INTRODUCTION
Trypanosoma evansi is a flagellate parasite of domestic and wild animals (Silva et al. Reference Silva, Seidl, Ramirez and Dávila2002), but it rarely parasitizes humans (Joshi et al. Reference Joshi, Shegokar, Powar, Herder, Katti, Salkar, Dani, Bhargava, Jannin and Truc2005). This protozoan has a wide geographical distribution, which allows it to be found in countries of Africa, Europe, Asia and Central and South America (Silva et al. 2002). In Brazil, the most common drug used to treat trypanosomosis in domestic animals is diminazene aceturate (DMZ), which has trypanocidal, bactericidal and babesicidal activity, of which manufacturers indicate a single dose of 3·5 mg kg−1 for horses, cattle, sheep and dogs, resulting in suppression of clinical signs within 24 h (Brender et al. Reference Brender, Pugh, Bywater and Jenkins1991). The mechanism of action of DMZ is not well understood, but it is known that it interferes with anaerobic glycolysis and synthesis of DNA and RNA of the parasites, as well as in the activity of enzymes, such as topoisomerases, nucleases and Ca++-ATPase (Peregrine, Reference Peregrine1994).
DMZ is capable of providing an elimination of trypanosomes in the bloodstream a few hours after administration (Peregrine and Mamman, Reference Peregrine and Mamman1993). However, it has no curative efficacy in many clinical situations, with relapses of parasitaemia sometimes occurring. This situation usually happens when the trypanosomes pass through the blood–brain barrier, finding accommodation in the central nervous system (CNS), a refuge area for T. evansi during the residual period (21 days) of the drug in the circulation. DMZ does not cross the blood–brain barrier in amounts sufficient to eliminate all the parasites (Lonsdale-Eccles and Grab, Reference Lonsdale-Eccles and Grab2002; Masocha et al. Reference Masocha, Rottenberg and Kristensson2007).
In order to seek alternative treatments, nanotechnology is a science that is driving research in the development of new pharmaceutical technologies. Among the nanostructure systems are the liposomes, which are vesicles that consist of one or more phospholipid bilayers that surround an aqueous compartment, serving as drug carriers, biomolecules or diagnostic agents (Batista, Reference Batista2007). They are used as an alternative in reducing systemic toxicity and cytotoxicity to normal cells, side effects associated with chemotherapy, since they are able to change the pharmacokinetics and biodistribution of antineoplastic drugs (Immordino et al. Reference Immordino, Brusa, Arpicco, Stella, Dosio and Cattel2003; Mamot et al. Reference Mamot, Drummond, Hong, Kirpotin and Park2003).
The treatment of trypanosomosis is based on specific drugs such as suramin, diminazene, quinapyramine, melarsoprol, homidium and isometamidium. In South America, the availability of trypanocidal compounds is limited, since some of them are not commercially available. In Brazil and Argentina, for example, only diminazene aceturate is marketed (Dávila et al. 1999). However, it is well known that it is sometimes ineffective, with recurrence of parasitemia in many of the animals infected with T. evansi and treated with DMZ. Therefore, this study aimed to develop a liposome formulation containing diminazene aceturate, as well as to evaluate its trypanocidal activity as a nanoencapsulated product, through the test of susceptibility in vitro and in vivo.
MATERIALS AND METHODS
Reagents
The reagents used for the preparation of culture medium, except for antibiotics and DMZ, were obtained from Sigma Chemical Co (St. Louis, MO, USA). DMZ (Ganazeg®) was purchased from Novartis, São Paulo, Brazil. Soybean phosphatidylcholine (100%) was obtained from Idealfarma (São Paulo, Brazil), while polysorbate 80 was supplied by Henrifarma (São Paulo, Brazil) and cholesterol was donated by Cristália (São Paulo, Brazil). Potassium phosphate monobasic and sodium phosphate dibasic were purchased from Vetec (Rio de Janeiro, Brazil), and ethyl acetate was obtained from F. Maia (São Paulo, Brazil). High performance liquid chromatography (HPLC) grade methanol and acetonitrile were acquired from Tedia (São Paulo, Brazil).
