INTRODUCTION
Leishmaniasis is a complex of diseases caused by different species of the intracellular protozoan parasites belonging to the genus Leishmania. Clinical manifestations of leishmaniasis depend on a fine interaction between the parasite and the host genetic backgrounds (Handman, Reference Handman2001). L. (L.) amazonensis has been isolated from various clinical forms of leishmaniasis including cutaneous, diffuse mucocutaneous and visceral clinical forms (Almeida et al. Reference Almeida, Barral-Neto, Jesus, Freitas, Carvalho and Barral1996; Gonçalves da Costa, Reference Gonçalves Da Costa and Coura2005).
Identification and characterization of macromolecules on the parasite may help select components for the development of a defined vaccine against leishmaniasis or for diagnostic purposes (Requena et al. Reference Requena, Alonso and Soto2000; Do Valle et al. Reference Do Valle, Gaspar, Souza-Lemos, Souza, Marquez, Baetas-da-Cruz, d'Escofier, Corte-Real, Calabrese and Gonçalves da Costa2007). The first demonstration of ATP diphosphohydrolase activity in the Leishmania genus was reported by Coimbra et al. (Reference Coimbra, Gonçalves-Da-Costa, Corte-Real, Freitas, Durão, Souza, Silva-Santos and Vasconcelos2002), and identified this protein on the external surface of L. (L.) amazonensis promastigote forms by ultrastructural cytochemical techniques. These enzymes, also named apyrases (EC 3.6.1.5), share several common features, such ability to hydrolyse di- and triphosphate nucleosides upon bivalent metal ion stimulus, and are members of the NTPDase family, that includes isoforms in the same species that are related in sequence, but that can differ in their solubilization, subcellular location and/or functions (Gendron et al. Reference Gendron, Benrezzak, Krugh, Kong, Weisman and Beaudoin2002). In parasites, the ATP diphosphohydrolases are associated with purine recuperation and/or to their protective mechanism towards the host organism, which involve ATP or ADP, such as platelet activation cytotoxicity and cytolytic T-lymphocyte reactivity (Bermudes et al. Reference Bermudes, Peck, Afifi, Beckers and Joiner1994; Vasconcelos et al. Reference Vasconcelos, Ferreira, Carvalho, De Souza, Kettlun, Mancilla, Valenzuela and Verjovski-Almeida1996; Coimbra et al. Reference Coimbra, Gonçalves-Da-Costa, Corte-Real, Freitas, Durão, Souza, Silva-Santos and Vasconcelos2002).
In this paper we demonstrate that a L. (L.) amazonensis ATP diphosphohydrolase isoform and potato apyrase have strong cross-immunoreactivity, suggesting that the parasite and vegetable proteins share conserved epitopes. The humoral response of experimentally infected BALB/c mice against ATP diphosphohydrolase and potato apyrase shared epitopes revealed the antigenicity of this parasite protein and, in addition, a correlation between the levels of IgG isotypes and disease progression.
MATERIALS AND METHODS
Chemicals
Nucleotides, ouabain, sodium azide, sodium orthovanadate, levamisole, protease inhibitors, protein molecular weight markers, 3-(N-morpholino) propanesulfonic acid (MOPS), Tween-20, Triton X-100, sodium deoxycholate (DOC), nonaethylene glycol monododecyl ether (C12E9), Protein A-Sepharose, and o-phenylenediamine (OPD) were obtained from Sigma Chemical Co. (St Louis, MO, USA). All other reagents were also of the highest analytical grade available. Serum of the tegumentary leishmaniasis patient was a gift from Dr Claude Pirmez (Laboratório de Imunopatologia/Instituto Oswaldo Cruz/FIOCRUZ, Rio de Janeiro, RJ). Potato apyrase was purified from a commercial strain of Solanum tuberosum (Kettlun et al. Reference Ketllun, Urra, Leyton, Valenzuela, Mancilla and Traverso-Cori1992). The purified enzyme presented a single protein band (52 kDa) as demonstrated by SDS/PAGE and Western blots, and was used for rabbit immunization as previously described (Faria-Pinto et al. Reference Faria-Pinto, Meirelles, Lenzi, Mota, Penido, Coelho and Vasconcelos2004).
