INTRODUCTION
The parasitic fly, Philornis downsi Dodge & Aitken, was recently identified from surveys of Darwin finch nests on the Galápagos Islands (Fessl et al. 2001). The adult fly is non-parasitic, but the 3 larval instars feed on the blood and tissues of nestlings (Fessl and Tebbich, 2002). Retrospective examination of museum specimens shows that the fly has occurred on the Galápagos Islands since at least 1964 (Causton et al. 2006), but it was not discovered as an avian parasite until 1997 (Fessl and Tebbich, 2002). In a comprehensive evaluation of invasive species affecting Galápagos biota, P. downsi was given the highest risk ranking (Causton et al. 2006).
The fly is currently found on 11 of 13 Galápagos Islands that were examined for Philornis prevalence, with the highest intensity found in birds' nests on the central island Santa Cruz, which also harbours the largest human population (Wiedenfeld et al. 2006). Philornis was found in nests of all 18 bird species examined, among them 3 native and 14 endemic species, including 11 species of Darwin's finches (Fessl et al. 2001; Wiedenfeld et al. 2006). Parasite intensity was higher on elevated islands and it has been proposed that the moist highlands act as a reservoir for the adult flies across years (Wiedenfeld et al. 2006). How Philornis was introduced to the Galápagos is speculative; adults possibly arrived with imported fruits or vegetables from the continent to the central inhabited islands and spread from there to other islands.
The genus Philornis Meinert (Diptera, Muscidae) comprises some 50 species occurring throughout South and Central America and extending north to southern North America (De Carvalho et al. 2005). Little is known about the biology of most species though almost all larvae are obligate haematophagous feeders that live subcutaneously on their avian host (Teixeira, 1999; Dudaniec and Kleindorfer, 2006). In contrast, the larvae of P. downsi are considered free-living in the nest of their host and externally feed on nestling blood (Couri, 1985). Philornis downsi predominantly feed during the night (on a nestling's abdomen, legs, and under the wings) and return to the thick bottom layer of the nest during the day. Pupation also occurs in the bottom layer of the nest. Thus, detection of parasitism (i.e. later instar larvae or puparia) can only be verified by dismantling the nests, which probably explains their late discovery on the Galápagos Islands. Unidentified fly larvae have been found in previous studies in the nostrils of finch nestlings but their significance was not recognized. Darwin's finches were surveyed in the subsequent study years for the presence of larvae in the nasal cavities. Here we (1) identify larvae from the nasal cavities, (2) describe the structure and biology of all 3 larval instars of P. downsi, (3) show their impacts on nestling development and survival and (4) discuss the consequences of P. downsi infestation for the survival and conservation of Darwin's finches.
MATERIALS AND METHODS
Detailed observations of larvae in the nasal cavities of Darwin's finches were conducted over 3 years (2000, 2004 and 2005) on the island of Santa Cruz (0 °37′S, 90 °21′W), Galápagos, Ecuador, during the avian breeding season from January to March. During this period, the arid zone of Santa Cruz receives on average 70 mm of rain per month (referred to as the wet season) (Grant, 1999). The year 2000 was moderately humid with average rainfall in January but then became progressively drier. Monthly rainfall data for the 2000 breeding season taken at the Charles Darwin Research Station in Puerto Ayora, Santa Cruz Island, at 6 m above sea level were 40·4 mm, 23·5 mm and 12 mm, respectively. The 2004 season was a dry year (18·8 mm, 9·9 mm and 19·1 mm monthly rainfall for January to March) and conditions remained very dry until March 2005 (13·2 mm, 0·2 mm and 109·2 mm monthly rainfall). The study site was located in the arid coastal zone, a semi-desert forest consisting of deciduous trees (mainly palo santo, Bursera gravolens Triana & Planch), shrubs (e.g. Croton scouleri Hook. f.) and cacti (mainly Opuntia echios gigantea J.T. Howell and candelabra, Jasminocereus thousarsii Weber). We observed nests of several different Darwin's finch species abundant in this zone, including: small ground finch, Geospiza fuliginosa, medium ground finch, Geospiza fortis, and cactus finch, Geospiza scandens (Table 1). These finches build dome-shaped nests preferably in Opuntia cacti at heights of 1·5 to 4 m. We accessed nests by ladder and determined nest status (number of eggs or nestlings) visually. During nest monitoring, we noted hatching date, presence of larvae in nasal and other cavities, nostril appearance, nestling mortality, and fledging success.
