in which Vt is hydrolysed by VTDCE and products of degradation precipitate while the enzyme remains soluble (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). Actually, it is hard to establish unambiguously the MW of pure VTDCE. Earlier studies described the presence of a 22- and a 17-kDa protein (Seixas et al. Reference Seixas, Dos Santos, Velloso, da Silva, Masuda, Horn and Termignoni2003). However, these characteristics may change as a result of further protein processing during autolysis, and only 1 subunit of low MW remains, suggesting that the 22-kDa protein corresponded to a distinct processing stage. Indeed, difficulties in isolation, purification and characterization of cathepsin-L, due to autolysis, are frequently observed in final purification steps (Cristofoletti et al. Reference Cristofoletti, Ribeiro and Terra2005). Concerning VTDCE, this problem is even more relevant, since autolysis is an essential step to digest Vt, and enzymes must subsequently be maintained in an acid medium to avoid eventual inactivation.
Experiments using 125I-radio-isotope labelled VTDCE confirm that VTDCE associates with Vt, its natural substrate. In fact, the results obtained here show that purified VTDCE is able to associate with Vt in a dose-dependent manner. It is important to stress the observation that the VTDCE-Vt association can occur regardless of the presence of a cysteine endopeptidase inhibitor (leupeptin). This finding proposes a possible mechanism that allows the co-existence of VTDCE and Vg/Vt with no premature Vg/Vt polypeptide cleavage during vitellogenesis. Association between Vg and a cysteine endopeptidase has previously been described in Blatella germanica (Yin et al. Reference Yin, Nordin, Lucches and Giorgi2001). In this cockroach, the enzyme co-localizes with Vg in fat body granules, suggesting that the enzyme and Vt are secreted together by this tissue, in order to be transported through the haemolymph and incorporated into the oocytes.
Some considerations help to understand the significance of Vg-VTDCE interaction. First, Vg may act as a potential inhibitor, maintaining the enzyme as a zymogen throughout vitellogenesis, as suggested in studies on the moth, Hemileuca oliviae and the cockroach, Blattella germanica (Kucera and Turner, Reference Kucera and Turner1981; Yin et al. Reference Yin, Nordin, Lucches and Giorgi2001). Second, enzyme activation may be delayed until pro-protease and Vg are fully dissociated from each other due to acidification of the yolk granules (Nordin et al. Reference Nordin, Beaudoin and Liu1990). Finally, a physiological inhibitor may be part of this complex, acting to control enzyme activity (Kucera and Turner, Reference Kucera and Turner1981). It is also important to consider that co-packaging of enzyme to Vg/Vt responds to the requirements of a general reproductive strategy, whereby the developing embryo is provided with a reserve protein and an associated enzyme able to mobilize this protein (Fagotto, Reference Fagotto1990; Giorgi et al. Reference Giorgi, Bradley and Nordin1999).
The VTDCE biological function can be clarified by the elucidation of its distribution in tick tissues. In the gut, the enzyme is present in basophilic cells and at high levels in the basal lamina. Gut basophilic cells in female ticks are derived from basal remnants of type 2 secretory cells (Agbede and Kemp, Reference Agbede and Kemp1987). The cytoplasm of these cells is filled with well-organized rough endoplasmic reticula, Golgi complexes and secretory granules, evidencing their synthetic capacity. Moreover, the contact of this cell with the underlying haemolymph is extended by basal labyrinth infoldings that facilitates material exchange. During the final rapid phase of engorgement, basophilic cells seem to take on an active role in water transport across the gut wall but, subsequent to this phase, its rough endoplasmic reticulum cisternae are re-organized and resume a secretory role. After the fat body, the gut epithelium is the second source of Vt synthesis in ticks (Coons et al. Reference Coons, Tarnowski and Ourth1982). Another enzyme involved in Vt digestion in eggs, the Boophilus aspartic pro-cathepsin (BYC), is also synthesized in the gut and fat body cells (Logullo et al. Reference Logullo, da Silva Vaz, Sorgine, Paiva-Silva, Faria, Zingali, De Lima, Abreu, Oliveira, Alves, Masuda, Gonzales, Masuda and Oliveira1998; Nascimento-Silva et al. Reference Nascimento-Silva, Leal, Daffre, Juliano, da Silva Vaz, Paiva-Silva, Oliveira and Sorgine2008). Thus, the distribution profile of VTDCE in the gut suggests that it can have the same synthesis site as Vt and BYC and it is very plausible that it is transported to the ovary through the haemolymph. The stronger signal in the basal lamina, as compared to basophilic cells, makes sense considering that the analysis was done on the 3rd day after detachment, the protein export stage.
