INTRODUCTION
Cutaneous leishmaniasis (CL) is a disease caused by protozoan parasites of the genus Leishmania, which are transmitted to humans by the bite of sandflies. In Mexico this disease is caused by Leishmania mexicana, resulting in either localized cutaneous leishmaniasis (LCL) or diffuse cutaneous leishmaniasis (DCL). Leishmania infects the vertebrate host, principally affecting phagocytic cells of the immune system such as macrophages and dendritic cells. Leishmania presents two phenotypically distinct stages during the life cycle: the promastigotes and the amastigote. The promastigote is the extracellular stage and responsible for infection of host cells. Within host cells promastigotes transform into amastigotes and initiate their replication, most importantly within macrophages (Liese et al. Reference Liese, Schleicher and Bogdan2008). During invasion, promastigotes tend to resist and modulate the host immune functions, due in part to a dense surface glycocalyx largely composed of glycosylphosphatidylinositol (GPI) anchored molecules. The most abundant glycoconjugate on the parasite surface is a virulence factor known as lipophosphoglycan (LPG). It has been shown that LPG is recognized as a PAMP (pathogen-associated molecular patterns) by Toll-like receptors, triggering signal transduction pathways that upregulate the production of pro-inflammatory cytokines (de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003; Kavoosi et al. Reference Kavoosi, Ardestani and Kariminia2009). Also, it has been reported that LPG from Leishmania major activates NK cells through TLR2 (Becker et al. Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, Gonzalez, Maldonado and Isibasi2003).
Early studies have shown that the control of infection of some Leishmania species requires different TLRs that recruit and activate signalling molecules of the innate immune response. TLR2 recognition has been shown for L. major (de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003; Kavoosi et al. Reference Kavoosi, Ardestani and Kariminia2009) as well as for Leishmania braziliensis, where TLR2-deficiency had an impact on disease outcome in mice infected with this parasite (Vargas-Inchaustegui et al. Reference Vargas-Inchaustegui, Tai, Xin, Hogg, Corry and Soong2009). TLR4 has also been shown to have a role in controlling L. major growth (Kropf et al. Reference Kropf, Freudenberg, Modolell, Price, Herath, Antoniazi, Galanos, Smith and Muller2004). Additionally, in Leishmania pifanoi-infected TLR4-deficient mice, an increase in parasite burdens was observed (Whitaker et al. Reference Whitaker, Colmenares, Pestana and McMahon-Pratt2008). TLR9 was found to be essential in the control of infection with L. major, L. braziliensis and Leishmania infantum (Liese et al. Reference Liese, Schleicher and Bogdan2008; Tuon et al. Reference Tuon, Amato, Bacha, Almusawi, Duarte and Amato Neto2008).
TLRs activate several signalling cascades, which include mitogen-activated protein kinases (MAPK) (Means et al. Reference Means, Golenbock and Fenton2000; Jung et al. Reference Jung, Yang, Lee, Shin, Jung, Son, Harding, Kim, Park, Paik, Song and Jo2006) that constitute a superfamily of serine/threonine kinases. Three major subgroups of MAP kinases are known in mammalian cells including extracellular signal-regulated kinases 1 and 2 (ERK), c-Jun amino-terminal kinases (JNK) and the p38 MAP kinase. ERK is activated by mitogens and growth factors, while cellular stress stimuli, such as UV light, osmotic changes, thermal shock and inflammatory cytokines induce the activation of JNK and p38 MAP kinases. MAPK modulate many cellular events, including cell cycle progression, regulation of embryonic development, cell movement and apoptosis (Vitale et al. Reference Vitale, Bernardi, Napolitani, Mock and Montecucco2000; Kyriakis and Avruch, Reference Kyriakis and Avruch2001; Ben-Othman et al. Reference Ben-Othman, Guizani-Tabbane and Dellagi2008). It is known that differential signalling triggered by ERK or p38 MAP kinase can play an important role in the type of response after stimulation (Feng et al. Reference Feng, Goodridge, Harnett, Wei, Nikolaev, Higson and Liew1999). For example, the activation of p38 MAP kinase is associated with the production of IL-12 and IL-10 can inhibit the activation of ERK (Lu et al. Reference Lu, Yang, Wysk, Gatti, Mellman, Davis and Flavell1999; Suttles et al. Reference Suttles, Milhorn, Miller, Poe, Wahl and Stout1999). These kinases are essential for the control of Leishmania infections (Junghae and Raynes, Reference Junghae and Raynes2002) and a reciprocal regulation of ERK and p38 MAP kinase favours a better response of pro-inflammatory cytokines during early stages of infection (Mathur et al. Reference Mathur, Awasthi, Wadhone, Ramanamurthy and Saha2004). It has been shown that Leishmania donovani promastigotes successfully suppress the production of pro-inflammatory cytokines in order to create a favourable environment in host cells for parasite survival (Chandra and Naik, Reference Chandra and Naik2008).
Our group previously reported that L. mexicana LPG induced a differential production of cytokines in human dendritic cells (DCs) and monocytes (Argueta-Donohue et al. Reference Argueta-Donohue, Carrillo, Valdes-Reyes, Zentella, Aguirre-Garcia, Becker and Gutierrez-Kobeh2008). Moreover, in monocytes of patients with LCL or DCL, LPG significantly reduced the production of TNF-α and IL-12p40 (Carrada et al. Reference Carrada, Caneda, Salaiza, Delgado, Ruiz, Sanchez, Gutierrez-Kobeh, Aguirre and Becker2007).
Although it has been shown that Leishmania possesses ligands for TLRs and that the engagement of these receptors is important for the infection, it is necessary to investigate the kinases that are involved in this activation and the cytokines that are produced.
The aim of the present study was to investigate the participation of TLR2 and TLR4 in the production of cytokines and to explore the possible phosphorylation of ERK and/or p38 MAP kinase in human macrophages stimulated with L. mexicana LPG. Our data show that L. mexicana LPG activates TLR2 and TLR4 in human macrophages leading to ERK and p38 MAP kinase phosphorylation and production of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10.
MATERIALS AND METHODS
Macrophage purification from peripheral blood
Peripheral blood monocytes were obtained from the buffy coats of blood from healthy donors (kindly supplied by the blood bank of the Centro Médico Nacional Siglo XXI, IMSS). Peripheral blood mononuclear cells (PBMC) were separated by using Ficoll–Hypaque (Sigma, St. Louis, MO, USA) density gradient centrifugation at 300 g for 20 min at 20 °C, and suspended in pyrogen-free and sterile phosphate-buffered saline pH 7·2. They were then incubated with CD14 MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) during 15 min and purified by magnetic sorting. CD14+ monocytes (1×106) were washed and left overnight in pyrogen-free and sterile RPMI-1640 medium (Life Technologies Laboratories, Gaithersburg, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) and 2 mm L-glutamine (Gibco Invitrogen Corporation Carlsbad, CA, USA). They were then cultured in 6-well tissue culture plates (Costar, Cambridge, MA) for 5 days at 37 °C, with 5% CO2 in a humidified atmosphere. Cell viability was assessed by trypan blue exclusion. Purity was analysed by flow cytometry, using the CD14, CD80, CD86, CD40 antibodies (BD, Bioscience, San Jose, CA, USA).
Parasites
Leishmania mexicana (MHOM/MX84/ISETGS) promastigotes isolated from a patient with DCL, were grown in RPMI-1640 medium (Life Technologies Laboratories) supplemented with 10% heat-inactivated FBS at 26 °C.
LPG purification
LPG was purified from L. mexicana promastigotes, as previously described (Delgado-Dominguez et al. Reference Delgado-Dominguez, Gonzalez-Aguilar, Aguirre-Garcia, Gutierrez-Kobeh, Berzunza-Cruz, Ruiz-Remigio, Robles-Flores and Becker2010). Briefly, parasites were sub-cultured every 4–5 days and grown to a density of 2×107 mL−1. Promastigotes were harvested from stationary-phase cultures, centrifuged at 3200 g for 10 min, washed three times with PBS, and finally counted after immobilization with glutaraldehyde (0·1%). The supernatant was removed and the pellet was extracted with chloroform/methanol/water (4:8:3, v/v) for 30 min at room temperature. The insoluble material (delipidated residue extracted and not soluble in chloroform/methanol) was used for LPG extraction with 9% 1-butanol in water (2×50 mL) and the pooled supernatants were vacuum-dried. LPG was purified from this fraction by high-performance liquid chromatography (HPLC), using two octyl-sepharose columns (each with a 1-propanol gradient of 5–60% in 0·1 m ammonium acetate) to optimize LPG purity. The LPG samples proved negative for the presence of endotoxin, evidenced by the Limulus sp. amebocyte lysate assay (E-Toxate Kit; Sigma). A sample was shown to be devoid of protein contaminants through analysis by SDS–PAGE followed by silver staining.