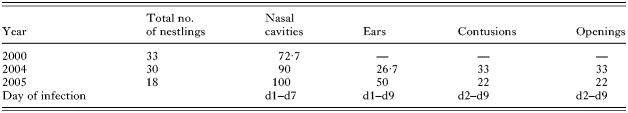
Table 1. Breeding biology parameters of Darwin's finches in the arid zone, Santa Cruz Island (Given are the numbers of observed nests per bird species (SG=small ground finch, MG=medium ground finch, CF=cactus finch), mean clutch size (±S.E.), number of hatchlings and number of fledglings (mean±S.E.) as well as hatching success (no. of hatched/no. of eggs), fledging success (no. of fledged/no. of hatched) and cause of nestling mortality (100%=all hatchlings).)

To examine larval infestation in nestling nasal cavities, we scored the nestling nostrils as: normal (no larvae seen, not enlarged), swollen (enlarged nostrils filled with larvae) or expanded (no larvae detected but nostrils considerably expanded) (Fig. 1). Some larvae were extracted from the nasal cavity and immediately preserved in 95% alcohol for later identification. Some recently dead nestlings were examined for larvae in nasal cavities and other body parts and then preserved in 95% alcohol. Some hours later, several tiny larvae were found floating in the solution, which were not detected previously. Once nest activity ceased, the nests were collected and carefully dismantled in the lab. The number of larvae and puparia were counted for calculating the median parasite intensity (no. of parasites/nestling). In each of several nests larvae were weighed to the nearest 0·0001 g.

Fig. 1. Effects of larvae causing myiasis in nasal cavities: (left) normal, non-infected; (middle) swollen with larvae inside the nostrils; and, (right) empty and expanded, after larvae have left the cavity.
The detailed anatomical study of larval specimens was performed on specimens cleared in hot lactic acid (85%) and examined in glycerine. Larval terminology of Courtney et al. (2000) and Skidmore (1985) was followed. The larvae and some reared adults of P. downsi were deposited in Museum Koenig, Bonn and vouchers have been sent to the Invertebrate collections of the Charles Darwin Research Station, Puerto Ayora, Santa Cruz Island.
RESULTS
Site-specificity of different larval instars
In 2005, we removed some larvae from the nostrils of living nestlings. These larvae were identified as first and second instar larvae of P. downsi. Some other first instar larvae were collected from freshly dead 2 to 3-day-old nestlings (see Materials and Methods section). Second instar larvae were also collected from nest material (nestlings aged 3 to 6 days). Third instar larvae were found in nesting material sheltering 3-day-old nestlings and older. Pupation occurred in the thick bottom layer of the nest, with the puparia protected by a frothy cocoon.
Parasitic phases of the life-cycle
First and early second instar larvae – myiasis
Larvae in the nasal cavities provided the first signs of parasitism by P. downsi in Darwin's finches (Table 2). The larvae caused a swelling of the entire nostril area, which was easily distinguishable from normal nostrils (Fig. 1). In the first phase of nostril infection the nostrils were swollen, slightly enlarged and contained larvae (referred to as swollen nostrils); while in the second phase the nostrils were expanded and without larvae (referred to as expanded nostrils). Larvae were found in the nasal cavities of 69 out of 81 nestlings. We did not determine the number of larvae as this would have required us to sacrifice the nestlings. Swollen nostrils were observed from day 1 to day 7 (median±quartiles: day 3<4>6), whereas expanded nostrils were observed from day 4 to day 9 (median±quartiles: day 5·5<7>8·75) (Wilcoxon signed Rank Test, P<0·0001, Fig. 1).
