INTRODUCTION
The tapeworm Ligula intestinalis (L.) has a wide global distribution (Bouzid et al. Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008) and a complex life cycle involving 2 intermediate hosts, a copepod and a fish, and a definitive piscivorous bird host (Loot et al. Reference Loot, Brosse, Lek and Guegan2001a; Dejen et al. Reference Dejen, Sibbing and Vijverberg2003, Reference Dejen, Vijverberg and Sibbing2006). Although typically reported from fishes of the Cyprinidae family, L. intestinalis utilizes a broad range of fish hosts, including members of the families Catostomidae, Salmonidae, Cobitidae and Galaxaxiidae (Bean and Winfield, Reference Bean and Winfield1992; Bean and Kirkwood, Reference Bean and Kirkwood1997; Groves and Shields, Reference Groves and Shields2001; Loot et al. Reference Loot, Brosse, Lek and Guegan2001a; Museth, Reference Museth2001; Chapman et al. Reference Chapman, Hobbs, Morgan and Gill2006). These published records, however, do not necessarily provide an accurate measure of host specificity in a given location, as host spectra differ between investigated fish communities (Poulin, Reference Poulin1998; Guégan and Kennedy, Reference Guégan and Kennedy1996). This conforms to the recent finding that L. intestinalis has genetically distinct and well-separated phylogenetic clusters across their global distribution, with these geographically isolated lineages being divergent due to physical isolation (Bouzid et al. Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008). This situation is likely to result in subsequent local adaptations to available host fauna (Gandon and Michalakis, Reference Gandon and Michalakis2002; Greischar and Koskella, Reference Greischar and Koskella2007; Bouzid et al. Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008).
The phylogenetic study of L. intestinalis by Bouzid et al. (Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008) revealed the existence of several clades, each linked to geographical origin, with some areas represented by a single monophyletic clade, such as Ethiopia in East Africa. The majority of studies investigating the host-specificity and the behavioural responses of L. intestinalis in its host have focused on Euro-Mediterranean clades (e.g. Orr, Reference Orr1966; Arme and Owen, Reference Arme and Owen1968; Wilson, Reference Wilson1971; Harris and Wheeler, Reference Harris and Wheeler1974; Sweeting, Reference Sweeting1976; Taylor and Hoole, Reference Taylor and Hoole1989; Loot et al. Reference Loot, Brosse, Lek and Guegan2001a, Reference Loot, Poulin, Lek and Guegan2002; Museth, Reference Museth2001). Within these, Loot et al. (Reference Loot, Brosse, Lek and Guegan2001a) tested the Parasite Increased Trophic Transmission (PITT) hypothesis, in which the parasite (L. intestinalis) modifies the behaviour of its intermediate host (fish) to facilitate transmission to the definitive host (piscivorous bird), with infected fishes utilizing habitats that increase bird predation (Barnard and Behnke, Reference Barnard and Behnke1990). In contrast to the Euro-Mediterranean clades, there have been few studies in East Africa of host-specificity and the impact of L. intestinalis on host-behaviour. Some studies suggested host-specificity for a very small number of Cyprinid species, such as Rastrineobola argentea (Pellegrin) in Lake Victoria (Marshall and Cowx, Reference Marshall, Cowx and Cowx2003; Cowx et al. Reference Cowx, Rollins and Tumwebaze2008), and Barbus humilis L. and Barbus tanapelagius (de Graaf) in Lake Tana, Ethiopia (Dejen et al. Reference Dejen, Vijverberg and Sibbing2006).
To overcome this deficiency in knowledge of L. intestinalis in East Africa, we investigated host specificity and parasite prevalence in Kenya, with a focus on the Lake Baringo and Naivasha catchments. These catchments provide a range of fish host families and species across lotic and lentic habitats for potential infection, enabling testing of the hypothesis that L. intestinalis has successfully adapted to the available local host fauna in Kenya which is revealed by host specificity. For the purposes of this study, L. intestinalis in Kenya was assumed to be of the same clade as present in Ethiopia (Bouzid et al. Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008) due to their geographical proximity and the absence of recordings in the introduced fishes of the two lakes (i.e. L. intestinalis was unlikely to have been introduced as a consequence of transfers of infected fish). For infected populations, we then investigated the dynamics of host-parasite interactions in fish, focusing on fish size and their spatial occupancy, providing insight into parasite prevalence and fish habitat utilization, and whether the East African clade of L. intestinalis may be conforming to the PITT hypothesis, similar to Euro-Mediterranean clades.