Cytokine assays
A total of 1×106 human macrophages were stimulated with 10 μg mL−1 of LPG from L. mexicana for 24 h. After this incubation time, macrophages were stimulated with 10 μg mL−1 L. mexicana LPG in 1 mL RPMI-1640 medium supplemented with 10% heat-inactivated FBS during 24 h at 37 °C and 5% CO2. For positive controls, cells were stimulated with 100 ng mL−1 of lipopolysaccharide (LPS). In some conditions macrophages were pre-incubated for 2 h, either with 40 μ m PD98059 (sc-3532), an inhibitor of ERK, or 20 μ m SB203580 (sc-3533), an inhibitor of p38 MAP kinase (both inhibitors from Santa Cruz, CA, USA) and dissolved in 1 m of dimethylformamide (Sigma). For experiments with the blocking antibodies for TLRs, macrophages were first pretreated with anti-human IgG antibody (500 μg mL−1) (Aventis Behering GmbH, Marburgo, Germany) for 1 h at 37 °C, in order to saturate Fc receptors and avoid unspecific attachment of anti-TLR mAbs. Then macrophages were treated with 5 μg mL−1 anti-TLR2 monoclonal antibodies (clone TL2.1) or 5 μg mL−1 anti-TLR4 (clone HTA125) (Imgenex, Biocarta, San Diego, CA, USA) for 1 h at 37 °C, followed by stimulation with LPG for 24 h. Cell-free culture supernatants were harvested and the concentration of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 were determined by standard sandwich enzyme-linked immunosorbent assay (ELISA) according to BD-Pharmingen Cytokine. In brief, 96-well microtiter plates (Costar, Corning, NY) were coated with an unconjugated anti-TNF-α capture antibody (clone Mab1, 6 μg mL−1), anti-IL-1β (clone Mab 1, 4 μg mL−1), anti-IL-10 capture antibody (clone JES3-19F1, 4 μg mL−1), anti-IL-12 p40/capture antibody (clone C8.3, 8 μg mL−1) or anti-IL-12 p70 (clone 20C2, 2 μg mL−1) in 100 mm Na2HPO4, pH 9·0 for 12 h at 4 °C, and blocked with phosphate-buffered saline pH 7·4, supplemented with 5% casein, dissolved in 0·1 N NaOH. Cell supernatants and recombinant hTNF-α standard, hIL-1β standard, hIL-10 standard, hIL-12 p40 standard, hIL-12p70 standard were incubated in RPMI-1640 medium supplemented with 10% FBS for 2 h at room temperature. Bound human TNF-α, IL-1β, IL-12 p40/p70 were detected using a biotinylated anti-mouse antibody in 1% BSA for 1 h. Human IL-10 was detected using a biotinylated rat anti-hIL-10. All antibodies and recombinant cytokines were from BD-Pharmingen, San Jose, CA, USA. The plate was developed using streptavidin alkaline phosphatase conjugate with p-nitrophenyl phosphate 4 mg mL−1 (Life Technologies) as substrate. Plates were read at 405 nm using a microtiter (EL 321e BIO-TEK instruments), and the concentrations were calculated from a standard curve of recombinant human TNF-α, IL-1β, IL-12 p40, IL-12 p70 and IL-10. The concentration of each sample was calculated by regression analysis using the mean absorbance (based on the average of triplicates of each sample).
RT-PCR of TLR2 and TLR4 mRNA
For RT-PCR analysis of TLR2 and TLR4 mRNA expression, 1×106 macrophages were stimulated with (2, 4, 6, 8 and 10 μg mL−1) of LPG in 1 mL RPMI-1640 medium supplemented with 10% FBS for 18 h at 37 °C with 5% CO2. Total RNA was extracted from macrophage cells with TRIzol and amplified with Super Script One-Step RT-PCR with Platinum Taq (Life Technologies). The cDNA products were PCR amplified with the TLR2 or TLR4 specific primers selected on the basis of the published human TLR2 and TLR4 sequence. The primers for TLR2 were: sense 5′-GCC AAA GTC TTG ATT GAT TGG-3′ and antisense 5′-TTG AAG TTC TCC AGC TCC TG-3′. The primers for TLR4 were: sense 5′ TGG AAG TTG AAC GAA TGG AAT GTG-3′ and antisense 5′-ACC AGA ACT GCT ACA ACA GAT ACT-3′ (Becker et al. Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, Gonzalez, Maldonado and Isibasi2003; Chandra and Naik, Reference Chandra and Naik2008). PCR products were separated on 1·5% w/v agarose gel and the densitometric analysis was performed by recording the intensity of the bands with a Multi Image Analyser. β-Actin was used as an internal control.
Flow cytometry analysis of cell-surface expression of TLR2 and TLR4
The extracellular expression of TLR2 or TLR4 was determined by flow cytometry. A total of 1×106 macrophages were stimulated with (2, 4, 6, 8 10 μg mL−1) of LPG for 18 h and re-suspended in blocking buffer (PBS containing total human IgG, 2% FBS, 5 mm EDTA and 0·1% sodium azide) and incubated on ice for 30 min. Afterwards, cell suspension was centrifuged 300 g and washed twice with PBS and stained with phycoerythrin (PE)-conjugated mouse anti-human TLR2 (sc-21759) and anti-human TLR4 (sc-13593) both of Santa Cruz Biotechnology. The isotype controls used to exclude non-specific staining were PE-conjugated anti-IgG2a (Santa Cruz Biotechnology). The cells were incubated for 20 min in the dark and washed twice with washing buffer (FACS). The cells were analysed by flow cytometry (FACSCanto II, Becton-Dickinson, San Jose, CA), and analysed with DIVA software (Becton Dickinson). The results are reported as Mean Fluorescence Intensity (MFI).
Western blot analysis
A total 1×106 macrophages were treated under different conditions: non-stimulated macrophages were used as the negative control for the kinetics of phosphorylation of ERK and p38MAP kinase; macrophages incubated with 10 μg mL−1 of L. mexicana LPG for different times: 5, 10, 15, 30 and 60 min; macrophages pre-incubated with the ERK inhibitor (40 μ m) or the p38 MAP kinase inhibitor (20 μ m), and subsequently stimulated with L. mexicana LPG; macrophages treated with 5 μg mL−1 of anti-TLR2 monoclonal antibodies (clone TL2.1) or 5 μg mL−1 of anti-TLR4 (clone HTA125) for 1 h 37 °C, followed by stimulation with LPG. All cell extracts from the different conditions were lysed with RIPA buffer (TrisHCI, pH 7·4 10 mm, NaCl 150 mm, EDTA 1 mm, NaF 10 mm, NP-40 1%, cocktail protease and phosphatase inhibitors; all obtained from Sigma) and then centrifuged at 150 g for 10 min at 4 °C. Afterwards, the pellet was removed and an aliquot was used for protein determination by the DC method (Biorad Laboratories, Hercules, CA, USA) compatible with detergents. Subsequently the samples were analysed by Western-blot assays.