Table 2. First and early second instar larval infection of Darwin's finches in the dry zone, Santa Cruz Island: sites and prevalence (Sites of larval infection on nestlings (nasal cavities, ears, contusions, openings) and percentage of infected nestlings. The last line gives the first and last observation day for the different infection sites (range in days after hatching).)
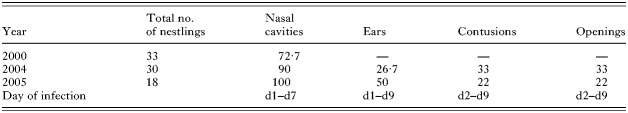
Infection sites and prevalence differed between the year 2000 and the other two study years (Table 2), possibly due to different climatic conditions. In 2000, we noticed larvae in the nasal cavities but no other signs of parasitism. In 2004 and 2005, we additionally encountered infection of the auditory canal, as well as contusions around the abdomen and openings under the wings, legs and backs of nestlings. Infection of auditory canals, wounds and contusions were observed throughout the nestling phase (Table 2). Five adult finches (at least 3 years of age) and 1 juvenile that showed severely expanded nostrils, were caught by S.K. Thus, the morphological deformation caused by P. downsi parasitism may persist into adulthood.
Late second and third instar larvae- nest-dwelling haematophagous phase
Second instar larvae left the nasal cavity around day 3 to day 9 and burrowed into the nesting material. The larvae were not visible during the day (e.g. while manipulating nestlings) being photophobic.
In 2005, we weighed third instar larvae from 7 different finch nests (including mature larvae). These ranged from 0·0014 g to 0·075 g (mean±S.E.=0·025±0·002, n=124 larvae). The mature larval instar was separated from 5 nests and weighed on average 0·046±0·002 g (n=46 larvae, range: 0·031–0·075), differences among nests were not significant (Kruskal-Wallis 6·43, D.F.=4, P=0·17). The size of the larvae appeared dependent on host body size. Mature third instar larvae from a mockingbird nest (average adult mass 51–56 g) reached on average 0·111±0·009 g (n=18, range 0·047–0·17) and were thus significantly larger than mature larvae from different Darwin's finch nests (average adult mass 16–20 g) (Wilcoxon, z=5·62, P<0·0001). No data on mass were available for second instar larvae.
Impact of parasites on nestling survival
In total, we observed 63 nests for which some or all eggs hatched in 54 nests (85·7% hatching success). For this study, we included data from 17 nests that were used in a separate study and had been treated with insecticide to eliminate P. downsi (Fessl et al. 2006); thus fledging success was not counted for these nests. Ten out of 37 nests had partial (40%) or total (60%) fledging success; the remaining nests failed due to predation (14·8%), abandonment (7·4%) or as a consequence of parasitism (77·8%) (see also Table 1). The climatic conditions resulted in lower clutch sizes during the dry years 2004 and 2005 (ANOVA, effect of year: chi-square: 14·6, P=0·001, effect of species: 2·31, P=0·32) probably due to low food availability (S. Kleindorfer, unpublished observations). For 54 nestlings, the age of death was known and differed significantly across the 3 study years (Kruskal-Wallis test, 12·63, D.F.=2, P=0·002). Nestlings in 2005 died at a significantly earlier age (day 4·85±0·6, n=20) compared to nestlings in 2004 (day 7·24±0·46, n=21) and 2000 (day 8±0·48, n=13).
Fledgling blood loss due to the larvae was calculated by converting larval biomass to blood consumption using a conservative conversion rate of 40% (Gold and Dahlsten, 1983). Average biomass of mature third instar larvae was 0·046 g, thus each larvae consumed 0·115 g of blood during its larval cycle. In birds, blood is approximately 6–8% of body mass (Sturkie, 1986). Using 6%, a fully-grown finch nestling (approximately 13 g at day 12) would have 9·36 g of blood available (0·78×12) during its nestling phase. According to this calculation, blood loss per nestling was 25% in 2000, 18% in 2004 and 26% in 2005 (the calculation is based on the median number of parasites per Darwin finch nestling per year which was 20, 15, and 21, respectively). For 2005, we were able to calculate nestling blood loss using the exact number and mass of larvae and nestlings at 4 nests, where nestlings died at the age of 3–7 days. The exact calculation of blood loss for these 4 nests varied between 32 and 55% and was thus much higher than the value calculated with average numbers and mass (Table 3).