MATERIALS AND METHODS
Lakes Baringo and Naivasha are shallow freshwater lakes in Kenya's Rift Valley. Lake Baringo lies 60 km north of the equator at an altitude of ~970 m above sea level. It is approximately 120 to 145 km2 in area, drains a catchment of 6820 km2 and had a mean depth of 2·65 m in 2002 (Hickley et al. Reference Hickley, Muchiri, Boar, Britton, Adams, Gichuru and Harper2004; Britton and Harper, Reference Britton and Harper2008). Lake Naivasha is located 190 km south of the equator at an elevation of 1890 m above sea level and is ~150 km2 in area and 3–6 m deep (Britton et al. Reference Britton, Boar, Grey, Foster, Lugonzo and Harper2007). The water levels of both lakes can vary considerably between years (Hickley et al. Reference Hickley, Muchiri, Boar, Britton, Adams, Gichuru and Harper2004; Britton et al. Reference Britton, Boar, Grey, Foster, Lugonzo and Harper2007, Reference Britton and Harper2008). Mean water temperatures, recorded hourly using deployed ‘Tiny-talk II’ data-loggers (Gemini data loggers, UK) between 25 June and 10 July 2008, were 19·5±1·6°C in Naivasha and 26·4±1·4°C in Baringo, and water temperatures generally remain relatively constant throughout the year (Hickley et al. Reference Hickley, Muchiri, Boar, Britton, Adams, Gichuru and Harper2004). Both lakes are fed by a number of catchment rivers. The principal tributaries of Naivasha are the River Malewa and the River Gilgil, and Baringo, the Rivers Ol Arabel, Perkerra and Molo (Hickley et al. Reference Hickley, Muchiri, Boar, Britton, Adams, Gichuru and Harper2004; Britton et al. 2006 a).
To determine the host specificity of L. intestinalis, fish samples were collected from both lakes during surveys conducted annually from 2002 (Naivasha) and 2004 (Baringo), and completed between June and October of each year. During each sampling period at each lake, when up to 10 consecutive daily sampling events were conducted, the fish community was surveyed by gangs of multi-meshed gill nets. These comprised three 60 m connected nylon nets of 1·5 m stretched depth, which in most of the sampling stations were sufficient to sample the entire lake depth. If the nets could not fish the entire depth, they were set from the surface. Each 60 m net comprised 12 panels of 5 m lengths of mesh size (knot to knot) 8, 10, 13, 16·5, 19, 22, 25, 30, 33, 38, 45 and 50 mm. Samples were collected by setting the nets at first light and then lifted after about 4 h of fishing. To supplement data on the host specificity of L. intestinalis, the principal tributary rivers of both lakes were sampled in June and July 2008 using gill nets of 8, 10 and 16 mm mesh sizes. The nets were set in pool habitats of 0·3 to 1·2 m depth.
During each sampling occasion, captured fish were identified to species, measured (fork length, L F, nearest mm), weighed (nearest g) and inspected visually for the presence of larval cestode infection in the body cavity. When L. intestinalis was recorded in a fish, the parasites were removed and their lengths and weights recorded to the nearest mm and 0·1 g. These data were analysed for each species to identify variations in parasitic prevalence (proportion of infected fish, expressed as a percentage), parasite abundance (number of parasites per fish) and their intensity of infection, quantified using the index of parasitization of Kennedy and Burrough (Reference Kennedy and Burrough1981). This was calculated from (parasite weight/somatic weight)×100, where somatic weight is total weight minus parasite weight.