Proteins were resolved by 10% SDS–PAGE in Tris/glycine/SDS buffer (25 mm Tris, 0·1% SDS) (Biorad Laboratories) and then electrotransferred onto Immobilon-P transfer membranes (Millipore, Billerica, MA, USA). Membranes were washed with TBS-T (20 mm Tris–HCl, 150 mm NaCl, 0·005% Tween 20) and blocked by treatment with 3% albumin in TBS-T for 1 h. They were then immunoblotted with polyclonal goat-anti mouse p38 MAP kinase (C-20), p-p38 MAP kinase (D-8), ERK (K-23) and p-ERK (E-4) (all from Santa Cruz) at a dilution of 1 : 5000 in 1% albumin in TBS-T overnight at 4 °C. After 1 h of washing with TBS-T, membranes were incubated with secondary HRP-conjugated goat anti-mouse IgG (Biomeda, Foster City, CA, USA; dilution 1/10 000) and washed five times in TBS-T. Bands were detected using enhanced chemiluminescence (Super- Signal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA), according to the manufacturer's instructions. Densitometric analysis was performed by recording the intensity of the bands of the corresponding gel with multiple image analyser (Biorad, Quantity One Analysis Software).
Western-blot for detection TLR2 and TLR4 in ODYSSEY infrared Imaging System
A total of 1×106 macrophages stimulated with different concentrations of LPG from L. mexicana (2, 4, 6, 8 and 10 μg mL−1) were lysed and analysed by Western-blot assay. Firstly, samples were resolved by 7·5% SDS-PAGE and then electrotransferred. The membranes were blocked with blocking buffer LiCor (Lincoln, NE) for 1 h at room temperature and incubated with specific antibodies (TLR2 sc-10739, TLR4 sc-30002 and Actin sc-1616, all from Santa Cruz) diluted in 0·1% Tween LiCor blocking buffer overnight at 4 °C. The membranes were washed with PBS tween and incubated with a secondary antibody IR dye 700 goat anti-rabbit IgG (H+L), and IR dye 680 conjugated donkey anti-goat IgG (H+L) at a dilution 1/10 000 for 1 h. The membranes were washed with PBS 1X and the proteins were analysed using Odyssey Infrared Imaging System (LI-COR, Lincoln, NE), according to the manufacturer's instructions.
Statistical analysis
All data are expressed as mean±s.d. Statistical evaluation of the data values was performed by the Mann–Whitney U-test. A value of P<0·05 was considered statistically significant.
RESULTS
Cytokine production induced by L. mexicana LPG in macrophages
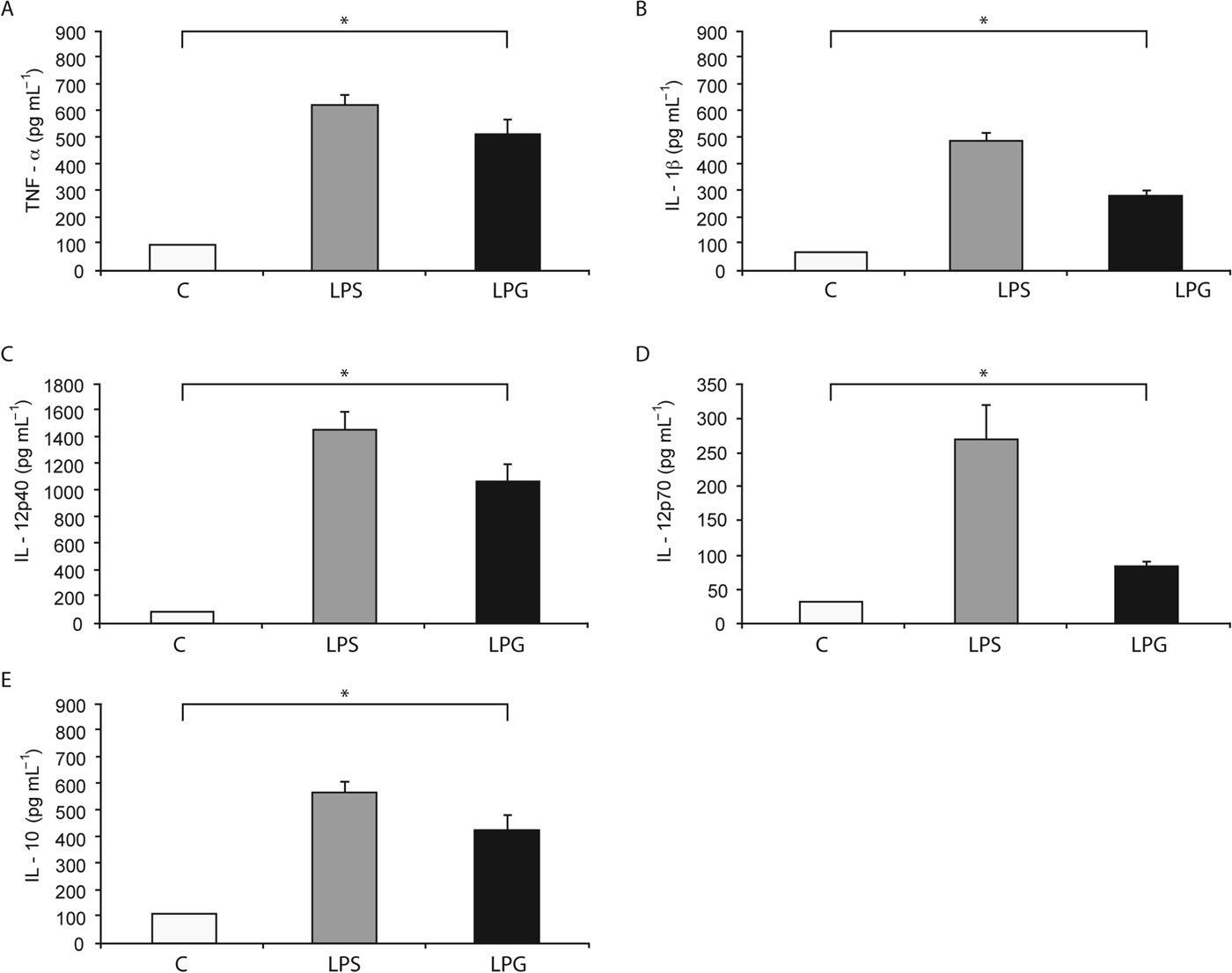
The effect of L. mexicana LPG on the production of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 by human macrophages was investigated. As shown in Fig. 1, LPG (10 μg mL−1) increased the production of TNF-α (511±53·87 pg mL−1) 5-fold, as compared to non-stimulated macrophages. LPG stimulation also led to an increase in the production of IL-1β (278±23·24 pg mL−1) (3-fold increase), IL-12p40 (1067±134·4 pg mL−1) (11-fold increase), IL-12p70 (84±9·2 pg mL−1) (2-fold increase) and IL-10 (421±56·21 pg mL−1) (3-fold increase), as compared with non-stimulated macrophages. Taken together, in all cytokines tested, we observed significant differences (P<0·05) with regard to controls using non-stimulated macrophages.