Table 3. Nestling blood loss calculation data for four Darwin finch nests in the dry zone, Santa Cruz Island (For the calculation of blood loss per nestling, we assumed conversion efficiency of 40% for blood to parasite biomass, and a nestling mass to blood ratio of 1[ratio ]0·06. Data per nest on larval and nestling mass, parasite intensity (no. of larvae/nestling), and age of death allowed an individual calculation of blood loss for 4 nests with 2 nestlings each in 2005.)

Morphology of larvae and puparium
Larva
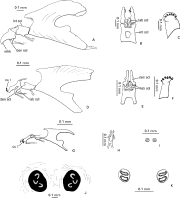
Instar 1 (Fig. 2G–I): Oral opening with 3–4 short, stout cuticular teeth-like spines, closely approximated with mouthhooks; rows of spicules absent. Body (excluding pseudocephalon) covered with spicules; spicule bands sparse on posterior margins of thoracic segments; abdominal segments 1–5 completely and densely clothed in spicules; abdominal segments 6–7 with fewer spicules ventrally; caudal segment with spicules confined mostly dorsally. Anterior spiracle absent. Posterior spiracles (Fig. 2I) brownish, lightly pigmented, rounded, separated by slightly more than their diameter; 2 oval spiracular slits present, lying opposite each other. Cephaloskeleton slender, with elongate intermediate sclerite fused to basal sclerite (Fig. 2G); mouthhooks elongate and slender, produced ventrally as tapered process with adductor apodeme inserted; apex of mouthhooks closely approximated (i.e. not easily separated) with teeth-like spines; labial sclerites absent (Fig. 2H).

Fig. 2. Philornis downsi, larval instars. (A) Third instar, cephaloskeleton, lateral view; (B) third instar anterior cephaloskeleton, ventral view; (C) third instar, anterior spiracle; (D) second instar, cephaloskeleton, lateral view; (E) second instar, anterior cephaloskeleton, ventral view; (F) second instar, anterior spiracle; (G) first instar, cephaloskeleton, lateral view; (H) first instar, anterior cephaloskeleton, ventral view; (I) first instar, posterior spiracles; (J) third instar, posterior spiracle; (K) second instar, posterior spiracles. Abbreviations: cu t – cuticular teeth; den scl – dentral sclerite; int scl – intermediate sclerite; lab scl – labial sclerite; mhk – mouthhook.
Instar 2 (Fig. 2D–F,K): Oral opening with 2–3 short rows of stout teeth-like spines on each side and rows of spicules. Body vestiture same as third instar (see below). Anterior spiracle semi-circular in shape, with 5–6 spiracular lobes (Fig. 2F). Posterior spiracles brown, lightly pigmented, rounded, separated 2–3 times their diameter (Fig. 2K); 2 oval spiracular slits present, lying opposite each other. Cephaloskeleton robust (Fig. 2D), with elongate intermediate sclerite; mouthhooks elongate and slender; dental sclerite greatly lengthened and slender, two-thirds length of mouthhook, tapered anteriorly to slender apex with adductor apodeme inserted; labial sclerites present, anterior pair (ligulate sclerites) quadrate with slender tapered apex from which muscle apodeme inserted (Fig. 2D), posterior sclerite (subhypostomal sclerite) positioned medially with central fenestrated region (Fig. 2E).