To determine the relationships between parasite prevalence and fish length and spatial occupancy, the fish host used was Barbus lineomaculatus (referred to hereafter as barbs) in Lake Baringo. This species was selected on the basis of a relatively high parasite prevalence compared with other fishes used in the study (cf. Results). For these analyses, repeat sampling (a minimum of 3 replicates per habitat type) was completed during two 4-day periods, in October 2007 and in June 2008, using the same gill net methodologies as described, but with fishing for 1 h per replicate sample at each site. Samples could only be collected during daylight, as there was an increased risk of net and fish damage caused by hippopotami (Hippopotamus amphibious L.) and Nile crocodiles (Crocodylus niloticus Laurenti) during darkness. For data analysis, the fish samples were sorted into their major habitat type and distance from the shore, providing 5 broad habitat classifications for analysing the spatial occupancy of parasitized barbs: rocky shore (1 to 15 m from shore; 1 to 1·5 m depth), shallow littoral (1 to 15 m from shore; 0·5 to 1·0 m), deep littoral (1 to 15 m from shore, more than 1 m depth), open water 1 (30 to 50 m from shoreline, more than 1 m depth) and open water 2 (more than 70 m from shoreline, more than 2 m depth). The shallow and deep littoral zones were characterized by a substratum consisting mainly of silt and led to a shoreline of shallow gradient, whereas the rocky shore substratum comprised principally stones and boulders of >0·5 m in diameter and led to a shoreline with steep cliffs of >40 m in height. To enable the analysis of parasite prevalence according to fish size, water depth and habitat, the sampled barbs from each of the 5 habitat classifications were separated into 4 length classes: <63 mm, 64–70 mm, 71–77 mm and 78–84 mm. To test for a significant association between parasite occurrence and habitat type, a binomial GLM was used, with parasite occurrence used (0 or 1) as the dependent variable and fish length and habitat type as the predictors, plus their two-term interaction. All statistics were completed in SPPS v. 14.0 (SPSS Inc., Chicago, Illinois, USA). If mean values are presented, their standard deviations were also provided.
RESULTS
Host specificity of L. intestinalis in Kenya
Ligula intestinalis in Kenya is highly host specific, with recordings in the study sites restricted to only 2 species of the Barbus genus (family Cyprinidae) and only in the lacustrine habitats (Table 1). In Lake Naivasha, recordings were only in B. paludinosus, and parasite prevalence was low, with only 7 of 8665 inspected individuals infected. The infected fish were between 68 and 81 mm in length, with this size range not significantly different from uninfected individuals (F1,8664=0·11; P>0·05). There was only 1 cestode recorded per infected fish. The mean length of each plerocercoid was 88±25 mm, mean weight 0·43±0·14 g and the index of parasitization ranged between 7 and 19%, mean 11·2±1·4%. Although B. paludinosus was also sampled from sites on the Rivers Malewa (n=94) and Gilgil (n=34) in 2008, L. intestinalis was not recorded. In Lake Baringo, there were 3 Cyprinid species captured in samples (including 2 Barbus species), but L. intestinalis was only recorded in B. lineomaculatus (Table 1). Compared with B. paludinosus, their parasite prevalence was relatively high. Only 1 cestode was recorded per infected fish, the threshold length for infection was 64 mm (with uninfected fish present between 44 and 63 mm) and the largest infected fish was 84 mm (Table 1). The mean length of each plerocercoid was 94±29 mm and mean weight 0·55±0·16 g, and the index of parasitization ranged from 4·1 to 20·2%, mean 10·9±2·1%. Similar to the Lake Naivasha tributary rivers, there were no recordings of fish infected with L. intestinalis in the Baringo river tributaries (Table 1), despite B. lineomaculatus being sampled (n=72) with Barbus intermedius australis (Banister) (n=54) and Labeo cylindricus (Peters) (n=274).
Table 1. Recordings of Ligula intestinalis in the fish communities of Lakes Naivasha and Baringo, Kenya, 2002 to 2008 (data combined for all years)
(Key: Present, species recorded in lake; n, total number of fish examined, np, number of fish recorded with infection of L. intestinalis.)

* Introduced species (Hickley et al. Reference Hickley, Muchiri, Boar, Britton, Adams, Gichuru and Harper2004).