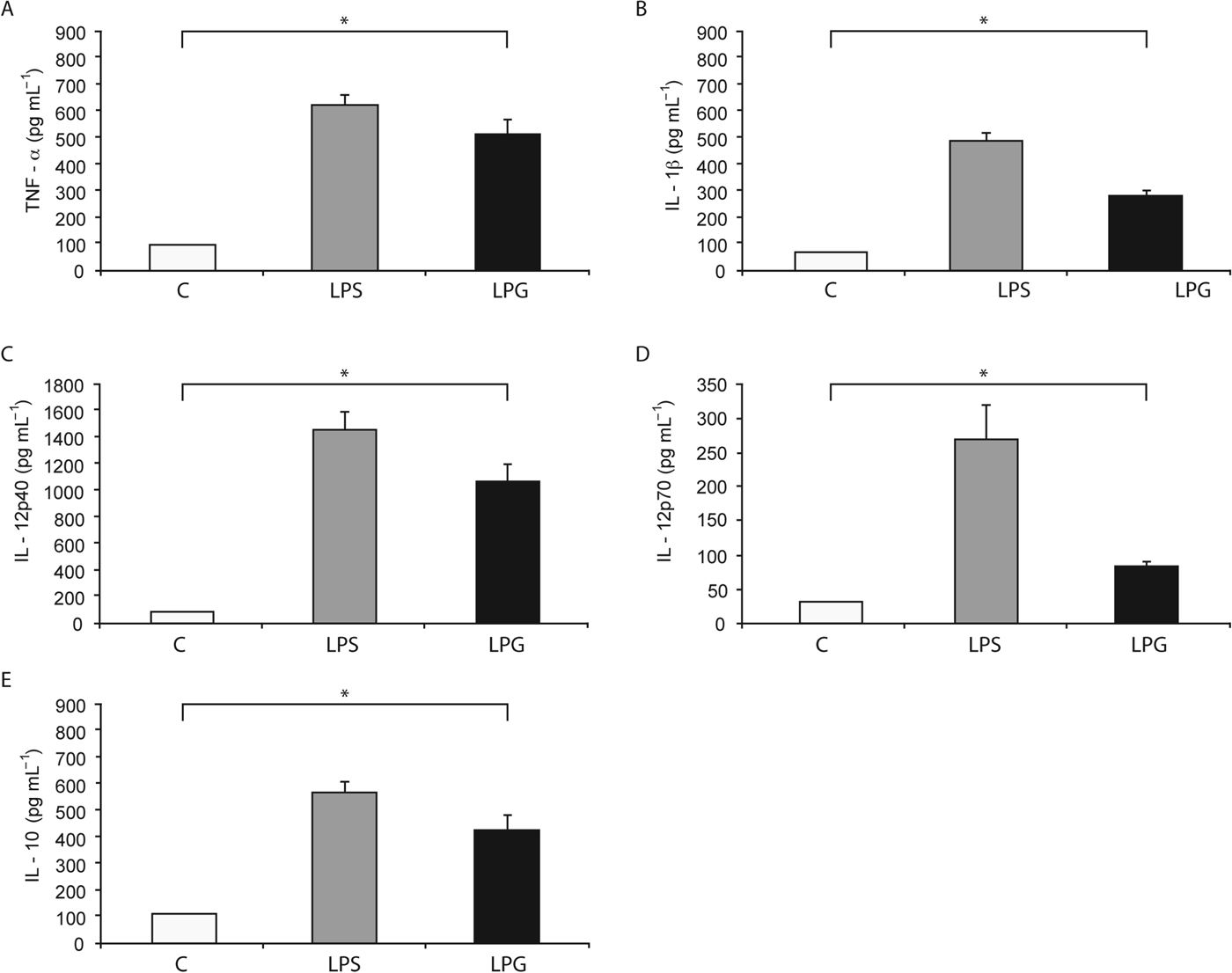
Fig. 1. Cytokines production induced by L. mexicana LPG in human macrophages. Macrophages (10×106 cell mL−1) were cultured in RPMI medium in the presence of LPS (100 ng mL−1) and LPG (10 μg mL−1). After 24 h, TNF-α (A), IL-1β (B), IL-12p40 (C), IL-12p70 (D) and IL-10 (E) production was analysed by ELISA. The bars represent mean±s.d. of five independent different experiments. Asterisks show the significant differences between macrophages stimulated with LPG and unstimulated macrophages (P<0·05).
Effect of L. mexicana LPG on TLR2 and TLR4 expression
The mechanism of induction of cytokines by Leishmania LPG is unknown. However, our group previously showed that L. major LPG induces cytokine production in human NK cells and that this LPG activation is through TLR2. In the present study we analysed the effect of L. mexicana LPG on TLR2 and TLR4 expression by evaluating the mRNA levels and protein presence in these receptors. Macrophages were incubated with different concentrations of LPG (2 to 10 μg mL−1), and all treatments resulted in a significant increase in TLR2 and TLR4 mRNA expression, as compared with control cells without stimulation (Fig. 2A). The protein presence of TLR2 and TLR4 was detected by Western blot and flow cytometry assays (Fig. 2B and C). The maximal expression of TLR2 and TLR4 mRNA and protein presence determined by Western blot was observed at 10 μg mL−1 of LPG, which was the maximal concentration tested. However when the surface protein presence of TLR2 and TLR4 was analysed by flow cytometry, the maximal expression was observed at 4 and 6 μg mL−1 of LPG for TLR2 and 8 μg mL−1 of LPG for TLR4 (Fig. 2C).

Fig. 2. Effect of L. mexicana LPG on TLR2 and TLR4 expression. Macrophages were stimulated with LPG at different concentrations (2, 4, 6, 8 and 10 μg mL−1) for 18 h. mRNA was isolated and RT-PCR performed for human TLR2, TLR4 and β-actin. Relative mRNA levels for TLR2 and TLR4 were calculated by densitometry analysis and the values in the histograms are the fold increase of TLR2/4 in treated macrophages with respect to untreated. Data are the mean±s.d. of three independent experiments (A). For protein analysis, the cells were lysed and processed for Western blot assays, the membranes were incubated with antibodies for TLR2, TLR4 and Actin and the proteins were analysed using Odyssey Infrared Imaging System (B). The blot image is representative of three independent experiments. Flow cytometry analysis of TLR2 and TLR4 extracellular expression shows the relative increase calculated by dividing the median fluorescence from stimulated macrophages by the median fluorescence from unstimulated macrophages, bars expression as mean±s.d. of three independent experiments (C). Asterisks show the significant differences between macrophages stimulated with LPG and unstimulated macrophages (P<0·05).
Participation of TLR2 and TLR4 in LPG-induced cytokine production
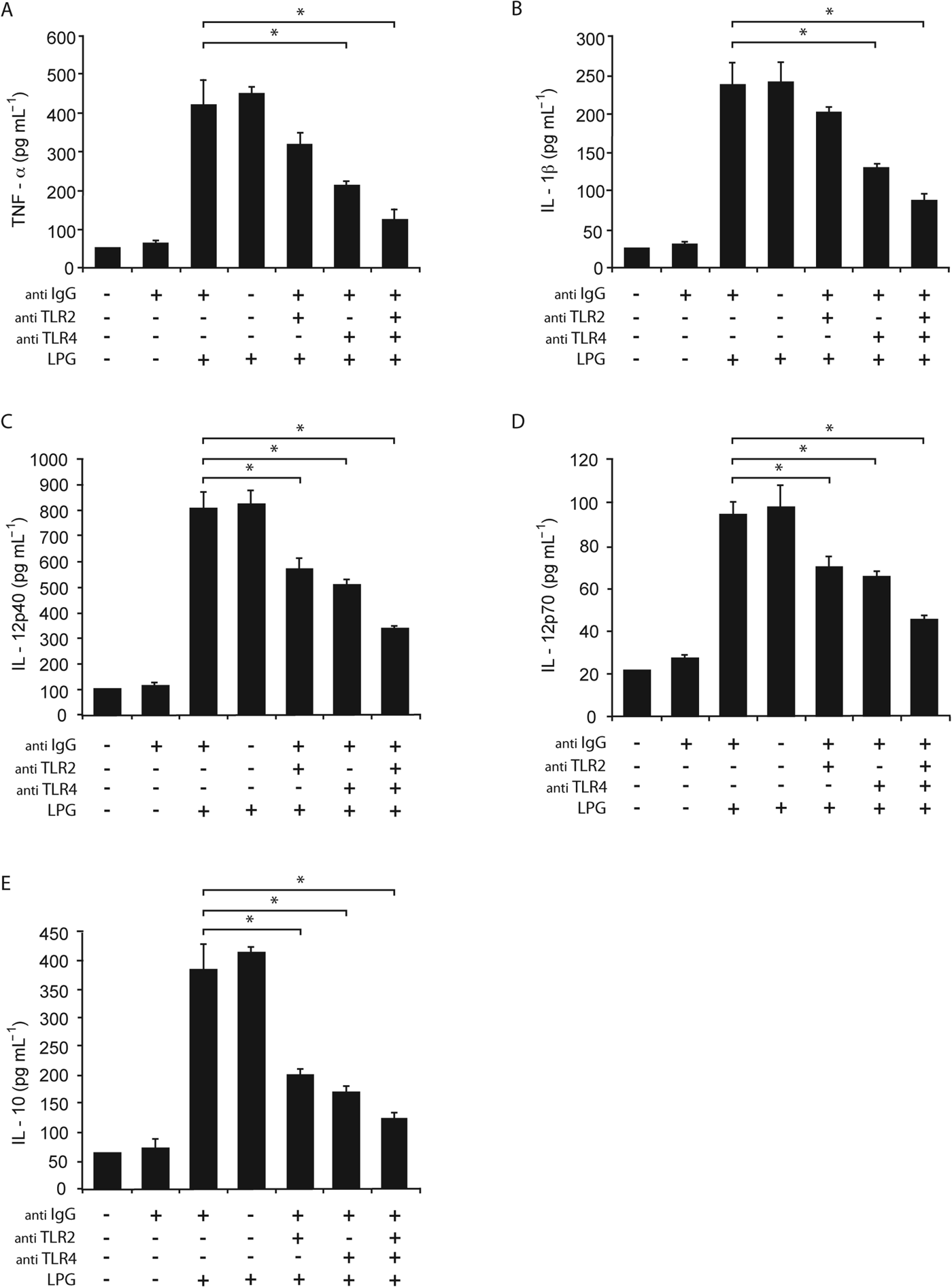
Once we determined that L. mexicana LPG induced the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10) and the expression of TLR2 and TLR4, we pursued to define whether the induction of pro-inflammatory cytokines is dependent on TLR2 and/or TLR4 activation. Macrophages were pre-incubated with an anti-TLR2 or anti-TLR4 mAb before being stimulated with L. mexicana LPG and afterwards cytokine production was measured. We found a decrease in the production of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 in macrophages incubated with anti-TLR2 or anti-TLR4 antibodies, or both, as compared with macrophages stimulated only with LPG (Fig. 3). The decrease in the production of cytokines after the pre-incubation with anti-TLR2 antibody, anti-TLR4 antibody or with both was 24, 46 and 70% for TNF-α, respectively; 19, 48 and 65% for IL-1β; 31, 38 and 59% for IL-12p40; 26, 32 and 52% for IL-12p70 and 48, 55 and 68% for IL-10, with regard to macrophages stimulated only by LPG. The major decrease in the production of cytokines was observed when macrophages were incubated with anti-TLR4 alone or in combination with anti-TLR2 (P<0·05).