Animals
Female BALB/c mice were originally obtained from the Jackson Laboratory, Bar Harbor, Maine (USA) and afterwards were propagated in the animal facilities of the Oswaldo Cruz Institute, Rio de Janeiro, Brazil.
Infection protocols
The L. (L.) amazonensis H21 MHOM/BR/76/MA-76 strain was isolated from a patient with diffuse cutaneous leishmaniasis and has been maintained in vivo by serial passages in mice. Normal female BALB/c mice (n=4) aged 6–8 weeks were subcutaneously infected by injecting 104L. (L.) amazonensis promastigotes in the left hind footpad. Blood was sampled prior to infection and 30 days after the infection. In another group of BALB/c mice aged 6–8 weeks, the female animals were subcutaneously infected by injecting 104L. (L.) amazonensis amastigotes in the left hind footpad. In this second group, blood was sampled prior to infection, and 20, 40, 60 and 90 days post-infection. The sera were frozen at −80°C until use. The kinetics of footpad swelling in amastigote-infected mice was evaluated by measurement at 20, 40, 60 and 90 days after infection, using a dial gauge caliper (Schnelltaster, HC Kroplin, GRBH, Hessen, Germany), as previously described (Gonçalves da Costa et al. Reference Gonçalves Da Costa, Barbosa-Santos and Lagrange1988). The results were expressed as arithmetic means of 8 or 10 mice for the analysis of the nodular lesion during the course of the infection and the standard error of the mean was calculated. These experiments were conducted in accordance with the guidelines for experimental procedures of Fundação Oswaldo Cruz (process no. L0001/07).
Isolation of plasma membrane fraction from promastigotes
Promastigote forms and plasma membrane fractions from L. (L.) amazonensis were obtained as previously described (Coimbra et al. Reference Coimbra, Gonçalves-Da-Costa, Corte-Real, Freitas, Durão, Souza, Silva-Santos and Vasconcelos2002). Isolated plasma membrane was stored until use at −80°C in the presence of 5 mm Tris-HCl, pH 7·4, 8% sucrose plus the protease inhibitors: leupeptin (0·5 μg/ml), pepstatin (0·07 μg/ml), soybean trypsin inhibitor (50 μg/l), and phenylmethylsulfonyl fluoride (2 μg/ml). Protein determination was performed by Lowry's method (Lowry et al. Reference Lowry, Roseborough, Farr and Randall1951).
Solubilization of the plasma membrane fraction from promastigotes, immunoprecipitation assays and activity measurement
An aliquot of plasma membrane fraction (4 mg protein/ml) from L. (L.) amazonensis was added to a medium containing 50 mm MOPS buffer, pH 7·4, 1 mm CaCl2, 1 mm MgCl2, supplemented with either 0·2% (v/v) Triton X-100, 0·2% (w/v) sodium deoxycholate (DOC) or 1mg/ml nonaethylene glycol monododecyl ether (C12E9), followed by centrifugation at 10 000 g for 10 min at 4°C. ATPase and ADPase activities were measured in the soluble supernatant (0·03 mg protein/ml) as previously described (Coimbra et al. Reference Coimbra, Gonçalves-Da-Costa, Corte-Real, Freitas, Durão, Souza, Silva-Santos and Vasconcelos2002), using standard reaction medium containing 50 mm MOPS buffer, pH 7·4, 1 mm CaCl2, 1 mm MgCl2, 1 mm orthovanadate, 1 mm ouabain, 1 mm sodium azide, and 4 mm of either ADP or ATP. The reaction was initiated by addition of substrate, allowed to proceed for 45 min at 37°C, and the amount of inorganic phosphate (Pi) liberated was determined according to the protocol described by Taussky and Shorr (Reference Taussky and Shorr1953) .