Liposomes preparation
Liposomes were produced in batches of 100 mL, in triplicate, employing the method of evaporation in reverse phase (Mertins et al. Reference Mertins, Sebben, Pohlmann and Silveira2005), according to the composition shown in Table 1. The phospholipid (phosphatidylcholine from soybean lecithin), cholesterol and alpha-tocopherol were dissolved in ethyl acetate with the aid of ultrasound. Later, an aqueous solution containing diminazene aceturate and polysorbate 80 was slowly added to the organic phase, leading to formation of two phases. This dispersion was subjected to ultrasonication aiming at the formation of reverse micelles which were concentrated by evaporation at reduced pressure until the obtaining of an organogel. The dispersion was subjected to ultrasonication for 5 min. In this method, it is necessary to add an initial aqueous portion to the formation of reverse micelles, and another portion in the final step for forming the liposomal vesicles. The reverse micelles are formed immediately when phosphatidylcholine is dissolved in a non-polar medium, due to interactions between the polar heads of phosphatidylcholine, which can interact with each other or with the water molecules after the first water addition. The subsequent addition leads to a unidimensional growth of spherical reverse micelles in long cylinders called worm-like micelles due to the hydrogen bonds between phosphate groups of phosphatidylcholine and water. After reaching a maximum length, extended micelles begin a process of aggregation forming a thermoreversible organogel. In the reverse phase evaporation method, after evaporation of the organic solvent, all other components are in the organogel. Dispersion of the organogel in pure water under shaking leads to nanovesicle formation.
Table 1. Evaluation of composition of liposomes (n = 3)

Finally, the remaining aqueous solution containing surfactant and the drug were added to the organogel and, under high agitation on a rotary evaporator, liposomes were formed. The size distribution of the vesicles was standardized through the technique of high pressure homogenization (two cycles, at 250 bar) (High-Pressure Homogenizer, Panda 2K, Niro-Soavi, Italy) followed by a sequential filtration on cellulose acetate membranes with porosity of 0·45 and 0·22 μm (Milex GV PVDF, Millipore Ireland Ltd, Ireland). This formulation was called DMZ-L. Blank liposomes were also prepared according to the same procedure described above (however, without DMZ in their formulation) as a parameter of comparison. This formulation was called B-L. All batches were stored in the dark and temperature controlled to maintain the stability of the formulations.
Drug content and encapsulation efficiency
Drug content was assayed by HPLC according to a previously described analytical method (Atsriku et al. Reference Atsriku, Watso, Tettey, Grant and Skellern2002). The chromatography system consisted of a Luna RP-18 column (250×4, 6 mm, 5 μm, Phenomenex, Torrance, USA) and a Shimadzu Instrument (LC-10AVP Pump, UV-Vis SPD-10AVP Module, LC Solution software, Shimadzu, Tokyo, Japan). The mobile phase at flow rate of 0·8 mL min −1 consisted of acetonitrile-methanol and ammonium formate buffer (20 mm, pH 4·0) at proportion 10:10:80% (v/v/v). The sample was prepared dissolving the liposomes (50 μL) in 5 mL of mobile phase before analysis (dilution factor of 100 times). The volume injected was 20 μL and the drug was detected at 254 nm. The method was linear (r 2 = 0·9986) in the range of 5–20 μg mL−1, accurate (recovery: 100±2%) and precise (R.S.D.: 1·10% for repeatability and 0·06% for intermediate precision). The specificity was tested in the presence of liposomal formulation components (without the drug), where the results demonstrated that these factors did not have influence on the drug assay. Figure 1 shows a chromatogram obtained from the standard solution at the concentration of 10·00 μg mL−1. Encapsulation efficiency was determined by an ultrafiltration-centrifugation technique. Free drug was separated from liposomes using a filter unit (Ultrafree- MC® 10 000 MW, Millipore, Bedford, USA) submitted to a centrifugation at 5000 rpm for 10 min. Afterwards, the drug content was determined in the ultrafiltrate by HPLC. Encapsulation efficiency (%) was calculated by the difference between the total and free drug concentrations.
Fig. 1. Chromatogram obtained from standard solution at the concentration of 10 μg mL−1.
Particle size analysis
Particle size and Span values were firstly determined by laser diffraction (Mastersizer, Malvern Instruments, Worcestershire, UK) over the volume of the particles (D4,3). Span values are a measure of the width of the size distribution. Afterwards, the mean particle size and polydispersity index (PDI) were determined by photon correlation spectroscopy (PCS) (Zetasizer® Nanoseries, Malvern Instruments, Worcestershire, UK) after dilution (1:500 v/v) of the liposome dispersion with purified water. pH values were determined by potentiometry directly in the dispersion using a calibrated potentiometer (Denver UB-10, Santo André, Brazil).
