Introduction
Strepsiptera is an endoparasitic order of Insecta that has a unique and complex life cycle caused by the extreme sexual dimorphism of the adult males and females. Adult males are winged and free-living, and actively seek females to mate. However, adult females are permanently endoparasitic (except those in Mengenillidae), and only their cephalothorax extrudes from the host's gaster (abdomen) to allow for copulation and release of mobile first-instar larvae (Kinzelbach, Reference Kinzelbach1971; Kathirithamby, Reference Kathirithamby1989, Reference Kathirithamby2009).
There are eight extant families of Strepsiptera, the three largest being Halictophagidae (115 species), Stylopidae (163) and Xenidae (117) (Kathirithamby, Reference Kathirithamby, Foottit and Adler2018). However, many species of the large genus Stylops may have been misidentified. Thus, based on molecular identification, the genus likely has around 67 valid species (Straka et al., Reference Straka, Juzova and Nakase2015), instead of 117 (Kathirithamby, Reference Kathirithamby, Foottit and Adler2018). Stylops stylopizes important bee pollinators of Andrena, causing the inhibition of ovary development (Pèrez, Reference Pérez1886; Smith and Hamm, Reference Smith and Hamm1914; Kathirithamby, Reference Kathirithamby1989, Reference Kathirithamby2009). Within Canada, Peck (Reference Peck and Bousquet1991) reported four species of Stylops, namely S. advarians, S. childreni, S. leechi and S. vicinae. Without disclosing their identity, Straka (Reference Straka2019) recently reported 15 species of Stylopidae.
Despite these established host–parasite relationships, there are surprisingly little data on standard life-history characteristics (prevalence, intensity, abundance) of strepsipteran parasites infecting their host populations, and much of the data cover a single year of investigation alone. Therefore, greater attention to annual studies at a common location to discover fluctuations in life-history traits over consecutive years is required. Strepsipterans that stylopize true bugs have been examined in this regard (Waloff, Reference Waloff1981; Melber, Reference Melber1989; Melber and Pohl, Reference Melber and Pohl1997; Roy and Hazra, Reference Roy and Hazra2016), whereas fewer studies have focused on Stylopidae and Xenidae (see Pierce, Reference Pierce1909 and references therein), which infect bees and wasps, respectively. Pierce (Reference Pierce1909) found that the intensity of stylopized bees was 1–3 parasites per host, but abundance data that include non-stylopized bees were not provided. In addition, for five Andrena species collected from several studies and locations, prevalence varied widely (35–90%) among species (Pierce, Reference Pierce1909). Furthermore, all Stylops were extruded dorsally, from between the host bee's 4th and 5th gastral segments, in all Andrena species examined (Pierce, Reference Pierce1909).
A single species, Stylops crawfordi, has been examined in different years, but at different locations. In Dallas, Texas, USA, Pierce (Reference Pierce1909) recorded that 35% of Andrena crawfordi were parasitized by S. crawfordi. Several decades later, Jones and Jones (Reference Jones and Jones1981) found the prevalence of S. crawfordi infesting A. crawfordi at College Station, Texas to exceed 25%, before declining to 10% a few days later. Whereas male puparia were readily found by Pierce (Reference Pierce1909), Jones and Jones (Reference Jones and Jones1981) were unable to collect males, which may have lowered the prevalence.
The general difficulty of finding males of Stylopidae could be due to their timing of emergence, as observed in Xenidae. Hrabar et al. (Reference Hrabar, Danci, McCann, Schaefer and Gries2014) observed the synchronous maturation of females and males of Xenos peckii; males emerge from their puparia concurrently when females protrude from their hosts' gasters, thereby increasing the likelihood of males finding female strepsipterans. Mating is also aided by a behavioural change observed in female Andrena infected by Stylops (Linsley and MacSwain, Reference Linsley and MacSwain1957; Straka et al., Reference Straka, Rezkova, Batelka and Kratochvíl2011). Male Andrena normally emerge earlier than females (Westrich, 1989, as cited by Straka et al., Reference Straka, Rezkova, Batelka and Kratochvíl2011), but stylopized females were observed emerging simultaneously with both non-stylopized and stylopized males (Brandenburg, Reference Brandenburg1953; Linsley and MacSwain, Reference Linsley and MacSwain1957; Straka et al., Reference Straka, Rezkova, Batelka and Kratochvíl2011). The advanced emergence of stylopized female bees ensures that all host bees would be outside of their nest, increasing the probability that when a male Stylops emerges, a mature female will be available to mate. Males of Stylops pacifica have been observed mating with a female while her host bee was foraging (Linsley and MacSwain, Reference Linsley and MacSwain1957; Daly et al., Reference Daly, Doyen and Purcell1998), whereas others were only found mating while bees were near their nest site (Ulrich, Reference Ulrich1933). The minute size and short life-span of these males also contribute to the difficulty in collecting them (Kathirithamby, Reference Kathirithamby2009).