For immunoprecipitation assays, an aliquot of plasma membrane fraction (4 mg protein/ml) from L. (L.) amazonensis was solubilized with 1mg/ml nonaethylene glycol monododecyl ether (C12E9), as described. Rabbit immune serum containing polyclonal antibodies against potato apyrase at a final dilution of 1:1000 was added to the aliquot of soluble supernatant (0·03 mg protein/ml) and incubated for 5 h at room temperature. Protein A-Sepharose was added and incubated for an additional 2 h. Control assays with pre-immune serum were run in parallel. The resin was sedimented by centrifugation for 5 min. Supernatants were used for determination of hydrolytic activity by addition of either ATP or ADP as described above.
Partial purification of an ATP diphosphohydrolase isoform by non-denaturing polyacrylamide gel electrophoresis
The purification of an ATP diphosphohydrolase isoform of L. (L.) amazonensis promastigote forms was performed as modified from Vasconcelos et al. (Reference Vasconcelos, Ferreira, Carvalho, De Souza, Kettlun, Mancilla, Valenzuela and Verjovski-Almeida1996) . Aliquots of 100 μg plasma membrane proteins were solubilized in standard reaction medium, supplemented with 0·2% Triton X-100. These samples were applied to a 6% polyacrylamide gel with 0·1% (v/v) Triton X-100 in the gel and running buffer and subjected to electrophoresis for 3 h at 130 V in the cold room using a Mini-Protean III Cell (Bio-Rad) apparatus. The gel was washed for 40 min in standard reaction medium without nucleotides, and incubated in fresh standard reaction medium containing 5 mm ADP or ATP, supplemented by 1 mm levamisole. After incubation at 37°C for approximately 60 min, the malachite green method (Zlotnick and Gottlieb, Reference Zlotnick and Gottlieb1986) was used to determine liberation of Pi. The green precipitate indicated a phosphohydrolytic activity in situ, which was photographed. Regions of the gels corresponding to the central portion of the reactive bands were cut out and electroeluted in an Electro-Eluter (Bio-Rad, model 422) according to the manufacturer's instructions. Eluted samples were precipitated with 10% trichloroacetic acid, washed by centrifugation with water and dissolved in gel loading buffer. Two samples were combined (eluted protein originated from 200 μg of plasma membrane) and submitted to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels (Laemmli, Reference Laemmli1970). Gels were either stained with Coomassie blue or electroblotted onto nitrocellulose membrane.
Western blots and dot blots
Samples of L. (L.) amazonensis promastigote ATP diphosphohydrolase electroeluted from non-denaturing gels or highly purified potato apyrase were applied on 10% SDS-PAGE and electroblotted onto nitrocellulose membrane, followed by blocking with non-fat dry milk using standard procedures (Harlow and Lane, Reference Harlow and Lane1988). Dilutions of rabbit serum (1:1000) containing polyclonal antibodies against potato apyrase, mouse serum (1:100) from experimentally L. (L.) amazonensis amastigote-infected BALB/c mice or human serum (1:100) from a patient with typical tegumentary leishmaniasis were incubated overnight. For dot blots, purified potato apyrase (1 μg) was spotted onto nitrocellulose membranes, which were blocked as already described, and incubated overnight with sera (1:250 to 1:1000) from experimentally L. (L.) amazonensis promastigote-infected BALB/c mice. Assays were developed by chemiluminescence with the specific secondary antibody coupled to horseradish peroxidase and Luminol as substrate using the ECL Kit (GE Healthcare) and exposed to X-ray film.