Trypanosoma evansi isolate
Trypanosoma evansi was originally isolated from a dog naturally infected (Colpo et al. Reference Colpo, Monteiro, Stainki, Colpo and Henriques2005), and kept cryopreserved in a laboratory environment. Initially, two Wistar rats (R1 and R2) were infected intraperitoneally with blood (cryopreserved in liquid nitrogen) containing 106 parasites animal−1 for in vitro tests.
Culture medium
Culture medium for T. evansi was formulated according to the method of Baltz et al. (1985), modified by Dalla Rosa et al. (2013). Once prepared, it was stored under refrigeration (10 °C) until the onset of the experiment. For that, 10 mL of medium was separated in a test-tube, with addition of 1 μL mL−1 of hypoxanthine 50 mm (dissolved in NaOH, 0·1 M) and 2 μL mL−1 of 2-mercaptoethanol 1·2 mm. After this procedure, the enriched culture medium was taken to a laboratory stove (37 °C at 5% of CO2), where it was equilibrated for 2 h prior to testing.
Obtaining trypanosomes
When the R1 rat was under high parasitaemia (107 trypanosomes μL−1), it was anaesthetized with isoflurane inside an anaesthetic chamber to perform collection of blood samples EDTA (ethylenediamine tetraacetic acid 10%). 200 μL of blood was employed for trypanosomes separation, diluted (1 v/v) in 200 μL of stabilized culture medium. It was stored in microtubes and centrifuged at 400 g during 10 min. The supernatant, containing the parasites and few red blood cells, was removed after centrifugation, with the protozoan collected and placed on the culture medium. The count of trypomastigotes was then performed using a Neubauer chamber, based on the methodology applied by Gillingwater et al. (Reference Gillingwater, Kumar, Mohamed, Arafa, Stephens, Boykin, Tidwell and Brun2010) with modifications.
Bioassays in vitro
Culture medium with the parasites was distributed in microtitre plates (270 μL well−1). Later, 0·25, 0·5, 1, 2 and 3 μg mL−1 of liposomal diminazene aceturate (L-DMZ) and conventional diminazene aceturate (C-DMZ) – unencapsulated, were added. Conventional C-DMZ was prepared by the dilution of the commercial drug (Ganazeg® – Novartis, Barueri, SP, Brazil) in dimethylsulphoxide (DMSO) (Gomes-Cardoso et al. Reference Gomes-Cardoso, Echevarria, Aguiar-Alves, Jansen and Leon1999). To validate the tests with C-DMZ, a control group was used (10 μL DMSO). The control group to validate the test with the liposomal formulation (L-DMZ) was composed of lipid vesicles without diminazene aceturate, characterized as the blank liposomes (B-L). After 1, 3, 6 and 12 h from the onset of the experiment, the counting of living parasites was performed in a Neubauer chamber (Baltz et al. Reference Baltz, Baltz, Giroud and Crockett1985). All the tests were carried out in triplicate.
Animals
This study used 42 Wistar rats (Rattus norvegicus), males at 60 days of age and around 170 g of weight. The animals were kept in cages with four animals each in an experimental room with temperature, humidity (23 °C; 70% UR) and luminosity (12 h light/dark) controlled. They were fed with commercial rations and water ad libitum. A period of 10 days was set as the adaptation period, in which they were submitted to clinical evaluation and antiparasitic treatment (pyrantel pamoate and praziquantel). The procedure was approved by the Animal Welfare Committee of Ethics in Animal Experimentation of Federal University of Santa Maria (UFSM), number 041/2011.
Animal inoculation
Animals from groups B, C, D, E, F and G were inoculated intraperitoneally with 0·1 mL of blood (from a rat previously infected with T. evansi) diluted in saline solution at 37 °C. The inoculum was quantified in a Neubauer chamber, with a parasite density of 106 parasites μL−1.
Evaluation of infection
Parasitaemia was estimated daily by microscopic examination of smears. Each slide was mounted with blood collected from the tail vein (Da Silva et al. Reference Da Silva, Doyle and Monteiro2006), stained by the Romanowsky method, and visualized at a magnification of 1000×.