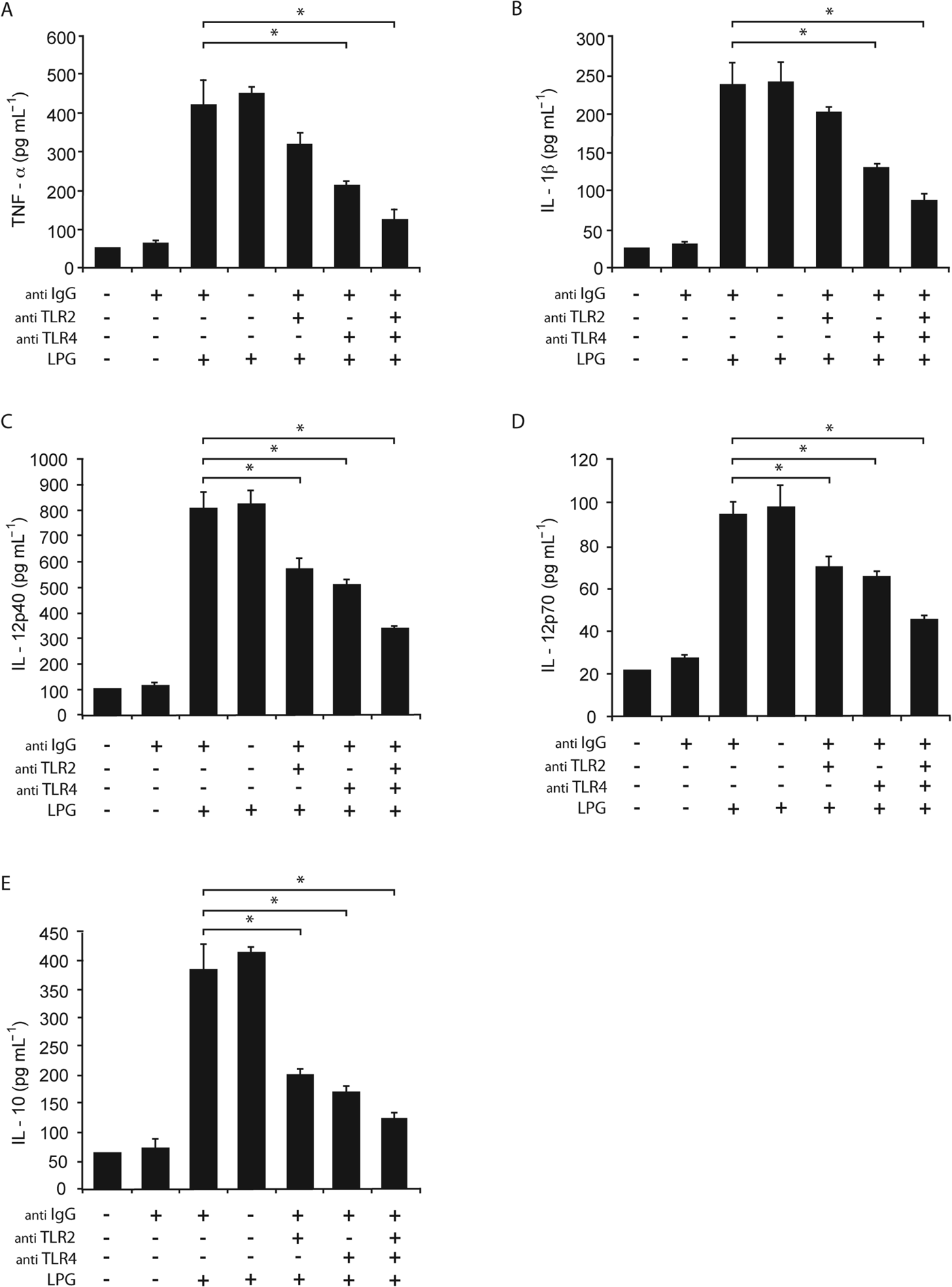
Fig. 3. Role of TLR2 and TLR4 in cytokine production induced by L. mexicana LPG. Macrophages were cultured in RPMI medium in presence of LPG (10 μg mL−1) with or without pre-treatment with anti-TLR2 (5 μg mL−1), anti-TLR4 (5 μg mL−1) or anti-IgG antibodies. After 24 h, TNF-α (A), IL-1β (B), IL-12p40 (C), IL-12p70 (D) and IL-10 (E) production was analysed by ELISA. The bars represent the mean±s.d. of six different experiments. Asterisks show the significant differences between macrophages stimulated with LPG and unstimulated macrophages (P<0·05).
Leishmania mexicana LPG induced the phosphorylation of ERK and p38 MAP kinase
We examined the activation of ERK and p38 MAP kinase in macrophages stimulated with L. mexicana LPG. Macrophages were stimulated with 10 μg mL−1 of LPG from L. mexicana for different times (5, 10 and 15 min for ERK and 5, 10, 15 and 30 min for p38 MAP kinase). As shown in Fig. 4A and B, the incubation of macrophages with LPG induced the phosphorylation of ERK and p38 MAP kinase, as determined by the densitometry analysis of ERK and p38 MAP kinase phosphorylation. The phosphorylation of ERK was observed after 5 min and reached a peak at 10 min (Fig. 4A). The highest phosphorylation of p38 MAP kinase was detected at 15 min of incubation (Fig. 4B). Macrophages were then pre-incubated for 2 h with either an inhibitor of ERK (PD98059), p38 MAP kinase (SB203580) or both inhibitors, and then stimulated with 10 μg mL−1 L. mexicana LPG for 10 min before p-ERK detection and 15 min before p38 MAP kinase (Fig. 4C and D). Western-blot analysis was used to detect the phosphorylation levels of these kinases. The phosphorylation of ERK induced by LPG in macrophages was suppressed by the ERK inhibitor, but not the p38 MAP kinase inhibitor (Fig. 4C). Likewise, the phosphorylation of p38 MAP kinase induced by LPG in macrophages was decreased by the p38 MAP kinase inhibitor but not the ERK inhibitor (Fig. 4D).