Antibody analyses by enzyme-linked immunosorbent assays (ELISA)
Potato apyrase (0·5 μg/well in 0·1 m NaHCO3, pH 9·6) was absorbed onto flat-bottomed Immunolon microtitre plates overnight. Following a blocking step (0·3% Tween-20, 5% nonfat dry milk, 0·15 m phosphate buffer solution, pH 7·2), sera from experimentally infected BALB/c mice were tested in duplicate, diluted 1:100 to 1:4000 in this blocking buffer without Tween-20. Antibodies bound to the potato apyrase-plate were detected using peroxidase-conjugated, isotype-specific, anti-mouse IgM (Sigma; St Louis, MO), anti-mouse total IgG (Sigma; St Louis, MO), anti-mouse IgG1, anti-mouse IgG2a or anti-human total IgG (PharMingen; San Diego, CA), and OPD/H2O2 as substrate. Subsequent colour reaction was read at 492 nm on a microplate reader (Molecular Devices Corp., Menlo Park, CA). The considered values of A492 were the means of 2–4 determinations with a variation of no more than 15% between them. Data were analysed statistically by the Student's t-test with the level of significance set at P<0·05. Excel software (Microsoft Corporation) was used for this statistical analysis. The significant results shown in the figures were expressed as arbitrary ELISA units i.e., optical density of each sample from infected mouse divided by mean of the optical density of uninfected mice samples plus one standard deviation [OD of each sample/(XOD control+1 s.d.)].
RESULTS
Plasma membrane solubilization
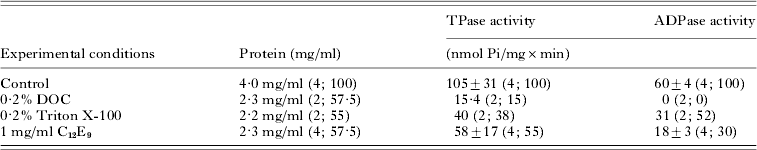
The effects of different detergents on the ATPase and ADPase activities of the plasma membrane fraction from L. (L.) amazonensis promastigotes were investigated as an attempt to obtain active ATP diphosphohydrolase. After treatment of the plasma membrane with 0·2% DOC, 0·2% Triton X-100 or 1 mg/ml C12E9, and centrifugation, most of the protein, approximately 56%, was located in the high-speed supernatant (Table 1). However, 0·2% DOC reduces approximately 85% ATPase activity and completely inhibits ADPase activity. On the other hand, 0·2% Triton X-100 or 1 mg/ml C12E9 maintains approximately 38% or 55% ATPase, and 52% or 30% ADPase activities, respectively, when compared to the activities of the crude membrane (Table 1). By this procedure, it is possible to observe that in Triton X-100- or C12E9-solubilized fractions the ATPase/ADPase activities ratios are about 1·3 and 3·2, respectively.
Table 1. Effects of detergents on the ATP diphosphohydrolase activity of plasma membrane from Leishmania (L.) amazonensis promastigotes
(Data are expressed as mean±standard deviation. The values in parenthesis represent the number (n) of experiments in duplicate and either the percentage (%) of solubilized total protein or the hydrolytic activity when compared to the Control in the absence of detergent.)
Partial purification of an ATP diphosphohydrolase isoform by non-denaturing gel electrophoresis
As an attempt to further separate active ATP diphosphohydrolase from other contaminants, several combinations and concentrations of detergents were tested in non-denaturing gels. The Triton X-100 alone gave the best resolution, and it was then selected as the most convenient detergent for routine use in subsequent purification steps. Figure 1 shows a non-denaturing polyacrylamide gel electrophoretic pattern of plasma membrane from promastigotes solubilized with 0·2% Triton X-100. After the electrophoretic run, the gel was incubated in the standard reaction medium containing 5 mm of either ATP (Fig. 1, left lane) or ADP (Fig. 1, right lane) for 1 h at 37°C. The gels were then assayed using the malachite green method, and the green precipitate was photographed. Phosphohydrolytic activity gave rise to the appearance of 1 band displaying identical electrophoretic mobilities when either ATP or ADP substrate was used (Fig. 1, left and right lanes). As previously observed in colorimetric or cytochemistry assays (see Coimbra et al. Reference Coimbra, Gonçalves-Da-Costa, Corte-Real, Freitas, Durão, Souza, Silva-Santos and Vasconcelos2002), addition of 1 mm ouabain, an inhibitor of Na+/K+-ATPase, 1 mm sodium azide, a mitochondrial ATPase inhibitor, 1 mm orthovanadate, a specific inhibitor of P-type cation transport ATPases, and 1 mm levamisole, an alkaline phosphatase inhibitor, did not inhibit ATP or ADP hydrolysis. These results suggest that ATP diphosphohydrolase protein is responsible for this hydrolytic activity observed in non-denaturing gels.