Over three consecutive seasons (2016–2018), we have undertaken an investigation of Stylops advarians interacting with its host, Andrena milwaukeensis, at a regular site within the western Canadian prairies. Phoresy and structure of the first-instar larvae (Balzer and Davis, Reference Balzer and Davis2019a) and adult male (Balzer and Davis, Reference Balzer and Davis2019b) of S. advarians have been described. The objectives of this article, however, are to provide standard life-history traits (prevalence, intensity and abundance) detailing the ecology of S. advarians within this population of A. milwaukeensis, and to investigate the seasonal dates of the parasite's activity, including initial emergence of the stylopized hosts and the emergence of the host-seeking first-instar Stylops larvae from the adult female parasites. Accordingly, this work also advances our general understanding of the potential impact of Strepsiptera on bees, which are particularly beneficial as pollinators.
Materials and methods
Study site
Specimens of S. advarians Pierce were collected by capturing their foraging host, bees of A. milwaukeensis Graenicher, from 2016 to 2018. In early May each spring, bees of A. milwaukeensis were found foraging on Shepherdia flowers at the northeast region of Cosmopolitan Park, a natural setting within Saskatoon, SK, near the South Saskatchewan River (52°07′43.8″N, 106°38′50.2″W). As the season progressed, bees of A. milwaukeensis were taken 1.1 km southwest (52°07′18.0″N, 106°39′25.2″W) several days later (Fig. S1). Moreover, two of four bees collected on 17 April 2019 for histological investigation near the newly constructed Collaborative Sciences Research Building (CSRB) at the University of Saskatchewan (52°07′56.4″N, 106°37′57.6″W) were found to be unusual in terms of parasite intensity and the host body's location of the extrusion of the parasite in the host body, and are included herein.
Specimen collection and analysis for parasitism
Adult bees of A. milwaukeensis were collected by sweep-netting and placed in vials. Bees of this species are discernible in the field due to their reddish-orange tergal hairs, and afterwards their identity was confirmed by their long clypeus and rounded pygidial plate (LaBerge, Reference LaBerge1980). Bees of A. milwaukeensis were examined for stylopization (the presence of strepsipteran parasites extruded from the gaster) using an Olympus SZ-ST dissecting microscope. Non-stylopized bees, and most of the stylopized bees, were euthanized using vapours of ethyl acetate; however, stylopized bees not immediately euthanized were used to collect first-instar larvae that emerged from the extruded female parasites in late May (Balzer and Davis, Reference Balzer and Davis2019a).
Results
Seasonal occurrence of S. advarians
From 2016 to 2018, neotenic females of S. advarians were encountered in foraging bees of A. milwaukeensis as early as 2 May around the northeastern area of the study site. Bees of this species were no longer found at this location after 8 May, but instead were collected further southwest 6–10 days later, where they were found visiting flowers of Cotoneaster and Syringa. Thus, bees of A. milwaukeensis, both stylopized and non-stylopized, were continually collected as the season progressed throughout May and most of June. Host bees of A. milwaukeensis do not die upon the emergence of males of S. advarians; indeed, bees with empty puparia were occasionally collected several weeks after the emergence of the male from the puparium. After 22 June, bees of A. milwaukeensis ceased to forage.
In 2016 and 2017, only female bees were collected. Upon micro-inspection of these hosts, only neotenic females of S. advarians were found with the exception of one female bee collected on 14 May 2018, which had two intact puparia (males) (Balzer and Davis, Reference Balzer and Davis2019b). In 2018, male bees also were caught until they ceased to be present from 18 May onward. Male bees were also stylopized.
Of 455 foraging bees of A. milwaukeensis captured from 2016 to 2018, the stylopized bees from our study site held between 1 and 3 parasites each (Tables S1–S3). However, our summarized data (Figs 1–3) do not differentiate between bees that had one, two or three female Stylops; instead, these figures simply illustrate the presence or absence of Stylops. The frequency of stylopized bees collected per day did not show a clear pattern over the 3-year sampling period (Figs 1–3), except for early in the season. Stylopized female bees were regularly caught around 2 May, with multiple days early each spring where all, or a relatively high proportion of collected bees, were stylopized (Figs 2 and 3). Indeed, non-stylopized bees were only occasionally seen from 2–4 May (Figs 2 and 3). In 2016, however, bee sampling was not undertaken in the northeast region of the study site, so the presence of stylopized bees in that area during early May is uncertain (Fig. 1).