Fig. 4. Effect of L. mexicana LPG on the phosphorylation of ERK and p38 MAP kinase. Macrophages were cultured in RPMI medium in the presence or absence of LPG (10 μg mL−1) for 5, 10 and 15 min for p-ERK, and 5, 10, 15 and 30 min for p-p38MAPK. Cell extracts were prepared (10 μg protein/well) and levels of phosphorylated ERK and p38 MAP kinase were detected by Western blot using specific antibodies for (A) p-ERK and T-ERK and (B) p-p38 MAP kinase and T-p38 MAP kinase. Macrophages were pretreated with PD98059 (an ERK inhibitor) or SB203580 (a p38 MAPK inhibitor) for 2 h (control cells were not pre-treated) and incubated with 10 μg mL−1 of LPG for 10 min for p-ERK and 15 min for p-p38 MAP kinase. Lysates were subjected to SDS-PAGE and immunoblotted for (C) p-ERK and T-ERK and (D) p-p38 MAP kinase and T-p38 MAP Kinase. Relative levels of phosphorylated ERK and p38 MAP kinase were measured by densitometric analysis and values represent the mean±s.d. of four different experiments. Asterisks indicate a significant difference with P<0·05 compared with unstimulated macrophages (A, B) and macrophages stimulated with LPG (C, D).
We also observed the effect of pre-incubation with the two inhibitors together in macrophages stimulated with LPG, which resulted in a decrease of ERK phosphorylation, as compared with phosphorylation of ERK induced in macrophages incubated only with LPG (Fig. 4C, lane 5), and a partial inhibition of p38 MAP kinase phosphorylation (Fig. 4D, lane 5). Macrophages were incubation only with both inhibitors (Fig 4C and D, lane 6).
Inhibition of ERK and p38 MAP kinase decreased cytokine production induced by LPG
ERK and p38 MAP kinase pathways are known to play a central role in the regulation of the innate response, including the production of pro-inflammatory cytokines. We evaluated whether the inhibition of ERK and/or p38 MAP kinase participated in the regulation of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 after stimulating macrophages with L. mexicana LPG. LPG was able to induce the production of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 (Fig. 5), but after macrophages were preincubated for 2 h with PD98059 (an ERK inhibitor) or with SB203580 (a p38 MAP kinase inhibitor) and subsequently stimulated with LPG, we observed that pre-incubation with the ERK inhibitor led to a significant decrease in cytokine production, being 45% for TNF-α, 50% for IL-1β, 33% for IL-12p40, 23% for IL-12p70 and 51% for IL-10, as compared with macrophages stimulated with LPG. In the case of the inhibitor for p38 MAP kinase only IL-1β production decreased significantly in 38% (P<0·05).

Fig. 5. Inhibition of ERK and p38 MAP kinase decreased cytokine production. Macrophages were cultured in RPMI medium in presence of 40 μ m PD98059 or 20 μ m SB203580, and LPG (10 μg mL−1). Control cells were only treated with LPG. After 24 h, TNF-α (A), IL-1β (B), IL-12p40 (C), IL-12p70 (D) and IL-10 (E) production was analysed by ELISA. Results are the mean±s.d. of six independent experiments. (*), (**) and (***) indicates a statistically significant difference (P<0·05, P<0·01 and P<0·001 respectively) compared to macrophages stimulated with LPG.
LPG activation of ERK and p38 MAP kinase is mediated by TLR2 and TLR4
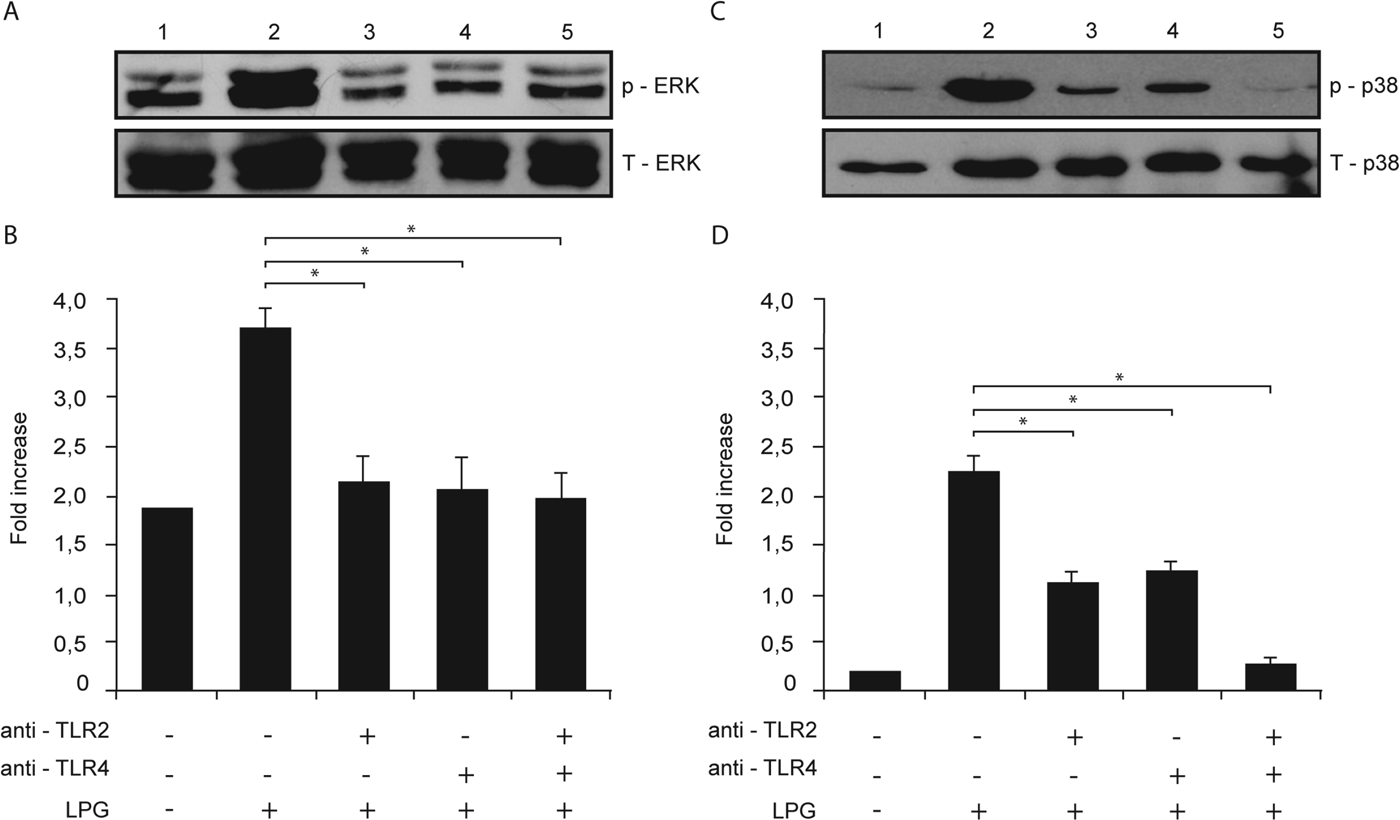
We studied the possible role of TLR2 and TLR4 in the phosphorylation of ERK and p38 MAP kinase in macrophages after stimulation with L. mexicana LPG. Macrophages were pre-incubated with anti-TLR2 or anti-TLR4 antibodies and stimulated with L. mexicana LPG, and then ERK and p38 MAP kinase phosphorylation was detected by Western-blot and a densitometry analysis was performed recording the intensity of the bands and total proteins were compared with phosphorylated ERK or p38 MAP kinase.
When macrophages were pre-incubated with either anti-TLR2 or anti-TLR4 (Fig. 6A and B) there was a similar decrease in ERK and p38 MAP kinase phosphorylation, as compared with macrophages stimulated only with LPG. Interestingly when macrophages were pre-incubated with both antibodies, p38 MAP kinase phosphorylation was almost completely inhibited (Fig. 6B).