In the ovary, VTDCE is located in the basal region and in membranes of vesicles present in ovarian pedicel cells. The pedicel is a structure resulting from the proliferation of ovary wall epithelial cells, which attach oocytes to the ovary (Till, Reference Till1961; Ricardo et al. Reference Ricardo, de Oliveira, Bechara and Mathias2007). The R. microplus ovary is devoid of nurse cells, a characteristic of panoistic ovaries (Saito et al. Reference Saito, Bechara, Nunes, de Oliveira, Denardi and Mathias2005). Thus, pedicel cells act producing and/or incorporating proteins from the haemolymph, which will then be transported to the oocytes. In Amblyomma triste, proteins are accumulated in the regions of contact among pedicel cells and in the pedicel cell/oocyte interface, showing that protein exchange among these cells does in fact occur (Ricardo et al. Reference Ricardo, de Oliveira, Bechara and Mathias2007). Therefore, the pedicel participates as an active structure that supplies yolk components for oocytes. In R. microplus oocytes, VTDCE is located at the basal lamina/chorium, in the cytoplasm and close to the germinal vesicle. No labelling was observed in the yolk granule, similar to the Aedes aegypti yolk-degrading cysteine endopeptidase (vitellin cathepsin B; VCB), which is present in a narrow layer between yolk and yolk body membrane in developing oocytes (Cho et al. Reference Cho, Tsao, Hays, Walter, Chen, Snigirevskaya and Raikhel1999). Therefore, the immunolocalization results, together with the presence of VTDCE in the haemolymph and other tissues, such as gut and fat body, suggest VTDCE could have an extra-ovarian origin, and could be internalized into the oocytes through the pedicel cells. However, a concomitant ovarian synthesis cannot be discarded.
Cysteine endopeptidase is a widely distributed group of enzymes with a broad range of putative functions working in an evolutionarily conserved network present in endo/lysosomal systems in most eukaryotes, including ticks. Biochemical screenings demonstrate that multiple similar activities are present in R. microplus (Mendiola et al. Reference Mendiola, Alonso, Marquetti and Finlay1996) and Ixodes ricinus (Sojka et al. Reference Sojka, Franta, Horn, Hajdusek, Caffrey, Mares and Kopacek2008; Horn et al. Reference Horn, Nussbaumerová, Sanda, Kovárová, Srba, Franta, Sojka, Bogyo, Caffrey, Kopácek and Mares2009). So we cannot dismiss the possibility that the antibody used here can recognize isoforms of cysteine endopeptidase. However, as this serum was prepared with a pure preparation of VTDCE, one can conclude that this protein is responsible for the major signal observed.
As mentioned above, cysteine endopeptidase activity is present in female haemolymph. This activity was found in partially and in fully engorged females, from the 1st day after detachment up to the 3rd day, when oviposition starts. The highest activity occurs in recently detached fully engorged female haemolymph. Interestingly, cysteine endopeptidase activity decreases gradually in the 2-day period before oviposition onset. The high activity in fully engorged female haemolymph on the 1st day after detachment could result from active synthesis by tissues such the gut, which secretes the enzyme into the haemolymph. In addition, the activity decrease in the following days could result from enzyme uptake by the ovary.
The haemolymph cysteine endopeptidase activity increases almost 100-fold after gel filtration chromatography, indicating the presence of a cysteine endopeptidase inhibitor. The existence of such an inhibitor was confirmed by inhibition of cysteine endopeptidase activity by whole haemolymph, as well as some gel filtration fractions. Indeed, a partly purified inhibitor preparation (pooled gel-filtration fractions) inhibits egg-VTDCE in a dose-dependent manner. This inhibitor would prevent premature degradation of Vt during transport through the haemolymph, as well as in oocytes. It has been suggested that the latency of yolk granules is reinforced by other regulatory mechanisms (Fagotto, Reference Fagotto1995). Actually, VTDCE and most lysosomal enzymes are active under mild acidic conditions and display residual activity even at pHs close to neutrality, while digestion of Vt polypeptides only occurs at quite low pHs. Thus, controlling the luminal pH alone is probably not enough to avoid all premature yolk granule proteolysis during the long period of oogenesis (Fagotto, Reference Fagotto1995). Indeed, Bmcystatin, a cystatin, was cloned from a R. microplus fat body cDNA library and the recombinant protein inhibits VTDCE (Lima et al. Reference Lima, Sasaki and Tanaka2006). The VTDCE inhibitory activity shown here eluted from gel filtration with a molecular mass between 34 kDa and 11 kDa, and the recombinant Bmcystatin has a molecular mass of 11 kDa (Lima et al. Reference Lima, Sasaki and Tanaka2006). It is possible that the cysteine endopeptidase inhibitory activity found in R. microplus haemolymph is the same Bmcystatin. Besides the well-described role of cystatins in haematophagy, these new data showing the presence of an active cysteine endopetidase inhibitor in R. microplus haemolymph are the first demonstration relating a cysteine endopeptidase inhibitor to vitellogenesis control.
In conclusion, VTDCE is a vitellin-associated enzyme, present in R. microplus eggs, larvae, partially and fully engorged females with a role in embryogenesis. Moreover, as VTDCE is the most active of all vitellin-degrading enzymes so far described in R. microplus eggs (Seixas et al. Reference Seixas, Leal, Nascimento-Silva, Masuda, Termignoni and da Silva Vaz2008) the strict regulation of its activity seems to play a key role in the control of yolk mobilization, ensuring nutrient provision, at the right time, to the developing embryo.
ACKNOWLEDGMENTS
We thank Dr Ulysses Lins (Instituto de Microbiologia, UFRJ) for the use of the fluorescence microscope and Dr Wanderley de Souza (Instituto de Biofísica, UFRJ) for the use of the transmission electron microscope. We are very grateful to Professor Joseph C. Polacco for several suggestions.
FINANCIAL SUPPORT
This work was supported by grants from Conselho Nacional de Pesquisa (CNPq), Programa de Núcleos de Excelência (PRONEX), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (INCT-EM).