Barbus lineomaculatus in Lake Baringo: parasite prevalence and spatial occupancy
There were significant differences recorded in parasite prevalence across the 4 barb length classes (χ2=37·217, d.f.=3, P<0·0001; Fig. 1). Parasite prevalence was highest in fish of the length range 71 to 77 mm, reaching 100% in some samples from the shallow littoral areas (Fig. 1). There was a marked reduction in parasite prevalence in fish between 78 and 84 mm, and no barbs of <64 mm were infected. No barbs of >84 mm were caught (Fig. 1).
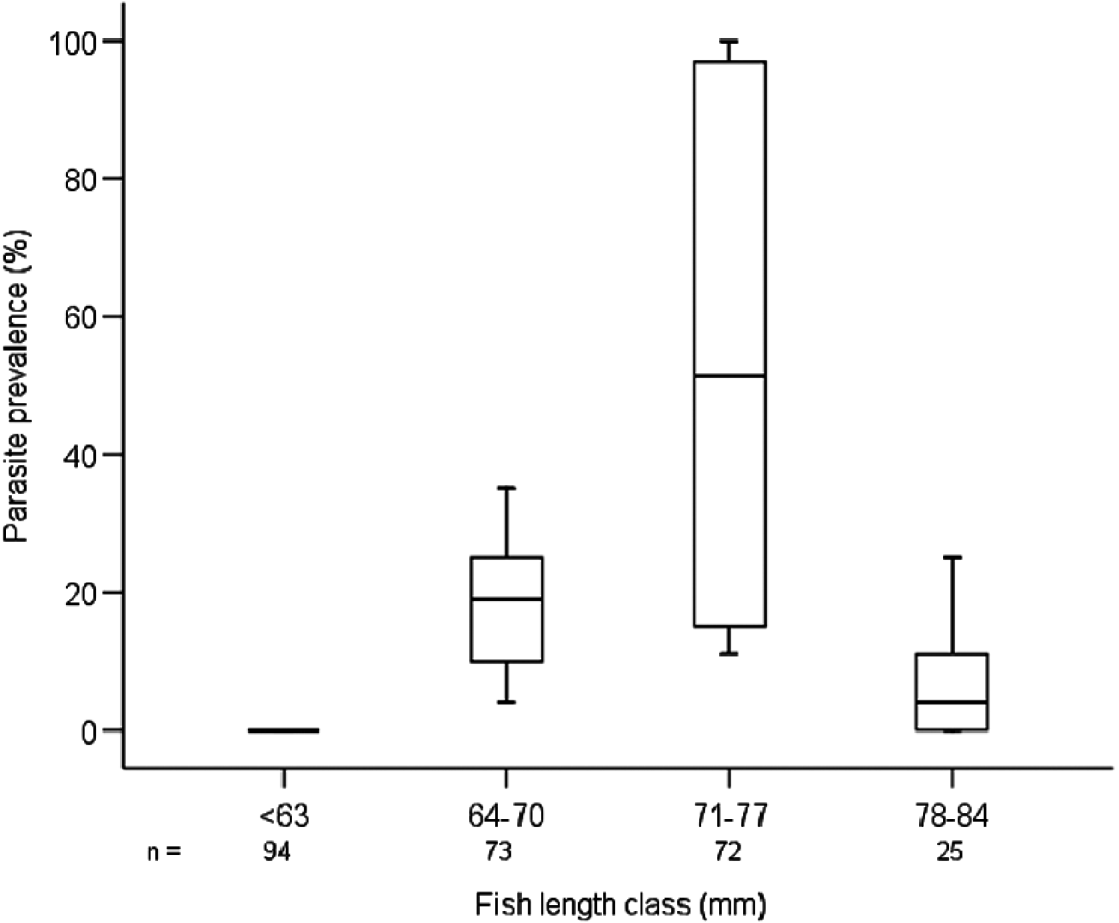
Fig. 1. Relationship between parasite prevalence and fork lengths of barbs sampled in 2007 and 2008 in Lake Baringo. Data are included only from sites where parasitized fishes were sampled. On all box plots, the top, mid-line and bottom of each box plot represent the 75th, 50th and 25th percentiles and the horizontal lines represent the 10th and 90th percentiles.