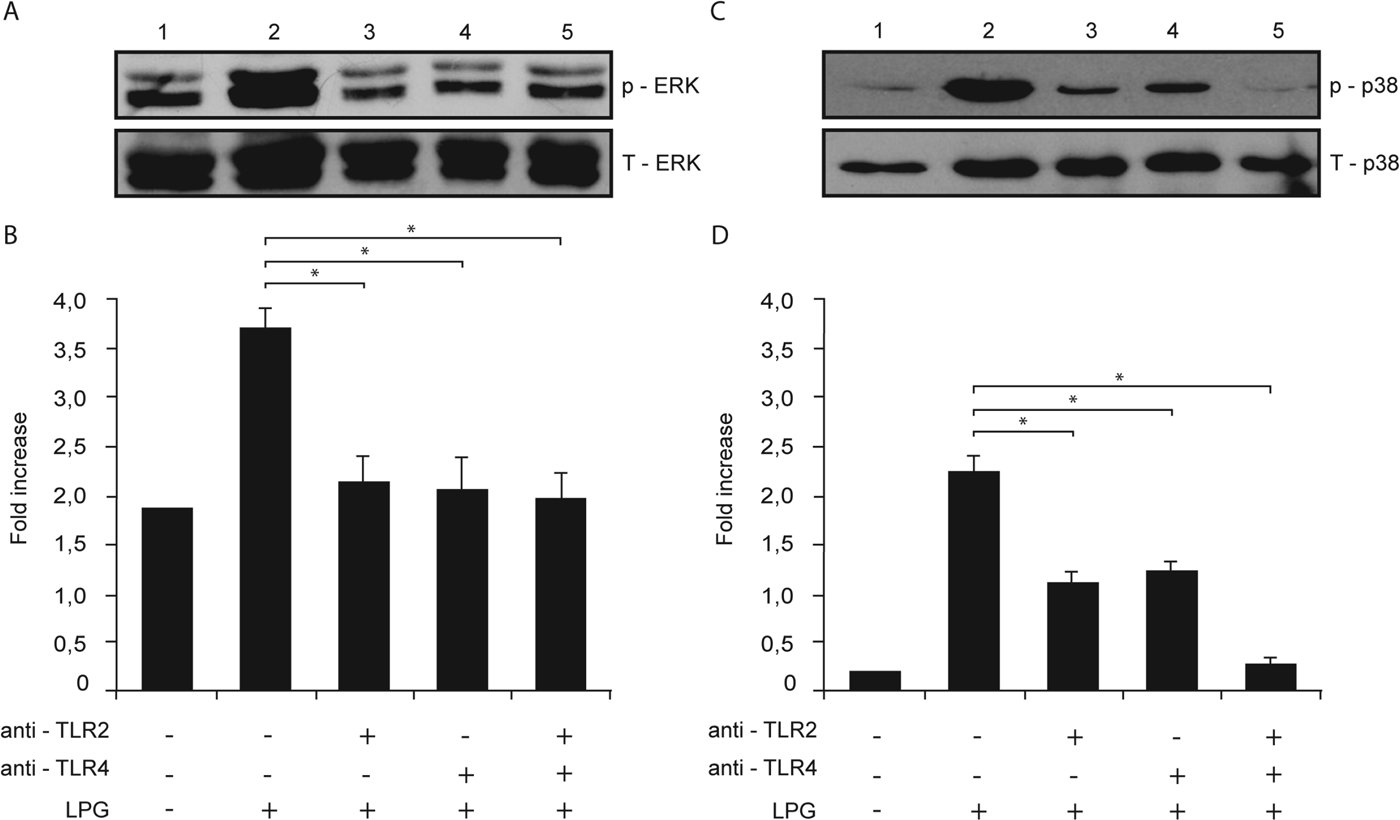
Fig. 6. LPG activation of ERK and p38 MAP kinase is mediated by TLR2 and TLR4. Macrophages were cultured in RPMI medium in presence of LPG (10 μg mL−1) with or without pre-treatment with anti-TLR2 (5 μg mL−1) or anti-TLR4 (5 μg mL−1) monoclonal antibodies. Whole cell lysates were prepared, separated by SDS-PAGE, and then assessed for (A) p-ERK and T-ERK and (B) p-p38 MAP kinase and T-p38 MAP kinase. Relative levels of phosphorylated ERK and p38 MAP kinase were measured by densitometry analysis, and values represent the mean±s.d. of four independent experiments. Asterisks indicate a significant difference with P<0·05 compared with macrophages stimulated with LPG.
DISCUSSION
It has been shown that Leishmania infection promotes an alteration in TLR signal transduction via phosphorylation mechanisms, which permits the parasite to create a favourable environment for its survival (Tuon et al. Reference Tuon, Amato, Bacha, Almusawi, Duarte and Amato Neto2008). One of the cells that are infected by Leishmania parasites are macrophages, that play a critical role in inflammatory responses through a variety of TLR receptors expressed on their surface that, upon contact with an infectious agent, trigger various signalling events that result in the activation of NF-kB and production of pro-inflammatory cytokines that contribute to effectively eliminate the parasite (Underhill and Ozinsky, Reference Underhill and Ozinsky2002).
All species of Leishmania express LPG, a GPI-anchored glycophospholipid, which is the most abundant glycoconjugate on the parasite surface (Naderer et al. Reference Naderer, Vince and McConville2004). LPG has been shown to be a ligand for TLR2 in different cells (Becker et al. Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, Gonzalez, Maldonado and Isibasi2003; de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003; Ibraim et al. Reference Ibraim, de Assis, Pessoa, Campos, Melo, Turco and Soares2013). These parasites can successfully establish an intracellular infection by employing various strategies, one of which is silencing TLR2 that decreases cytokine production of the cell, thus hampering the host defence (Chandra and Naik, Reference Chandra and Naik2008). The differential regulation of pro-inflammatory cytokine production exerted by Leishmania plays a crucial role in determining disease control or disease chronicity (Del Vecchio et al. Reference Del Vecchio, Bajetta, Canova, Lotze, Wesa, Parmiani and Anichini2007; Sharma and Singh, Reference Sharma and Singh2009). Yet little is known of the molecules and pathways involved in the modulation of the innate immune response by the parasite.
In order to analyse the effect of L. mexicana LPG on cytokine production by human macrophages and on the possible involvement of MAP kinases and TLR2 and TLR4 receptors in this event, we first analysed TNF-α, IL-1β, IL-12p40, IL-12 p70 and IL-10 produced by macrophages after stimulation with L. mexicana LPG. Our results show that LPG significantly enhances TNF-α, IL-1β, IL-12p40 and IL-10 production, with only a slight increase of IL-12p70. These results agree with previous studies of our research group, showing that L. mexicana LPG differentially induced IL-12p40, IL-12p70, TNF-α and IL-10 production in monocytes and monocyte-derived dendritic cells (moDC): whereas moDC produced higher levels of IL-12p40, monocytes produced more IL-10, yet both cells produced similar amounts of TNF-α (Argueta-Donohue et al. Reference Argueta-Donohue, Carrillo, Valdes-Reyes, Zentella, Aguirre-Garcia, Becker and Gutierrez-Kobeh2008). Additionally, our group has previously analysed cytokine production by monocytes of patients with LCL or DCL and healthy donors, stimulated with L. mexicana LPG and found that monocytes from healthy donors produced higher amounts of TNF-α, IL-12p40, IL-15, but not IL-18, as compared with monocytes from LCL and DCL patients (Carrada et al. Reference Carrada, Caneda, Salaiza, Delgado, Ruiz, Sanchez, Gutierrez-Kobeh, Aguirre and Becker2007). Our current data on cytokine production by macrophages are in accordance with the literature, where cytokine production (TNF-α, IL-10 and IL-6) has been reported in human macrophages or Raw 264·7 infected with L. major amastigotes. It is noteworthy however, that this was not observed after stimulation with L. major promastigotes (Ben-Othman et al. Reference Ben-Othman, Dellagi and Guizani-Tabbane2009). Variations in the effector responses of macrophages may be related to diverse origins of the cells or to the different Leishmania strains, whose amastigotes and promastigotes can have modifications in their surface molecules (Teixeira et al. Reference Teixeira, Fernandes, Teixeira, Andrade, Pompeu, Santana da Silva, Brodskyn, Barral-Netto and Barral2005). Additionally, TNF-α production has been shown in U-937 macrophages after stimulation with Leishmania panamensis promastigotes (Gallego et al. Reference Gallego, Golenbock, Gomez and Saravia2011). The role of TNF-α in leishmaniasis has been extensively studied in mouse models showing that this cytokine induces reactive oxygen intermediates and nitric oxide (NO), leading to macrophage activation and parasite control (Wilhelm et al. Reference Wilhelm, Ritter, Labbow, Donhauser, Rollinghoff, Bogdan and Korner2001).