Instar 3 (Fig. 2A–C,J): Oral opening with 2–3 short rows of stout cuticular teeth-like spines on each side. Thoracic segments with complete anterior spine bands composed of numerous rows of spicules. Spine bands complete on anterior margins of abdominal segments 1–5; spine bands mostly restricted ventrally and dorsally on abdominal segment 6–7; caudal segment with spicules over entire surface, although not as pronounced as on anterior segments, arranged in short arcuate rows. Ventral welts not well defined. Caudal segment obliquely truncate, with posterior spiracles lying in very slight depression; pair of low, ventrolateral perispiracular lobes present (mostly on young instar). Anus with pair of conical papillae. Anterior spiracle semi-circular in shape, with 5–6 spiracular lobes (Fig. 2C). Posterior spiracles black, darkly pigmented, rounded, separated by about their diameter (Fig. 2J); spiracular slits C or U-shaped, radiate from median ecdysial scar (although not visible). Cephaloskeleton robust (Fig. 2A), with broad intermediate sclerite and stocky mouthhooks; dental sclerite triangular; labial sclerites present, anterior pair (ligulate sclerites) subtriangular, posterior sclerite (subhypostomal sclerite) positioned medially with central fenestrated region (Fig. 2B).
Puparium
Previously described by Skidmore (1985). Characterized by conspicuous, strongly raised carina or acute cuff-like margin encircling deeply concave perispiracular field. Posterior spiracles blackish, rounded, separated by about their diameter. The puparia were observed encased in a frothy cocoon presumably produced by the mature larva. As far as known, all puparia of Philornis are always contained in these cocoons (Dodge and Aitken, 1968; Skidmore, 1985). Cocoon production is known to occur in at least three subfamilies of Muscidae and consists of sand grains, soil and other particles surrounding the larva, stuck together with sticky secretions from the mouth of the larva which later hardens (Skidmore, 1985; Ferrar, 1987). Cocoons are formed in a number of different families of Cyclorrhapha (e.g. Anthomyiidae, Lauxaniidae, Playpezidae, Sarcophagidae, Sepsidae and Tephritidae) (Ferrar, 1987).
General
Externally the larval instars are very similar except in size and development of spiracles. First instar larvae lack anterior spiracles and the posterior spiracles have only 2 slits each. Second instars possess anterior spiracles and the posterior spiracles are like first instars. Third instar larvae also have anterior spiracles, but the posterior spiracles are darkly pigmented and have 3 C-shaped slits (Fig. 2J). The larval instars can also be identified on the basis of the cephaloskeleton. The intermediate sclerites of first instar larvae are fused to the basal sclerite and the mouthhooks comprise a single component (i.e. lack dental sclerites). The intermediate sclerite is separated from the basal sclerite in second and third instar larvae; however, the mouthhooks distinctly differ. The dental sclerite of second instars is greatly prolonged and slender, tapered and arched to a slender apex, whereas the dental sclerite is triangular and not greatly lengthened in third instar larvae.
The cephaloskeleton and mouthhooks of all 3 instars are very similar to those of Philornis torquans Nielsen. However, this is the only other species in which all larval instars are well known (see Skidmore, 1985). The dilated mouthhooks described in the latter species are likely in reference to the cuticular teeth-like spines near the oral opening. Blood was observed in the gut of third instar larvae.
Male flies collected during this study differed greatly in size (due to host size, see below) and colouration of the legs and body. Large individuals normally had bright yellowish legs, whereas the legs of smaller males were darkened apically or were mostly entirely dark. The postpronotal lobes also varied from yellowish to greyish.
Adults have not been observed near nests and are only known from reared or malaise trapped specimens (Fessl et al. 2001).
DISCUSSION
Since its discovery in 1997, P. downsi has been considered a potential threat to the endemic and native avifauna of the Galápagos Islands (Fessl and Tebbich, 2002; Dudaniec et al. 2006; Wiedenfeld et al. 2006). Despite the massive impacts the fly appears to be having on Darwin's finches, little is known about its life-cycle or that of the genus in general (but see Teixeira, 1999; Arendt, 2000). Philornis downsi is possibly closely related to P. nielseni Dodge and P. mimicola Dodge, on the basis of the cuff-like caudal segment of the puparium (Skidmore, 1985). The larvae of P. nielseni are subcutaneous feeders forming tumours on the nestlings skin (Skidmore, 1985). The marked shift in the host site specificity of P. downsi (phase 1: first and second instar larvae cause myiasis inside nasal cavities; phase 2: second instar larvae exits cavity and develops into third instar larvae living as nest-dwelling haematophagous larvae), is, to our knowledge, unique for Philornis. This marked shift in the host site specificity is likely to be more widespread in the genus since the habits of many species are unknown. A similar life-cycle is reported in some species of bird blow flies, Protocalliphora Hough (Calliphoridae) (Sabrosky et al. 1989).