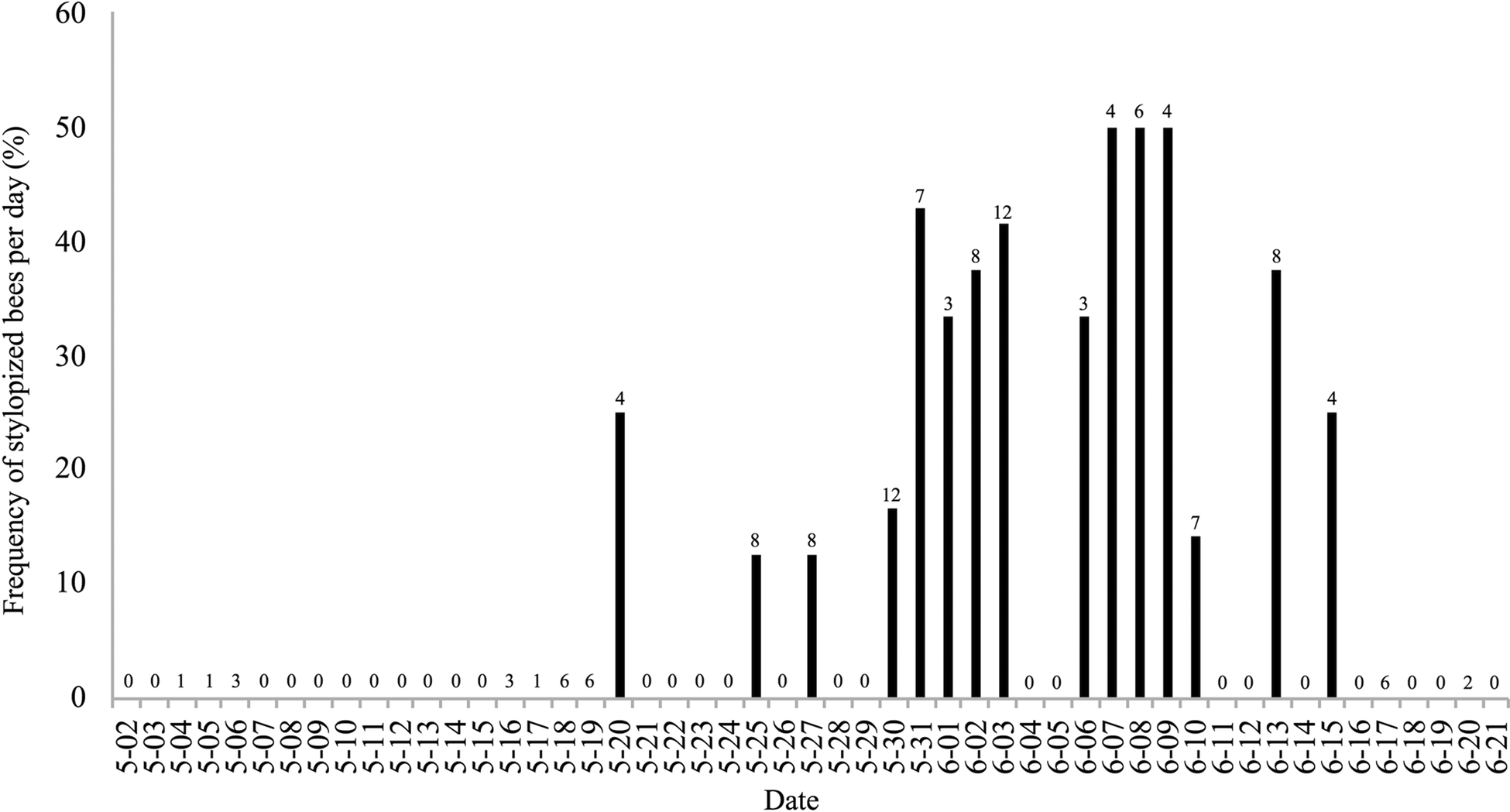
Fig. 1. Bar graph showing the frequency of stylopized bees of Andrena milwaukeensis collected each day in 2016. The number above each bar represents the total bees collected that day. Dates without bars are either days where collection did not occur (0), or stylopized bees were not encountered.
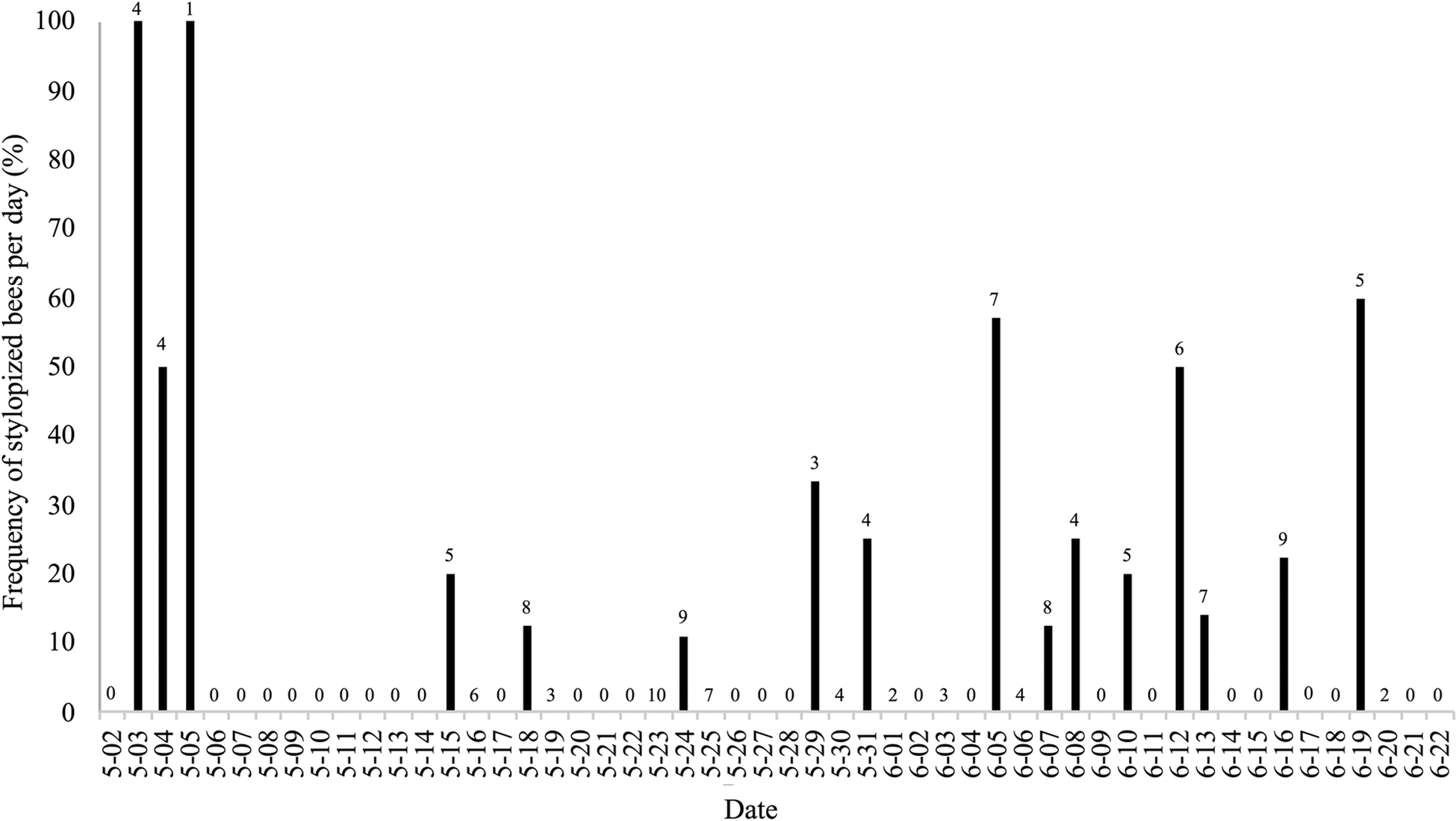
Fig. 2. Bar graph showing the frequency of stylopized bees of Andrena milwaukeensis collected each day in 2017. The number above