Treatments
The rats were divided into 7 groups of 6 animals each (A, B, C, D, E, F and G). The efficacy of liposomal diminazene aceturate (L-DMZ) was evaluated through longevity, when compared with the treatment with conventional diminazene aceturate (C-DMZ). Thus, Group A was composed of healthy or uninfected animals (negative control); Group B was used as a positive control (not-treated); in Group C the animals were infected and then treated with L-DMZ (3·5 mg kg−1); Group D was composed by animals infected and treated with C-DMZ (3·5 mg kg−1) in a single dose; In Group E, the infected animals were treated with L-DMZ during 5 days (3·5 mg kg−1 day−1); Group F represented the animals that were infected and treated with C-DMZ (following the same protocol); and in Group G, the infected animals were treated with blank liposomes (B-L) for 5 days. The same volume was used in groups E and F (1 mL).
The treatments started 24 h post-inoculation (pi), when the parasitaemia was estimated from 0 to 1 trypomastigotes/field. L-DMZ, C-DMZ and B-L were administered intraperitoneally.
Polymerase chain reaction (PCR) detection of T. evansi in brain and blood
Blood and brain samples were collected in animals of groups E and F, since the animals of these groups did not show trypanosomes in their blood smears on the 40th day pi. For this, the animals were anaesthetized with inhaled anaesthetic (isoflurane). Blood was taken by cardiac puncture and the following volumes were utilized: 1 mL was stored in microtubes with EDTA 10%. Blood and brain samples were preserved in ethanol (1:1), in order to perform PCR to detect the presence or absence of the parasite in the CNS (Ventura et al. Reference Ventura, Takeda, Silva, Nunes, Buck and Teixeira2002), indicating the effectiveness of the treatment.
Data analysis
The results of tests were analysed by ANOVA followed by Duncan test (P>0·05). Statistical analyses were performed using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Physicochemical characterization of liposomes
Liposome formulations were analysed by laser diffractometry in order to attest the presence of particles only at the nanoscale. Granulometric profiles of formulations containing or free of the DMZ are showed in Fig. 2. Mean particle size was 120±3 nm and 123±2 nm for DMZ-L and B-L, respectively. Span values were lower than 2 (0·88 and 0·92, respectively). Drug content assayed by HPLC was 1·02±0·22 mg mL−1. Encapsulation efficiency (%) calculated by the difference between the total and free drug concentrations was 53±10%. Further characterization was carried out to determine the particle size and PDI by PCS and pH. Results are presented in Table 2.

Fig. 2. Granulometric distribution of profiles obtained by laser diffractometry of (A) blank liposomes and (B) diminazene-loaded liposomes.