The relationship between fish length (parasitized and not parasitized) and each habitat category was not significant (F4,469=1·109, P>0·05). However, infected barbs did occupy different habitats when compared to uninfected fish, as they were only present in the littoral zone (Fig. 2). Parasite prevalence was increased in the shallow littoral compared with the deep littoral zone (Fig. 2). No infected fish were recorded from the rocky shore or open water habitats, despite uninfected barbs being captured there in all length classes (Fig. 2). The effects of fish length and habitat on parasite prevalence were explored using the GLM. It revealed there was a significant effect for habitat (F4,469=49·4, P<0·0001) and fish length (F3,469=8·35, P<0·0001), but also their two-way interaction (F12,469=20·27, P<0·0001). The interaction plot of parasite prevalence with habitat and fish length further illustrated this output (Fig. 3); infected fish were only recorded at lengths between 64 and 84 mm, and these infected fish were only significantly associated with the littoral zone, particularly the shallow littoral zone.
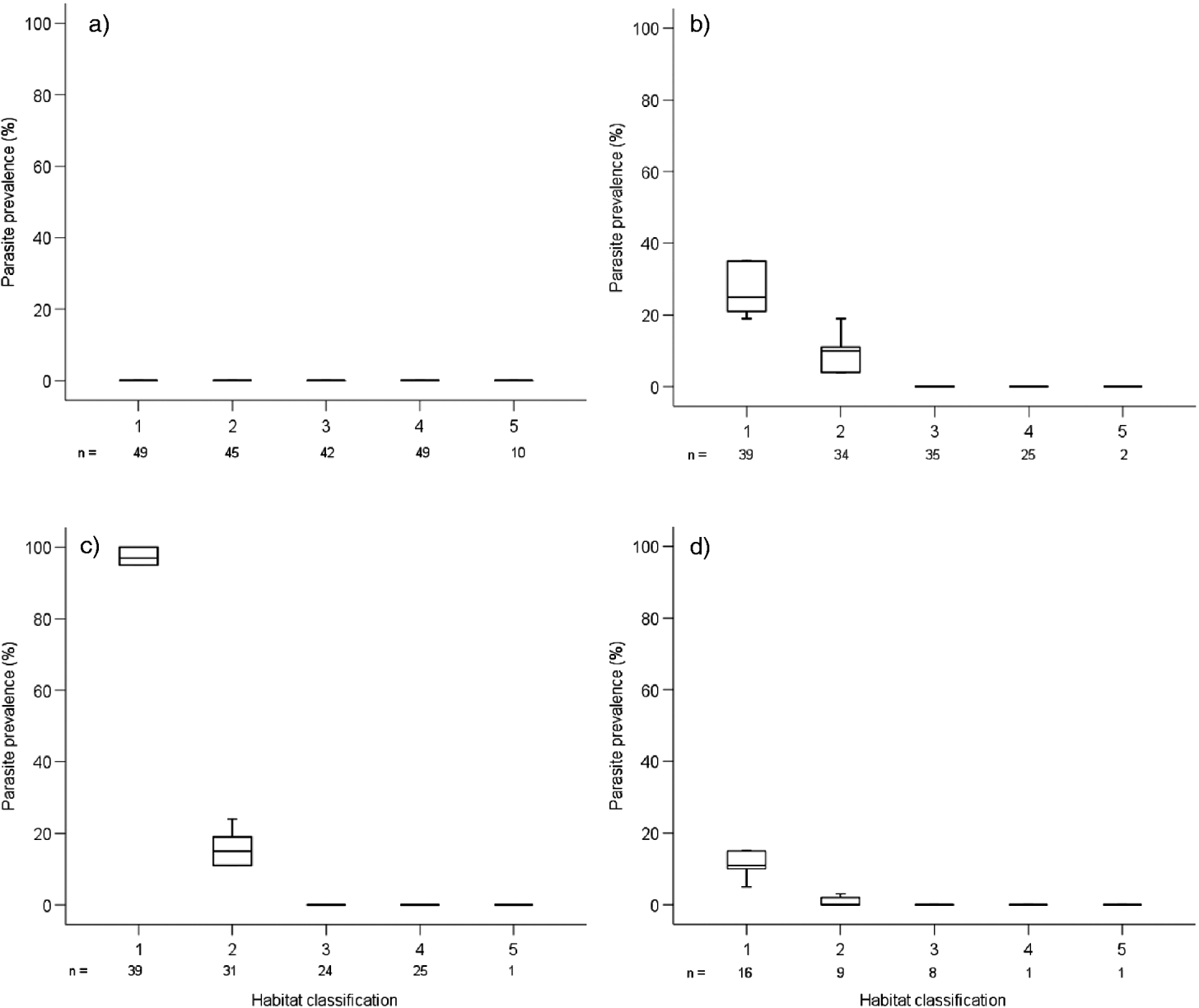
Fig. 2. Relationship between parasite prevalence and habitat classification for Lake Baringo barbs sampled in 2007 and 2008 for each size class (a=less than 63 mm; b=64 to 70 mm; c=71 to 77 mm; d=78 to 84 mm), where 1=shallow littoral; 2=deep littoral; 3=open water; 4=open water 2; and 5=rocky shore. Data have been combined across 2007 and 2008 and sampling in each habitat classification was replicated at least 3 times. On each box plot, the top, mid-line and bottom of each box plot represent the 75th, 50th and 25th percentiles and the horizontal lines represent the 10th and 90th percentiles.