each bar represents the total bees collected that day. Dates without bars are either days where collection did not occur (0), or stylopized bees were not encountered.
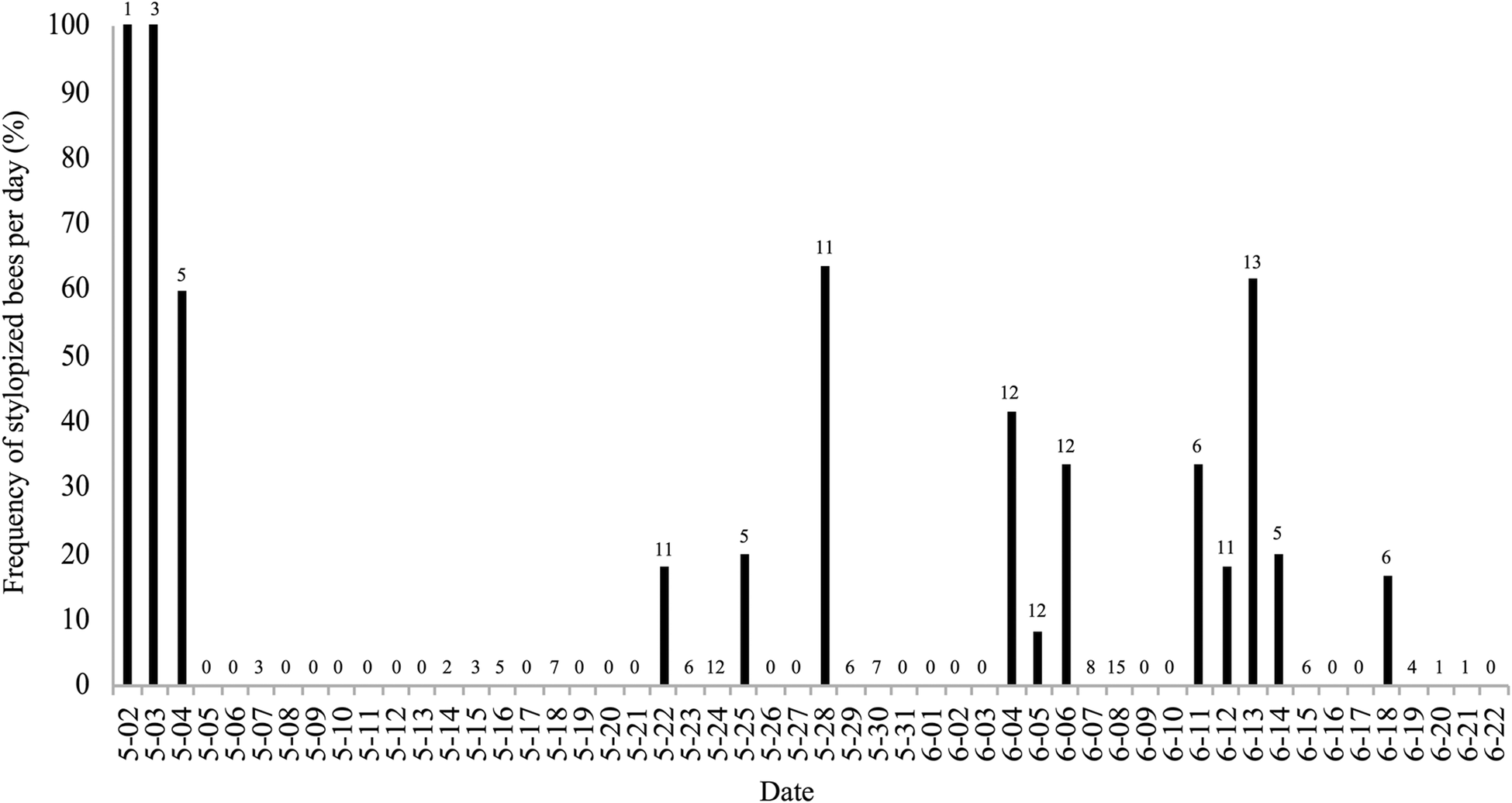
Fig. 3. Bar graph showing the frequency of stylopized bees of Andrena milwaukeensis collected each day in 2018. The number above each bar represents the total bees collected that day. Dates without bars are either days where collection did not occur (0), or no stylopized bees were not encountered.