One important aspect of the life-cycle of P. downsi that remains unknown is whether the larvae in the nasal cavities of the nestlings are deposited as eggs or if P. downsi is viviparous, laying first instar larvae as reported for P. torquans Nielsen (Skidmore, 1985). The mouthhooks of the first instar larvae with the tooth-like spines near the oral opening certainly appear well adapted to penetrating into the host. Some authors suggest that females of Philornis lay directly on the nestlings (Arendt, 1985a), while others suggest that eggs/larvae are laid in the nest material and larvae then seek out their hosts (Couri, 1999; Teixeira, 1999). Protocalliphora is believed to lay eggs either in clusters directly on the nestlings or along the edge of the nest (Sabrosky et al. 1989). In any case, development must be very rapid, since larvae were found in the nasal cavities of 1-day-old nestlings. Similarly, Arendt (1983) observed parasitism by P. deceptivus Dodge & Aitken on 1-day-old nestlings, and Snyder et al. (1987) reported larvae on 2-day-old nestlings.
Fessl and Tebbich (2002) found that the number of parasites per nest differed according to nestling age (e.g. fewer parasites were found in nests with nestlings younger than 8 days compared to nests with nestlings more than 8 days of age). We now know that this difference in parasite intensity with nestling age is due to first or second instar larvae living as myiasis producers and thus remaining undetected in nests with young nestlings. Even by carefully examining the body of a dead nestling, these tiny larvae can be difficult to find. In many cases, we only discovered the first and second instar larvae after the nestling body had been submerged in alcohol for several hours (usually 3–12 h). The discovery of the myiasis agent in the life-cycle of P. downsi is important to better understand and thereby develop efficient control methods for this parasitic species. Interestingly, nests treated with insecticide at an early nestling phase (day 2) were not found to be re-infested thereafter (Fessl et al. 2006).
The tiny larvae in the nasal cavities likely affect the breathing abilities of nestlings. In many cases, the nostrils of nestlings and fledglings were much larger than normal. S.K. had caught several adult ground finches with expanded nostrils. Possible negative secondary effects of expanded nostrils are unknown. In addition to larvae in nasal cavities, we observed larvae in feather quills in 2004 and 2005, as well as wounds under wings, on legs and on the abdomen (probably caused by larvae). These openings were often places of secondary infection, leading to infestation with larvae from the flesh flies Sarcodexia lambens Wiedemann and Blaesoxipha plinthopyga Wiedemann (Fessl et al. 2006).
The very dry conditions in these two study years and the consequent strenuous rearing conditions for birds may have reinforced the impact of Philornis parasitism. Other researchers have found – contrary to this statement – an augmentation of Philornis parasitism in wetter seasons or years (Arendt, 1985b; Nores, 1995). Santa Cruz harbours a humid highland zone, which stays relatively wet even during dry years. The humid zone might act as a reservoir for flies, as non-parasitic adult flies may find enough food to persist and to disperse from the moist highlands to the arid lowlands for nidification (discussed by Wiedenfeld et al. 2006). In Protocalliphora, the adults are believed to overwinter (Sabrosky et al. 1989).
Philornis infestation was shown to have a strong effect on nestling mortality in Darwin's finches (Fessl et al. 2006). Nests that were parasite-free following the use of an insecticide had more than twice the fledging success compared to nests with non-manipulated parasite intensity (88·6% versus 33·9% fledging success, respectively). Parasite-induced mortality in relation to parasite intensity was found in other studies (all these Philornis species are sub-cutaneous parasites) (Arendt, 1985b; Delannoy and Cruz, 1991; Nores, 1995), but numerous factors interact with parasite numbers to affect mortality including host species, nestling age, rainfall, and larval site specificity (reviewed by Dudaniec and Kleindorfer, 2006).