Fig. 3. Interaction plot (estimated marginal means) of parasite prevalence with fish length and habitat type (1: •; 2: ○; and 3: ▪; 4 and 5 are obscured by the output for habitat classification 3, and are identical).
DISCUSSION
The requirement for more region-specific studies on L. intestinalis: fish interactions was exemplified by Bouzid et al. (Reference Bouzid, Stefka, Hypsa, Lek, Scholz, Legal, Hassine and Loot2008), who revealed geographically isolated lineages and suggested clade host-specificity was a direct result of adaptations to local host fauna rather than co-speciation processes. We revealed high host specificity in this L. intestinalis clade in the Lake Baringo and Naivasha catchments. Across 12 fish species covering 6 families, recordings of L. intestinalis were limited to 2 species of cyprinid, B. lineomaculatus and B. paludinosus. It was perhaps not surprising that Micropterus salmoides (Lacepéde), Clarias gariepinus (Burchell) and Protopterus aethiopicus (Heckel) were uninfected, given their dietary reliance on fish (Hickley et al. Reference Hickley, North, Muchiri and Harper1994; Britton et al. Reference Britton and Harper2006a, Reference Britton, Ng'eno, Lugonzo, Harper, Odata, Olago, Ochola, Ntiba, Wandiga, Gichuki and Oyiekeb), providing little interaction with intermediate copepod hosts. There were, however, 3 other cyprinid species present in the lakes in which no parasitized individuals were recorded, despite some dietary reliance on zooplankton during their life cycles (Britton et al. Reference Britton and Harper2006a, Reference Britton, Boar, Grey, Foster, Lugonzo and Harper2007).