The number of stylopized bees collected before 8 May in 2017 and 2018 was significantly higher than the number of stylopized bees collected afterward (2017: ![]() $\chi _{1\,{\rm df}}^2 $ = 22.216; N = 151 bees, P < 0.01; 2018:
$\chi _{1\,{\rm df}}^2 $ = 22.216; N = 151 bees, P < 0.01; 2018: ![]() $\chi _{1\,{\rm df}}^2 $ = 10.66; N = 199, P < 0.01).
$\chi _{1\,{\rm df}}^2 $ = 10.66; N = 199, P < 0.01).
No seasonal pattern for the occurrence of multiple S. advarians per collected host was apparent. Instead, such hosts were encountered randomly. For example, in 2017, the four bees with two S. advarians in their gaster were taken on 5, 15 and 29 May and 12 June (Table S2). Corresponding collection dates for eight similar hosts in 2018 were 2, 4, 22 and 28 May and 4, 6, 12 and 18 June (Table S3).
Stylops advarians is viviparous, and from year to year, the host-seeking first-instar larvae were observed emerging from the brood canal opening of several of the endoparasitic, neotenic females at similar dates. First-instars were initially observed on 25 May 2016, on 24 May 2017 and on 22 May 2018. Larvae exhibited asynchronous development inside their mother's body (Kathirithamby, Reference Kathirithamby2009). Thus, many more larvae emerged at dates thereafter, as well as were found within the same adult female's body, following these initially recorded emergences each year.
When mean temperature data (J. Diefenbaker International Airport, Saskatoon; 6 km from the study site) for spring–summer of 2016–2018 were plotted (Fig. S2), we could not discern correlations between temperature and the dates of advanced emergence of stylopized bees, nor dates of appearance of first-instar larvae.
Prevalence, intensity and abundance of S. advarians
Table 1 displays the annual sampling of bees of A. milwaukeensis collected from 2016 to 2018, as well as the parasite load of the stylopized bees. These results include only bees that held female parasites; bees with intact or empty male puparia were very rare (<0.7%).
Table 1. Number of bees of Andrena milwaukeensis sampled each year, including the number of neotenic females of Stylops advarians extruding per host gaster

Prevalence, mean intensity and abundance for each year of the collection are also indicated.
The maximum number of Stylops females found per bee gaster during the collection period was three (Fig. 4), with the parasite intensity ranging from 1.17 to 1.24 and averaging 1.2 (Table 1). Of the total of 455 bees of A. milwaukeensis collected during May and June of 2016–2018, 356 (78%) lacked a female parasite (Fig. 4). Eighty-two bees (18%) had one female parasite (Figs 4 and 5A). Thirteen bees (3%) had two female parasites (Figs 4 and 5B), and four bees (1%) had three female parasites (Figs 4 and 5C). Thus, most bees lacked any sign of parasitism by a female S. advarians. Thus, the mean abundance of S. advarians was 0.27 (range 0.26–0.28; Table 1).

Fig. 4. A bar graph showing the intensity of infection by adult females of Stylops advarians within the Andrena milwaukeensis population of Cosmopolitan Park in Saskatoon, Saskatchewan sampled from 2016 to 2018. Percentages are shown above each bar.

Fig. 5. Photographs of dorsal (A–E) and ventral (F) surface of gasters of Andrena milwaukeensis infected with Stylops advarians. (A) Gaster stylopized by one neotenic female. Note protruding cephalothorax of the adult female parasite. (B) Gaster stylopized by two neotenic females. (C) Gaster stylopized by three neotenic females. (D) A neotenic female at the midline of the host bee's gaster. (E) Partially dissected gaster with two neotenic females and evidence of two male puparia. (F) Gaster showing an empty Stylops puparium. cph, cephalothorax of adult female Stylops; ep, empty puparium; p, intact puparium.