Fig. 1. Active ATP diphosphohydrolase isoform separated by non-denaturing gel. Plasma membrane (100 μg of protein) from Leishmania (L.) amazonensis promastigotes was solubilized in non-ionic detergent Triton X-100 and separated by polyacrylamide gel electrophoresis in buffer containing the same detergent. Two lanes were immersed in standard reaction medium containing either ATP (left) or ADP (right) as substrate. After 1 h of incubation at 37°C, phosphohydrolytic activity in situ was revealed by the malachite green method.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was developed (Fig. 2) with the material electroeluted from the active band from non-denaturing gels (Fig. 1). Considerable purification was achieved as compared to total proteins from plasma membrane (Fig. 2, panel CB, lanes PM and A), and diffuse bands of approximately 50 to 63 kDa were revealed by Coomassie blue staining (lane A). In Western blots (Fig. 2, panel WB), anti-potato apyrase polyclonal antibodies reacted strongly with 1 diffuse band of approximately 58–63 kDa (panel WB, lane A), and 1 additional diffuse band of higher molecular weight (>110 kDa), which coincide with the immunoreactive bands in total proteins from plasma membrane (lane PM). The 58-63 kDa bands are not the major plasma membrane proteins as seen by Coomassie blue staining (Fig. 2, panel CB, lanes PM and A). Furthermore, the immunoreactive band of >110 kDa (panel WB, lanes PM and A) represents only a minor fraction of the total amount of proteins since no corresponding band was clearly visible on the stained gels (panel CB, lane A), suggestive of aggregated proteins or oligomeric structures.

Fig. 2. Separation by SDS-PAGE of proteins extracted from active bands from non-denaturing gels. Both bands developed with ATP and ADP were extracted and electroeluted from non-denaturing gels similar to that shown in Fig. 1, and the resultant samples (A) were separated by SDS-PAGE. On panel (CB) the gel was stained with Coomassie blue and on panel (WB) the proteins were electroblotted onto nitrocellulose and developed with rabbit polyclonal antibodies against potato apyrase. Lanes (PM) show a control of 80 μg of the total plasma membrane from Leishmania (L.) amazonensis promastigotes.
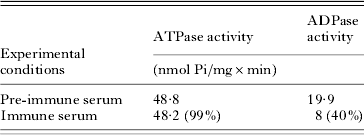
Anti-potato-apyrase antibodies were then tested for their ability to immunoprecipitate ATP-diphosphohydrolase from detergent-solubilized membrane fraction obtained from L. (L.) amazonensis promastigote forms. The C12E9 was selected as the most convenient for use in the immunoprecipitation assays, since this non-ionic detergent, different from the Triton X-100, maintains a clear reaction medium for colorimetric measurements. Table 2 shows that antibody against potato apyrase immobilized on Protein A-Sepharose was able to deplete about 60% of the ADPase activity while the ATPase activity remaining in solution was not affected. The Western blots and immunoprecipitation results confirm the identity of an ATP diphosphohydrolase isoform in the membrane fraction from L. (L.) amazonensis promastigote forms that shares conserved epitopes with potato apyrase.