The outputs of the GLM provided evidence that parasite prevalence was significantly correlated with habitat occupancy. There are 2 mechanisms that may explain this: (i) infection by the parasite resulted in a behavioural modification that increased the opportunity of bird predation (PITT hypothesis); and (ii) fish were more exposed to parasite infection in certain habitats, resulting from either a horizontal gradient of infected copepods or increased parasite egg-hatching success and larval survival. Although it was not possible to determine which of these mechanisms were operating in Lake Baringo, the pattern of host specificity and pathogenicity in this L. intestinalis clade was highly consistent with outputs from field and experimental studies on Euro-Mediterranean clades and is independent of their phylogenetic history (e.g. Lester, Reference Lester1971; Giles, Reference Giles1983, Reference Giles1987). The debilitating effects exerted by Euro-Mediterranean clades on fish hosts and the modification of host-behaviour has been directly related to infection (e.g., Orr, Reference Orr1966; Wilson, Reference Wilson1971; Harris and Wheeler, Reference Harris and Wheeler1974; Sweeting, Reference Sweeting1976; Bean and Winfield, Reference Bean and Winfield1992; Museth, Reference Museth2001) and conforms to the PITT hypothesis (Loot et al. Reference Loot, Brosse, Lek and Guegan2001a). The modified behaviour of fish infected with L. intestinalis is thought to arise from the increasing host energy demands caused by plerocercoid development within the body cavity (Loot et al. Reference Loot, Francisco, Santoul, Lek and Guegan2001b). Whilst this may stimulate foraging behaviour through increased feeding motivations in the infected fish (Pascoe and Mattey, Reference Pascoe and Mattey1977; Giles, Reference Giles1983; Milinski, Reference Milinski, Barnard and Behnke1990; Godin and Sproul, Reference Godin and Sproul1988), the distended body cavity impacts on swimming and foraging, and reduces their competitive ability with uninfected con-specifics (Loot et al. Reference Loot, Brosse, Lek and Guegan2001a). The infected fish are then forced to occupy the most productive lake areas, such as in the littoral zone, in order to forage successfully, irrespective of any increase in predation risk (Fisher and Eckmann, Reference Fisher and Eckmann1997; Loot et al. Reference Loot, Brosse, Lek and Guegan2001a). Uninfected fish, whose swimming and foraging abilities would not be impaired, continue to utilize less productive areas, such as off-shore areas, in a trade-off with reduced predation risk (Persson and Eklov, Reference Persson and Eklov1995; Loot et al. Reference Loot, Brosse, Lek and Guegan2001a).
Whilst there were some similarities in the effects of L. intestinalis on their fish hosts between the East African and Euro-Mediterranean clades, differences were apparent in the parasite abundance of infected fish. In this study, only 1 parasite was recorded per infected fish, with this also found for infected Barbus species in Lake Tana, Ethiopia (Dejen et al. Reference Dejen, Vijverberg and Sibbing2006). However, although Cowx et al. (Reference Cowx, Rollins and Tumwebaze2008) did report finding up to 8 plerocercoids in Rastrineobola argentea (pellegrin) in Lake Victoria. Studies have revealed that multiple infections are common in fish infected with Euro-Mediterranean clades. For example, Loot et al. (Reference Loot, Brosse, Lek and Guegan2001a) recorded up to 11 plerocercoids per host in roach Rutilus rutilus, with no infected fish encountered of <2 years of age. There was also an associated decrease in parasite prevalence with host age of >3 years, attributed to parasite-induced mortality. Parasite-induced mortality was also suggested in this study by the high parasite prevalence in the length class 71–77 mm, but much reduced between 78 and 84 mm. In Euro-Mediterranean clades, plerocercoids may attain lengths up to 300 mm (Arme and Owen, Reference Arme and Owen1968), whereas in this study, maximum plerocercoid length was only 176 mm and in R. argentea in Lake Victoria, mean plerocercoid length did not exceed 120 mm in any size class of infected fish (Cowx et al. Reference Cowx, Rollins and Tumwebaze2008). However, these apparent differences between the clades may not just be a function of differential clade-pathogenicity. Compared to species such as R. rutilus, B. lineomaculatus is small bodied and of shorter life span, with infected fish only measuring between 64 and 84 mm fork length, i.e., development in the body cavity of a 300 mm plerocercoid, or of multiple plerocercoids, may not be possible.
In conclusion, this study on the East African clade of L. intestinalis revealed host-specificity to only 2 species of the family Cyprinidae, and its effects on fish hosts were similar to those induced by Euro-Mediterranean clades. Although both lakes support regionally-important commercial fisheries, neither infected species is exploited and so the socio-economic impact of the parasite may be limited. However, these fishes may be considered an important ecological component of the fish community and the food web in which they are situated. As such, the heavy infestation in the barb population of Lake Baringo may be a concern, particularly through the impaired reproduction that may result through infection.
Sponsors of the work include the ‘Lakes of the Rift Valley’ project of the Universities of Leicester and Nairobi, funded by the Earthwatch Institute, Boston, USA and Oxford, UK, with logistics supported by the Darwin Initiative 2003 to 2008, and the Percy Sladen Memorial Fund. We thank the Ministry of Science and Technology of the Government of Kenya for research permissions. The views expressed are those of the authors and not their parent organizations.