Most neotenic females of S. advarians protruded between the 4th and 5th tergites on the left or right side of the host bee's metasoma. A female parasite occupied the left or right side of the host's gaster, without significant bias (![]() $\chi _{1\,{\rm df}}^2 $ = 1.634; N = 42 bees, P = 0.439). When three females were present per host, one female occupied each of the left and right sides of the host, whereas the third parasite protruded near the midline of the bee's gaster (Fig. 5C). Rarely was a bee found with a single female parasite residing at the centre of its gaster (Fig. 5D). When a bee infected with one central female parasite was found, that parasite was wider than those that resided laterally within a bee's gaster.
$\chi _{1\,{\rm df}}^2 $ = 1.634; N = 42 bees, P = 0.439). When three females were present per host, one female occupied each of the left and right sides of the host, whereas the third parasite protruded near the midline of the bee's gaster (Fig. 5C). Rarely was a bee found with a single female parasite residing at the centre of its gaster (Fig. 5D). When a bee infected with one central female parasite was found, that parasite was wider than those that resided laterally within a bee's gaster.
Unexpectedly, two abnormal specimens were acquired very early in spring (17 April 2019) at a different site (CSRB), during the intended collection of bees of A. milwaukeensis for histological investigations. One bee supported four Stylops: two puparia (one empty, one intact) and two neotenic females (Fig. 5E). The empty puparium and two females extruded between the 4th and 5th tergites, but the intact puparium extruded between the 3rd and 4th. Another host gaster held a vacated puparium on its ventral side, extruding between two sternites (Fig. 5F). Both host bees were captured while foraging on Salix catkins.
Discussion
The relatively advanced seasonal emergence of overwintered female bees of A. milwaukeensis that are stylopized corroborates previous observations (Brandenburg, Reference Brandenburg1953; Linsley and MacSwain, Reference Linsley and MacSwain1957; Straka et al., Reference Straka, Rezkova, Batelka and Kratochvíl2011). As suggested by the latter, a Stylops female may physiologically manipulate its bee host – if also female – to emerge from her nest earlier than non-stylopized female bees. Consequently, stylopized female bees emerge concurrently with stylopized and non-stylopized male bees, thereby increasing the overlap of strepsipteran activity within a vicinity; as a result, the opportunity for a male of S. advarians to find a female should increase.
Over three consecutive years of sampling, however, free-living adult males of S. advarians remained elusive, not being encountered at the study site. This difficulty was likely due to factors such as their small size, the synchronization necessary between male emergence from their puparia coinciding with female maturation (Hrabar et al., Reference Hrabar, Danci, McCann, Schaefer and Gries2014), and their short life-span (Kirkpatrick, Reference Kirkpatrick1937; Kathirithamby, Reference Kathirithamby2009). As a result, free-living males unnoticed during their short lives go undetected for that entire season. In California, however, males of S. pacifica were recorded mating while the female's host bee was foraging on flowers of Ranunculus californicus (Linsley and MacSwain, Reference Linsley and MacSwain1957; Daly et al., Reference Daly, Doyen and Purcell1998). Stylopized bees of another Andrena species were observed to emerge earlier than normal, but did not leave their nest site, presumably because flowers had not yet begun blooming (Ulrich, Reference Ulrich1933). We suggest that at this study location, stylopized A. milwaukeensis emerge before the flowers they feed on bloom; thus, mating of S. advarians may take place at the bees' nest sites, rather than at open flowers. Unfortunately, nests of A. milwaukeensis were not found, so this supposition awaits confirmation.
Nonetheless, the annual mating period of S. advarians at this site likely occurs on, or shortly before, 2 May, when stylopized bees were first observed. This conclusion is based on inaugural observations of stylopized bees recorded in spring, the synchronization of male emergence with female maturation (Hrabar et al., Reference Hrabar, Danci, McCann, Schaefer and Gries2014), the lack of intact puparia detected within bee hosts after 2 May, the detection of eggs in female Stylops collected in early May (unpublished results), and the emergence of first-instar larvae around 3 weeks after initial observation of the stylopized bees. Capture of a single individual bearing two intact puparia on 14 May 2018 very likely reflects that these two males died in situ (Balzer and Davis, Reference Balzer and Davis2019b), rather than that the mating period of S. advarians extends over a 2-week span. After exposure of these puparia to the bright light of a microscope lamp failed to stimulate their emergence (James et al., Reference James, Nandamuri, Stahl and Buschbeck2016), the males were declared deceased, due to the short life-spans of male Stylops (Kathirithamby, Reference Kathirithamby2009) and, for unknown reasons, having been unable to escape their puparia. The study by Jones and Jones (Reference Jones and Jones1981) also suggests that all males emerge and mate around the same time, as they could only detect empty puparia. Pierce (Reference Pierce1909) found 44 bees of A. crawfordi hosting males of S. crawfordi, potentially within their puparia, though this possibility was not stated. Unfortunately, no dates of collection were given for those bees and their parasites.