Table 2. Physicochemical characteristics of diminazene aceturate-loaded liposomes (DMZ-L) and blank liposomes (B-L) (n = 3).

In vitro assay
A dose-dependent effect of both formulations tested was reached, as shown in Fig. 3. However L-DMZ led to a greater mortality of trypanosomes in a dose-dependent effect when compared with C-DMZ, since at 1, 2 and 3 μg mL−1 L-DMZ was capable of killing all the parasites after 1 h of the assay (Fig. 3b). The same effect was not observed when equal concentrations of C-DMZ were used (Fig. 3a).
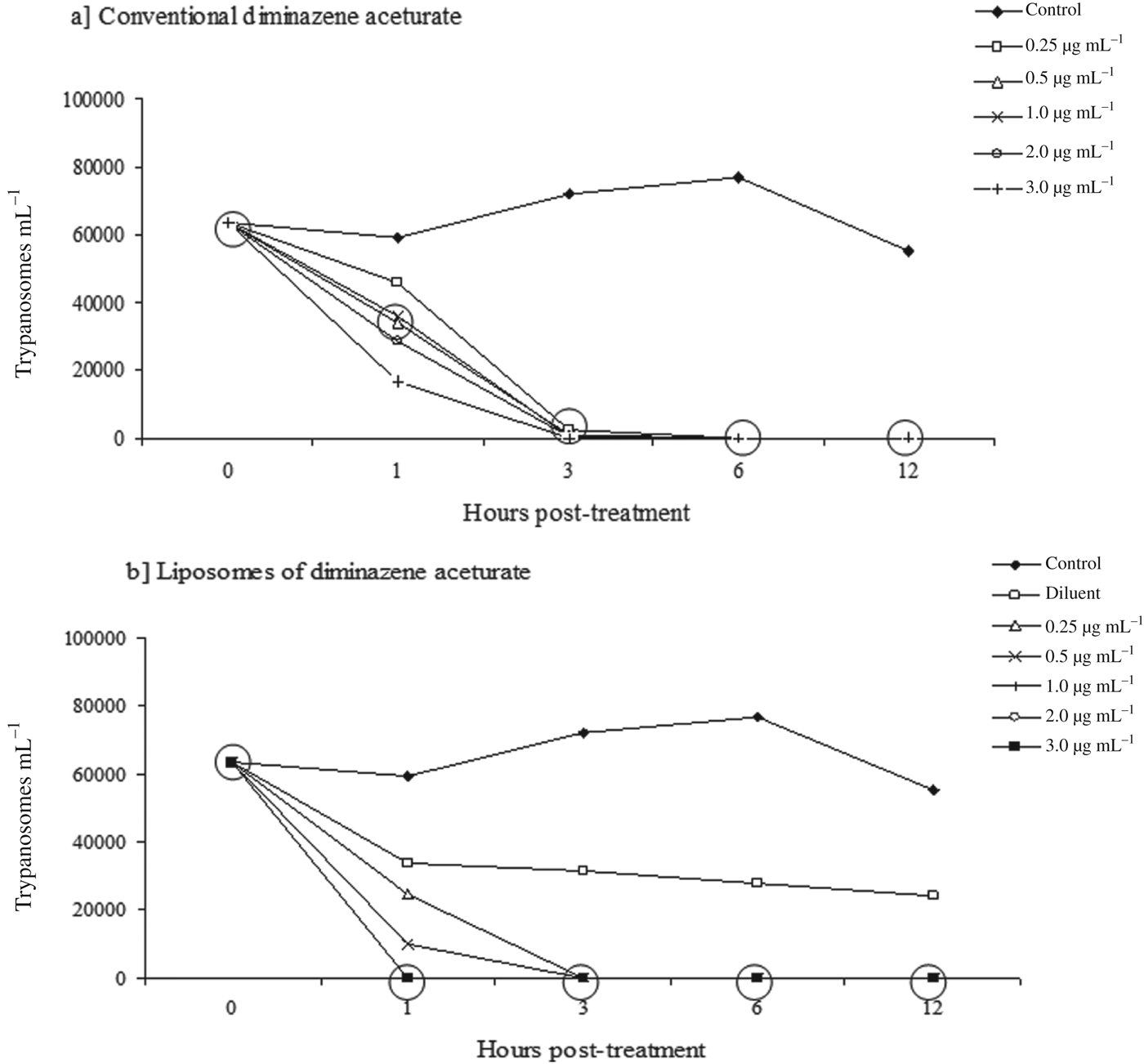
Fig. 3. Trypanocidal activity in culture medium of diminazene aceturate in its conventional (a) and liposomal (b) forms on Trypanosoma evansi. The analyses were performed at 1, 3, 6 and 12 h post treatment. In the same column, within the circle results not statistically different from each other in Duncan test (P>0·05).
Three hours after the onset of the experiment almost all the parasites subjected to C-DMZ were dead, exception at 0·25 μg mL−1. However, at 6 h of assay, the unencapsulated drug killed all trypanosomes, in comparison to the control group used for test validation, where the parasites were kept alive until 12 h (Fig. 3a).
After the same 3 h of assay, there were no live trypanosomes at all the concentrations of L-DMZ tested, when compared with the control group (Fig. 3b). When only the diluents (liposome dispersion) were used, there was an initial reduction of parasites living during the first hour of the experiment; however, this number was kept constant for 12 h, with a similar pattern as observed for the control group.
In vivo assay
Result of longevity and in vivo tests are shown in Table 3. A single dose of 3·5 mg kg−1 of liposomal and conventional diminazene aceturate (groups C and D) controlled the infection, but a recurrence of parasitaemia was observed 26 and 31 days later, respectively.
Table 3. Mean and standard deviation of the longevity, mortality and success of therapy using treatment with conventional diminizane aceturate (C-DMZ) and liposomal diminazene aceturate (L-DMZ) in rats experimentally infected with Trypanosoma evansi.

Means followed by same letters in the same column do not differ significantly in the Duncan test T (P<0·05). *Considered a therapeutic success for drug-treated rats that survived for 40 days and remained negative for the parasite by examination of their blood smears and PCR.
Treatment with L-DMZ and C-DMZ at a dose of 3·5 mg kg−1 for 5 consecutive days showed an absence of parasite movement during the experiment. Specific PCR assays from blood and brain of these animals were negative for the presence of T. evansi. Animals treated with the solution containing blank liposomes showed no statistical difference when compared with the infected rats (Group B).