In this study, we report on exceedingly high levels of blood loss – 18 to 55% – due to Philornis parasitism. Gold and Dahlsten (1983) found that daily blood loss of over 10% was likely to lead to physical deficiencies and severe health problems, whereas losses higher than 25% were lethal. Kovach et al. (1969) reported blood losses from 35 to 50% prior to mortality. Blood loss was over 10% for all 3 study years and far greater than 25% for the few unsuccessful nests in 2005. Dudaniec and Kleindorfer (2006) have shown a significant relationship between high parasite intensity and low haemoglobin-levels as well as reduced fledgling success. The difference in haemoglobin values of nearly 40% of nestlings from infested and experimentally non-infested nests also provides evidence for anaemia in parasitized Darwin's finch nestlings (Fessl et al. 2006).
No behavioural observations are available for adult flies, an important parameter for understanding the life-cycle and for developing efficient control methods. Arendt (2000) invested much effort to study the distribution of adult Philornis in a tropical forest and the question of how females find the host nests, but with inconclusive results. Females of some species seem to lay their eggs/larvae irrespective of nestling age (Young, 1993) but, in most studies, there was a peak in infestation during the middle of the nestling-period and no larvae attached close to the time of fledging (Oniki, 1983; Arendt, 1985a). In P. downsi, nasal cavities were not infested after day 7 of nestling age (half of the nestling period) and after day 9 at other sites of myiasis. We often found fly larvae from different size classes in nests, e.g. larvae in nasal cavities, plus second and third instar larvae in the nest or mature third instar larvae ready to pupate together with third instar larvae still requiring bloodmeals. This suggests multiple infection, also known from other Philornis species (Hector, 1984; Arendt, 1985b; Young, 1993). R. Dudaniec and S. Kleindorfer (personal communication) are currently investigating this important aspect of Philornis biology.
The finding of high fitness costs due to the introduced parasitic fly that is now widely distributed across the Galápagos archipelago is of high international conservation significance. The introduced fly is a significant threat to small bird populations including the medium tree finch, Camarhynchus pauper, which only occurs on Floreana Island (about 300 breeding pairs left), and the mangrove finch, Cactospiza heliobates, which only occurs on Isabela Island, with an estimate of only 50 breeding pairs (Dvorak et al. 2004). Nests of the mangrove finch were monitored between 1996 and 2005 and Philornis-induced chick mortality increased during that period (personal communication H. Vargas). Nesting success in the medium tree finch was monitored for the first time in 2006; nestling mortality was high (1 fledgling from 6 nests) and parasitism by P. downsi severe (around 40 larvae per nestling) (Kleindorfer and O'Connor, unpublished observation). Urgent efforts are required to protect Darwin's finches and other endemic passerines from the current massive threat of parasitism.
This work was supported by grants from the Galápagos Conservation Trust to S.K. and B.F., the Basler Stiftung to B.F. and the American Bird Conservancy and Flinders University Establishment Grant to S.K. A Darwin Foundation Grant (awarded to Cardiff Museum, Wales) aided B.J.S. in examination of the CDRS Invertebrate collections. Charles Griswold (California Academy of Sciences) kindly arranged a loan of Galápagos Muscidae to B.J.S., which included the earliest record of P. downsi. We thank the Galápagos National Park Service and the Charles Darwin Research Station for the opportunity to work on the Galápagos Archipelago and especially the Invertebrate Department for their support. TAME airlines provided reduced airfare to the Galápagos. We thank Rachael Dudaniec, Jeremy Robertson, Rebekah Christensen, Sarah Huber, Jeff Podos, David Wiedenfeld, Gustavo Gimenez, Jorge Carrion Tacuri and Carlos Vinueza for field assistance, and Jeremy Robertson, André Mauchamp and two referees for helpful comments on the manuscript.