Owing to the short life-span of adult males of S. advarians, only females were regularly collected. Because females of S. advarians reside within their host as neotenic adults, A. milwaukeensis bees were collected continuously throughout their foraging season to sample their female parasites. Stylopized bees were observed earliest on flowers of Shepherdia, whereas non-stylopized bees were much less commonly encountered at this time. Both stylopized and non-stylopized bees were later found further southwest, visiting Syringa and Cotoneaster. The bees' presence at different foraging areas at the study site was likely stimulated by the successive depletion of pollen and nectar sources from each taxon upon which they visited.
Inaugural issuance of first-instar larvae of S. advarians from their mothers occurred on similar dates each year. This comparable emergence in three consecutive springs suggests that mating of S. advarians occurs at similar times each year, assuming the length of development from embryo to the emergence of first-instar larvae is consistent. Linsley and MacSwain (Reference Linsley and MacSwain1957) estimated that the development of first-instars of S. pacifica, from insemination to emergence, takes 30–40 days. Moreover, Jones et al. (Reference Jones, Williams and Jones1980) reported an incubation period of 2–3 weeks for the eggs of S. bipunctatae. The histological study of eggs within adult females of S. advarians taken around 2 May (Balzer, Reference Balzer2019), as well as first emergence of the viviparous first-instar larvae around 22 May, corroborates that the duration of egg incubation of S. advarians resembles that of S. bipunctatae (Jones et al., Reference Jones, Williams and Jones1980). Like in other Stylops species (Ulrich, Reference Ulrich1956; Linsley and MacSwain, Reference Linsley and MacSwain1957), the first-instar larva of S. advarians is deposited on flowers. By phoresy, it may be transported either internally or externally by an adult bee of A. milwaukeensis (Balzer and Davis, Reference Balzer and Davis2019a) to its nest, where the parasitic larva eventually can invade its host.
This study's prevalence data pertain only to stylopization by at least one female of S. advarians, the single bee with two puparia (males) being excluded. Thus, the true level of prevalence is undoubtedly higher than 22%. Beyond the fortuitous discovery of additional host bees with puparia (males), another line of research that would indirectly estimate the proportion of males of S. advarians in this population would be the determination of the sex ratio of the first-instar larvae. Assuming that both sexes have an equal probability of successfully parasitizing a host, this approach may clarify the true prevalence of S. advarians within this population of A. milwaukeensis.
The annual prevalence of stylopized bees of A. milwaukeensis remained consistent (21–24%) for three consecutive years of sampling. These values accord to two independent studies from different locations in Texas, USA, with an initial level of prevalence (25%) for S. crawfordi parasitizing A. crawfordi (no males), before a recorded decline to below 10% (Jones and Jones, Reference Jones and Jones1981). Sampling during the foraging season of S. crawfordi spanned around 30 days but actually was curtailed to only 13 days of collection, due to rain (Jones and Jones, Reference Jones and Jones1981). In the other study of this same bee–parasite relationship, wherein male parasites were included, the prevalence was 35% (Pierce, Reference Pierce1909). In a separate strepsipteran–host relationship, Hughes et al. (Reference Hughes, Kathirithamby and Beani2004) found that 7% of Polistes dominula at their nest were stylopized, but found 25% of overwintering wasps to be stylopized. The low percentage of stylopized wasps on the nest were workers which usually abandon the nest, whereas the higher percentage was of gynes that aggregate to overwinter. The consistency in the prevalence of S. advarians from 3 years at the same site may reflect the vast numbers of larvae that each female parasite produces, as well as the minute probability that each first-instar successfully parasitizes a host. Since so many first-instar larvae are produced per female, annual fluctuation in the proportion of these larvae that successfully find a host evidently remains low. This presumably low success rate would cause the number of bees that become infected with S. advarians to remain similar each year, leading to similar prevalences.
Prevalence may be different between strepsipteran species due to several factors. The particular environment that these parasites and their hosts occupy, plus their resultant behaviour, may govern each parasite's success. For example, the behaviour of stylopized eusocial wasps of Polistes is different than that of the stylopized solitary bees of Andrena. Stylopized wasps of the former forego their social tasks and leave their colony to aggregate at the same site, facilitating mating by the parasites (Hughes et al., Reference Hughes, Kathirithamby and Beani2004). Andrena infected by Stylops, however, continue their normal behaviour of foraging and nest making (Linsley and MacSwain, Reference Linsley and MacSwain1957). Strepsipterans may also manipulate their host, as seen with Xenos females potentially causing their wasp host to spend more time on specific plants in order to increase the success of their larvae being deposited in that favourable place. Makino and Yamashita (Reference Makino and Yamashita1998) found that Xenos moutoni manipulates its Vespa host to visit holes and scars in trees that produce sap in order to facilitate the dispersal of first-instar larvae. More recently, Beani et al. (Reference Beani, Cappa, Manfredini and Zaccaroni2018) found that Polistes wasps stylopized by a female Xenos spent more time at Campsis radicans flowers, likely for a similar reason. The prevalence of species within most other strepsipteran families is unclear and needs further study.