Table 2. Depletion of detergent-solubilized ATP diphosphohydrolase activity from the medium by anti-potato apyrase antibodies immobilized on Protein A-Sepharose
(Pre-immune or immune serum was used at a dilution of 1:1000. The results in parenthesis represent the percentage of hydrolytic activity when compared to the pre-immune serum. The experiments were repeated twice with similar results.)
Antigenicity analysis
At 30 days after infection sera from L. (L.) amazonensis promastigote-infected BALB/c mice were tested in dot blots (Fig. 3A) and by ELISA technique (Fig. 3B), using potato apyrase as antigen. Figure 3A shows typical positive dot blots developed with serum diluted at 1:500 from infected mice (M1, M2, M3 and M4), while serum obtained prior to infection did not show any reaction (C). As observed in Fig. 3B, the total IgG antibody serum level was about 1·8-fold higher than that found in uninfected mice, suggesting that the epitopes shared between potato apyrase and L. (L.) amazonensis ATP diphosphohydrolase isoform are antigenic.

Fig. 3. (A) Dot blots of potato apyrase (1 mg) developed with serum diluted at 1:500 from experimentally L. (L.) amazonensis promastigote-infected BALB/c mice (M1, M2, M3, M4) 30 days post-infection, or from uninfected mice (C), incubated overnight and developed by chemiluminescence. (B) Reactivity of total IgG antibody in sera diluted 1:500 (***P<0·001) from promastigote-infected mice, using potato apyrase as coating antigen in the ELISA technique, when compared to the sera from uninfected mice (Control). As described in the Materials and Methods section, the arbitrary value 1 for the Control (base level) corresponds to the mean of the optical density of uninfected mice samples (0·038) plus 1 standard deviation (0·011).
The infection progress was then monitored along a period of 90 days in amastigote-infected mice. In preliminary analyses, it was observed by Western blotting that the ATP diphosphohydrolase isoform isolated from the plasma membrane fraction of the promastigote forms was recognized by diluted sera (1:100) from amastigote-infected BALB/c mice (panel A, lane I), but not by sera obtained prior to infection (Fig. 4, panel A, lane NI). In addition, strong reactivity was observed between diluted serum (1:100) from a typical tegumentary leishmaniasis patient and either ATP diphosphohydrolase isoform from promastigotes (Fig. 4, panel A, lane P) or potato apyrase (panel B, lane P), while serum from healthy individuals did not react with any protein band in these preparative gels (panels A and B, lanes H). Moreover, no other antigenic band was observed in the samples of the partially purified ATP diphosphohydrolase (Panel A, lanes I and P). The band of >110 kDa (Fig. 2, panel WB, lanes PM and A) was not visible in this preparative gel, confirming that this band represents only a small amount of the total purified proteins. In addition, the high purity of the potato apyrase could also be observed, since no other background was present when rabbit polyclonal antibodies against this protein were used as control (Fig. 4, panel B, lane C).

Fig. 4. Sera from leishmaniasis individuals react with Leishmania (L.) amazonensis ATP diphosphohydrolase isoform and potato apyrase. Partially purified parasite protein (panel A) and pure potato apyrase (panel B) samples were electrophoresed by preparative 10% SDS-PAGE followed by electroblotting onto nitrocellulose membrane using standard conditions. Membranes were incubated overnight with sera diluted 1:100, either from experimentally amastigote-infected mice 20 days post-infection (I), uninfected mice (NI), tegumentary leishmaniasis patient (P) or healthy individuals (H). Rabbit polyclonal antibodies against potato apyrase were used as control (C). Membranes were developed by chemiluminescence with the specific secondary antibody coupled to horseradish peroxidase and Luminol as substrate.