DISCUSSION
There are many ongoing studies using nanotechnology, especially on development of drugs for treatment of diseases caused by microorganisms, and liposomes have been suggested as effective carriers of antiprotozoal drugs (Alving and Richards, Reference Alving and Richards1990). Liposomes composed of stearylamine/phosphatidylcholine (SA/PC) have shown cytolytic activity against Trypanosoma cruzi, Trypanosoma brucei and Toxoplasma gondii (Souto-Padrón et al. Reference Souto-Padrón, Carvalho, Chiari and Souza1984; Tachibana et al. Reference Tachibana, Yoshihara, Kaneda and Nakae1988).
During our study, liposomes containing diminazene aceturate were produced, presenting vesicles with monomodal particle and size distribution in the nanometer scale, according to analysis by laser diffraction. The exclusively nanometric population shows the suitability of the composition employed. The presence of micrometric clusters was not verified, suggesting that none of the components is above the concentration required. Moreover, the absence of micrometric particles suggests that the drug is associated with the liposomes and/or dissolved in the external medium, excluding the possibility of precipitation in the external medium.
The determination of the size of the vesicles through the technique of PCS is more accurate for the particles/vesicles in the nanometer scale. The results from these analyses support those obtained by laser diffraction, showing vesicles with an average diameter close to 100 nm and low index of polydispersity. This low value of PDI reflects the narrow distribution of vesicle sizes. The DMZ used in the formulation followed the therapeutic suggested value (1·0 mg mL−1) with low variation between batches, demonstrating the accuracy and reproducibility of the preparation. Furthermore, although DMZ is a compound with hydrophilic characteristics, the encapsulation efficiency was around 50%. It can be explained by the ability of liposomes to retain hydrophilic drugs into the central aqueous phase or between its bilayers (Polozova et al. Reference Polozova, Yamazaki, Brash and Winnik1999).
Although the particle diameter and measures of uniformity of particle size distribution have not been altered by the presence of the drug, when comparing the formulations DMZ-L and B-L, the same does not apply to pH values. While the pH of the formulation containing no drug (B-L) was near neutrality, the addition of DMZ (DMZ-L) led to a significant reduction in the pH value. This decrease in pH could be explained by the 50% fraction of the drug that was not associated to the liposomes, but dissolved in the external aqueous phase, as previously shown by the results of encapsulation efficiency. The pH of the DMZ is around 5·8–6·5, the minimum value we have obtained an approximate value of the liposome (L-DMZ). This pH value may interfere with the in vivo response, since it can influence the stability of the nanostructure (Campbell et al. Reference Campbell, Prankerd, Davie and Charman2004), but the coating of a lipid vesicle could also protect against degradation in acidic medium. To confirm this finding we would carry out more studies on the degradation of L-DMZ in acidic medium.
The interaction between the parasite and the liposome depends upon several factors, including the size of the liposome, its physicochemical characteristics, the surface charge and fluidity of the phospholipid membrane (Lopez-Berestein, Reference Lopez-Berestein1987). In this study, the in vitro efficacy of L-DMZ was higher than C-DMZ, leading to a faster mortality of protozoans incubated with L-DMZ. The greater efficacy of the liposome has been demonstrated in other studies with T. cruzi and Leishmania major (Yoshihara et al. Reference Yoshihara, Tachibana and Nakae1987; Badiee et al. Reference Badiee, Jaafari and Khamesipour2007). This result might be attributed to a higher affinity of the liposomes by the parasite, as already suggested by Kroubi et al. (Reference Kroubi, Daulouede, Karembe, Jallouli, Howsam, Mossalayi, Vincendeau and Betbeder2010). These authors tested porous nanoparticles with lipid core (70DGNP+) of diminazene aceturate, observing greater interaction of these particles with the parasite. This increase in the interaction was explained by the different charges present, since, while the nanoparticles had a positive charge, the outer surface of trypanosomes has a negative charge, promoting an electrostatic attraction.