In addition to the cytokine production by macrophages, we analysed the ability of L. mexicana LPG to activate TLR2 and TLR4 receptors in human macrophages. Our results show that LPG from L. mexicana induces the expression of TLR2 and TLR4 on the surface of macrophages. We further analysed if the cytokine production was related to TLR2 and TLR4 activity and found that anti-TLR2 and anti-TLR4 antibodies significantly reduced TNF-α, IL-1β, IL-12p40, IL-12 p70 and IL-10 production, after LPG stimulation. Similar results were found by Srivastav et al. (Reference Srivastav, Kar, Chande, Mukhopadhyaya and Das2012), who showed that purified L. donovani LPG induced the production of IL-12 and TNF-α in macrophages, whereas L. donovani promastigotes suppressed the production of pro-inflammatory cytokines, despite having LPG on their surface. In contrast to our study, these authors also showed that the increased production of IL-12 induced by LPG in macrophages was blocked by peptidoglycan, a well-known TLR2 agonist (Srivastav et al. Reference Srivastav, Kar, Chande, Mukhopadhyaya and Das2012). The inhibitory effect on IL-12 production after stimulation with a TLR2 agonist reported by this author may be due to the amounts of the stimulus used, since excessive stimulation can lead to the expression of inhibitory molecules. The production of cytokines (IFN-γ , IL-12 and IL-10) in PBMC after TLR2 stimulation was also analysed with L. major promastigotes, where partial inhibition of TLR2 by antibodies were shown to reduce cytokine production (Kavoosi et al. Reference Kavoosi, Ardestani and Kariminia2009). Other studies performed in a murine model of leishmaniasis demonstrated that the activation of TLR4 by L. major during immunotherapy against leishmaniasis was associated with an increase in the cure rate, since mice with mutation in the TLR4 gene were unable to cure skin lesions (Kropf et al. Reference Kropf, Freudenberg, Modolell, Price, Herath, Antoniazi, Galanos, Smith and Muller2004).
The activation of TLR receptors triggers different signal transduction cascades, which include ERK and p38 MAP kinases (Means et al. Reference Means, Golenbock and Fenton2000; Jung et al. Reference Jung, Yang, Lee, Shin, Jung, Son, Harding, Kim, Park, Paik, Song and Jo2006), which lead to the production of pro-inflammatory cytokines (Robinson and Cobb, Reference Robinson and Cobb1997). We here show the first evidence that L. mexicana LPG activates TLR2 and TLR4, leading to ERK and p38 kinase phosphorylation and pro-inflammatory cytokine production in human macrophages. The specific participation of both kinases in cytokine production was proven by inhibition studies, showing that phosphorylation of ERK induced by LPG in macrophages was suppressed by the ERK inhibitor (PD98059) but not by the p38 MAP kinase inhibitor (SB203580). Likewise, the phosphorylation of p38 MAP kinase was decreased by the p38 inhibitor, but not by the ERK inhibitor, thus demonstrating the specificity of these inhibitors. The inhibition of ERK and p38 MAP kinase partially blocked the production of pro-inflammatory cytokines, which was most notorious in the presence of the ERK inhibitor that achieved a reduction of TNF-α, IL-1β, IL-12p40 and IL-10, but not of IL-12p70 production. For additional evidence to show that activation of TLR2 and TLR4 receptors leads to phosphorylation of ERK and p38 MAP kinases, we used anti-TLR2 and anti-TLR4 antibodies. We observed a decrease in the phosphorylation of both kinases, as well as of the cytokine production, suggesting that these kinases participate in the activation of TLR2 and TLR4 by L. mexicana LPG. Our results are consistent with those of Shweash et al. (Reference Shweash, Adrienne McGachy, Schroeder, Neamatallah, Bryant, Millington, Mottram, Alexander and Plevin2011), who reported that infection of macrophages deficient in TLR2, TLR4, or in both, with L. mexicana promastigotes led to slight decrease in the phosphorylation of p38 in TLR2 -/- macrophages, whereas in the TLR4 -/- macrophages, the phosphorylation of ERK and JNK was abolished (Shweash et al. Reference Shweash, Adrienne McGachy, Schroeder, Neamatallah, Bryant, Millington, Mottram, Alexander and Plevin2011). Our current report is in accordance with various reports that have shown that LPG of various Leishmania species leads to activation of MAP kinases (de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003; Balaraman et al. Reference Balaraman, Singh, Tewary and Madhubala2005). Thus, L. major LPG was shown to simultaneously stimulate three classes of MAP kinases (ERK, p38 and JNK) in J774A.1 macrophages, whereas ERK and p38 MAP kinases were stimulated in RAW-ELAM macrophages (de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003). Both L. major and L. donovani promastigotes have been shown to induce activation of p38 MAP kinase in human macrophages (Junghae and Raynes, Reference Junghae and Raynes2002; Ben-Othman et al. Reference Ben-Othman, Guizani-Tabbane and Dellagi2008), whereas Leishmania amazonensis amastigotes induce ERK phosphorylation during the infection (Yang et al. Reference Yang, Mosser and Zhang2007).
However, contradictory data have shown that wild-type L. donovani promastigotes failed to activate phosphorylation of ERK, JNK and p38 MAPK in mouse bone marrow-derived macrophages (BMDM), yet after IFN-γ treatment, the infected BMDM showed phosphorylation of ERK and p38 MAP kinases, leading to TNF-α production (Prive and Descoteaux, Reference Prive and Descoteaux2000). Furthermore, L. donovani promastigotes were also shown to inhibit TLR2 and TLR4 related phosphorylation of p38 MAP kinase, yet ERK showed significant increase in phosphorylation. Thus, the parasite seems to be able to counter regulate p38 MAP kinase and ERK phosphorylation, leading to suppression of IL-12 and induction of IL-10 (Chandra and Naik, Reference Chandra and Naik2008). Again, these discrepancies might reflect that macrophages from various origins respond differently or that various Leishmania species or surface molecules trigger different signalling pathways.
Previous studies suggested that MAPK and NF-kB pathways transduce signals triggered by TLRs, after Leishmania recognition (Becker et al. Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, Gonzalez, Maldonado and Isibasi2003; de Veer et al. Reference de Veer, Curtis, Baldwin, DiDonato, Sexton, McConville, Handman and Schofield2003; Flandin et al. Reference Flandin, Chano and Descoteaux2006; Ben-Othman et al. Reference Ben-Othman, Guizani-Tabbane and Dellagi2008). As Leishmania seems to target molecules upstream in NF-kB and MAPKs signalling pathways, it will be interesting to analyse different molecules involved in the TLR signalling pathway, including TLR itself (Nomura et al. Reference Nomura, Akashi, Sakao, Sato, Kawai, Matsumoto, Nakanishi, Kimoto, Miyake, Takeda and Akira2000; Li et al. Reference Li, Wang and Redmond2006; Ben-Othman et al. Reference Ben-Othman, Dellagi and Guizani-Tabbane2009), as well as negative regulators, that might be targeted by various Leishmania species.
In conclusion, the present results demonstrate for the first time that L. mexicana LPG can induce the production of TNF-α, IL-1β, IL-12p40, IL-12p70 and IL-10 by binding to TLR2 and TLR4 receptors which leads to the phosphorylation of ERK and p38 MAP kinases in human macrophages and that the inhibition of TLR2 and TLR4, as well as of ERK and p38 MAP kinases, decreases the production of pro-inflammatory cytokines. The present study helps to clarify the mechanisms by which L. mexicana LPG induces the production of pro-inflammatory cytokines. The results are interesting for further research in order to explore the mechanisms by which whole Leishmania promastigotes vs purified LPG molecules modulate human macrophages and especially those of patients with different disease severity as observed with LCL and DCL.
ACKNOWLEDGEMENTS
Araceli Rojas Bernabé is a student of Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México and supported by CONACyT, México fellowship. The authors are especially grateful to Alma Escalona-Montaño for technical assistance in the Western-blot assays and analysis in ODYSSEY infrared Imaging System. Thanks to Norma Salaiza Suazo and Adriana Ruiz Remigio for technical assistance.
FINANCIAL SUPPORT
This work was supported by CONACyT 45052-M, 152433; and PAPIIT-DGAPA, UNAM IN218412.