The intensity of parasitism throughout the collection period of 2016–2018 never exceeded three females per host. However, early in 2019, a specimen was captured with four parasites of S. advarians within its gaster (Fig. 5F), which we have not found documented for any Stylops species. Most of the 455 bees (78%) were not stylopized, and the majority of those that were had just one parasite (18%). Occasionally, bees were found with two female parasites (3%), but very rarely (1%) were three female parasites present in a host. Thus, the mean abundance of parasitism was 0.27 and remained annually consistent (range 0.26–0.28). The low mean intensity (1.2) of S. advarians in A. milwaukeensis bees is most likely due to the difficulty of a first-instar successfully finding and surviving within a permanent host. It is likely rare for a single larva of S. advarians on a flower to successfully travel with an adult bee to its nest and parasitize one of that phoretic host's offspring. It is therefore a successively rarer event for two, three or even four strepsipteran first-instar larvae to be carried by a single adult bee to the bee's nest, and then successfully parasitize the same immature bee in that nest. That intensity evidently rarely exceeds three strepsipterans per host also suggests that the gaster of an individual host may reach its carrying capacity. Supportive indirect evidence includes the expanded state of a centrally-located single parasite, compared to laterally-positioned parasites (see below).
Females of S. advarians were most often found protruding between the 4th and 5th tergites of their host's gasters, even when three females were present. The consistent location of the females within their host may signify that this position provides the female with the greatest reproductive success (Maeta et al., Reference Maeta, Gôukon, Kitamura and Miyanaga2001). This location within the host allows the female parasite to grow to the anterior end of her host's gaster, without impacting important organs of the host in a debilitating way. A longer body may allow the strepsipteran female to produce more offspring. Female parasites were also often found in the lateral area of their host's abdomen in single- and double-parasite infections.
Occasionally, bees were found to have just one female Stylops that protruded from the midline of their gasters and, unlike the findings of Linsley and MacSwain (Reference Linsley and MacSwain1957), we did not find undeveloped Stylops on either side of this single, central female. The few centrally-residing females observed were wider than those found laterally, and perhaps this larger space permits increased growth of the parasite that allows more larvae to be produced. However, if the number of eggs a single female produces is genetically based, as observed in Drosophila (Robertson, Reference Robertson1957), or is governed by nutrient acquisition (Rivero et al., Reference Rivero, Giron and Casas2001), the lateral or central placement within the host would be irrelevant in regard to the production of larvae. That most females were found laterally, rather than centrally within their host's gaster, likely signifies how the female parasite is supported by her host's organs (unpublished results). However, there was no preference to which side of the host that a neotenic female occupied.
The consistency in the annual values of several life-history traits (prevalence, intensity and abundance), as well as their narrow ranges for 2016–2018, suggests that the host–parasite system of S. advarians and A. milwaukeensis at this location in western Canada is currently in balance. On the other hand, collections of several specimens of A. milwaukeensis in 2019 near the newly-constructed CSRB at the University of Saskatchewan on April 17, as they foraged on Salix catkins, revealed some unique features. This date is about 2 weeks earlier than bees of A. milwaukeensis were normally collected during 2016–2018 at the main study site. On that same date (17 April 2019), the main study site was examined and no A. milwaukeensis were observed. Foraging bees found near the CSRB may have nests near the building, and the soil may have warmed more quickly than the ground at the main site, perhaps related to the recent construction or presence of the building. Temperature increases are known to advance the emergence of ground-nesting bees, elsewhere (Schwartz and Karl, Reference Schwartz and Karl1990; Schenk et al., Reference Schenk, Mitesser, Hovestadt and Holzschuh2018). One of these bees was infected with four Stylops parasites. The presence of four Stylops in one bee of A. milwaukeensis is likely an extremely rare event, as this is the only specimen with four Stylops found in over 3 years of sampling. Another of these collected bees had an empty puparium on the ventral side of its gaster. This strategy for ventral protrusion by a male did not preclude its successful emergence from this host body's position, but it would seem detrimental for the females of S. advarians because of a potential impediment for a male to mate with a female residing in this ventral position on the host. Protruding from the tergites rather than the sternites is most often seen and hence likely the most successful strategy. As far as we know, this is the first recorded event of four Stylops parasites within a single Andrena bee, and the first time a Stylops puparium has been detected on the ventral side of the gaster of an Andrena bee. On the other hand, dorsal- and ventral-protruding strepsipterans are a common occurrence in wasps stylopized by xenid strepsipterans (Maeta, Reference Maeta1963, Reference Maeta1971; Kifune and Maeta, Reference Kifune and Maeta1975; Maeta et al., Reference Maeta, Gôukon, Kitamura and Miyanaga2001).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020000037.
Acknowledgements
We thank Kelton Braun for his help collecting several stylopized bees. Additionally, we thank three anonymous reviewers for their constructive critiques of this submission.
Financial support
This work was supported by a University of Saskatchewan Graduate Scholarship, and an Arthur Brooks Award from the Entomological Society of Saskatchewan (ZSB), plus an NSERC Discovery Grant (ARD), for which we are grateful.
Conflict of interest
None.
Ethical standards
Not applicable.