The difference of electric charges is also indicated by Yoshihara et al. (Reference Yoshihara, Tachibana and Nakae1987), when they assessed the in vitro efficacy of liposomes with stearylamine (SA-liposomes) against T. cruzi. The authors observed a rapid mortality of protozoa, as well as differences between the forms of the parasite. The trypomastigotes have a higher susceptibility, since their surface has a more negative charge than epimastigotes and amastigotes (Romero and Morilla, Reference Romero and Morilla2010). However, there was no addition of component in the formulation prepared in the present study that could provide a positive charge to the liposomes. Thus, the hypothesis of an electrostatic interaction between the liposomes and the parasites, which would be an explanation to the higher efficacy obtained during the in vitro tests, can be discarded. In the liposome developed in this study, the addition of phosphatidylcholine of soybean lecithin may influence its surface potential. This component allows the liposome to become as negatively charged as phosphatidylserine or phosphatidylglycerol (Lopez-Berestein, Reference Lopez-Berestein1987). Particularly, in this case, this greater effectiveness could be explained by the greater absorption of L-DMZ, due to the reduced size of lipid vesicles through purinergic receptors when compared with C-DMZ. The P2 receptor of the parasite is involved in the transport of nucleosides and trypanocidal drugs (Anene et al. Reference Anene, Onah and Nawa2001). This might have led to a greater accumulation of L-DMZ by pathogen (Gillingwater et al. Reference Gillingwater, Kumar, Mohamed, Arafa, Stephens, Boykin, Tidwell and Brun2010).
In in vivo test it was possible to observe that treatment with a single dose controlled the parasitaemia, but it did not provide an effective and curative treatment with both C-DMZ and L-DMZ. The therapeutic dose of 3·5 mg kg−1 is the recommended protocol, however this lack in efficacy was already observed in other studies with dogs, horses and rodents (Tuntasuvan et al. Reference Tuntasuvan, Jarabrum, Viseshakul, Mohkaew, Borisutsuwan, Theeraphan and Kongkanjana2003; Colpo et al. Reference Colpo, Monteiro, Stainki, Colpo and Henriques2005; Doyle et al. Reference Doyle, Da Silva, Monteiro, Santurio and Graça2007). In the case of L-DMZ, our data differ from the results of Yongsheng et al. (Reference Yongsheng, Yongchun, Chengmai, Yuanguo and Fenqin1996) who tested liposomal diminazene in mice and observed a greater longevity of the animals, with a dose 12× higher than the therapeutic recommendation. Additionally, the negative charges of the liposomes developed differently in this study. The results observed in animals treated with liposomes could be attributed to the low stability of liposome, influenced by chemical, physical and biological agents post administration, since these nanostructures can be captured by the mononuclear phagocytic system, decreasing the active contact with the parasite. Frézard et al. (Reference Frézard, Schettini, Rocha and Demicheli2005) affirmed that liposomes, when administered intravenously, are naturally captured by the macrophages of the reticulo-endothelial system, particularly from the liver, spleen and bone marrow. In this study, the drug was administered intraperitoneally and could have been at an increased concentration in the mentioned organs, reducing its local concentration, allowing less direct contact with the parasite, and therefore, providing a lower bioavailability of liposomal diminazene in places with high concentration of the parasite.
Another factor to be considered is that the molecules of the drug, in the therapeutic dose, did not cross the blood–brain barrier (Kaminsky and Brun, Reference Kaminsky and Brun1998) even though nanostructured. The groups treated with the therapeutic dose for 5 days had curative efficacies with both L-DMZ and C-DMZ. This treatment protocol demonstrates greater efficiency due to the passage of these drug molecules through the blood–brain barrier, leading to the higher concentration of an active ingredient, and thus eliminating the trypanosomes in the CNS (Zanette et al. Reference Zanette, Da Silva, Costa, Monteiro, Santurio and Lopes2008; Howes et al. Reference Howes, Da Silva, Athayde, Costa, Corrêa, Tavares, Miletti, Lopes, Amaral and Schmidt2011).
CONCLUSION
In conclusion, the liposomes developed by reverse phase evaporation have suitable characteristics as a nanotechnological formulation, suggesting they could be alternative approaches for the administration of diminazene aceturate in chemotherapy of infectious diseases, especially trypanosomes. Furthermore, our results demonstrate that L-DMZ has greater efficacy in vitro against T. evansi, when compared with the conventional drug formulation (unencapsulated). In in vivo tests, these data suggest that treatment with the encapsulated and conventional drug showed similar efficacy. The potential of the formulation developed in this study was clearly demonstrated, as it increased the efficacy of the treatment against trypanosomosis, but more studies are needed to increase the effectiveness in vivo. Subsequent studies will be performed to assess the in vivo course of these formulations, evaluating their potential for targeting and crossing of the blood–brain barrier.
Funding
The authors acknowledge funding from the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS) for financial support for this study.