The primary lesion kinetics in amastigote-infected mice showed progressive large lesions at 20, 40, 60 and 90 days after infection (Fig. 5A). Antibody levels were quantified in diluted sera samples from amastigote-infected mice and compared to the levels found prior to infection. The representative data in sera diluted 1:100 are shown in Fig. 5 (B and C). Significantly (P<0·001) higher serum levels of IgM antibody, about 50% above of the control, were present at the beginning of the disease, and they were maintained significantly elevated and at similar levels up to the end of the experience (Fig. 5B). Significantly higher (P<0·001) total IgG antibody levels were found at 20 and 40 days post-infection, about 3-fold higher than that found prior to infection, and the levels were reduced at 60 and 90 days post-infection (Fig. 5B). The IgG antibody subtypes were then tested (Fig. 5C). Significantly (P<0·001) higher IgG2a antibody levels were observed at 20 and 40 days post-infection (Fig. 5C), when compared to the levels found prior to infection. At 60 and 90 days post-infection, IgG2a antibody levels significantly (P<0·001) decreased as compared to the levels found at 40 days post-infection (Fig. 5C). No response of IgG1 antibody subtype could be observed at 20, 40 and 60 days post-infection, with values remaining close to the background level (Fig. 5C). On the other hand, significant (P<0·001) elevation of the IgG1 antibody level, about 2-fold higher than that found prior to infection, was observed 90 days post-infection (Fig. 5C).

Fig. 5. (A) Kinetics of footpad lesion formation in BALB/c mouse infected with 104Leishmania (L.) amazonensis amastigotes. (B) Kinetics of specific IgM and total IgG antibody. (C) Specific IgG1 and IgG2a antibody against L. (L.) amazonensis ATP diphophohydrolase and potato apyrase shared epitopes, analysed by the ELISA technique, using potato apyrase as coating antigen, in sera diluted 1:100 from experimentally amastigote-infected mice, at 20, 40, 60 and 90 days post-infection. \\, Data are not to scale.
DISCUSSION
An L. (L.) amazonensis ATP diphosphohydrolase isoform was partially purified from the plasma membrane from promastigote forms, and identified as diffuse bands of about 58–63 kDa, possibly glycosylated forms of this protein. The analysis of effects of the Triton X-100 and C12E9 on the hydrolytic activities of plasma membrane showed ATPase/ADPase activity ratios of about 1·3 and 3·2, respectively, suggesting that each detergent is able to extract and/or to activate different ATP diphosphohydrolase isoforms. In addition, antibody produced against highly purified apyrase isolated in its native state from S. tuberosum, which has a low ATPase/ADPase ratio as recently demonstrated (Penido et al. Reference Penido, Resende, Vianello, Bordin, Jacinto, Dias, Montesano, Nelson, Coelho and Vasconcelos2007), was able to immunoprecipitate about 60% of ADPase activity with no effect on ATPase activity, leaving in the soluble fraction an isoform with high ATPase/ADPase ratio. Therefore, although we cannot discount that the solubilization of the plasma membrane fraction by these detergents could be stimulating others enzymes, these results, associated with the presence of the ATPase inhibitors in the standard reaction medium, favour the hypothesis that our purification procedures were capable of isolating only an ATP diphosphohydrolase isoform, with very low ATPase/ADPase ratio. The presence of 2 or more ATP diphosphohydrolase isoforms in the same organism has been amply reported, and each one of them can co-exist either as soluble or membrane-associated protein, in glycosylated or deglycosylated form, and as a monomer, dimer or tetramer (Ketllun et al. Reference Ketllun, Urra, Leyton, Valenzuela, Mancilla and Traverso-Cori1992; Bermudes et al. Reference Bermudes, Peck, Afifi, Beckers and Joiner1994; Vasconcelos et al. Reference Vasconcelos, Ferreira, Carvalho, De Souza, Kettlun, Mancilla, Valenzuela and Verjovski-Almeida1996; Gendron et al. Reference Gendron, Benrezzak, Krugh, Kong, Weisman and Beaudoin2002).
This ATP diphosphohydrolase isoform found in L. (L.) amazonensis promastigote forms is antigenic for both infected mice and tegumentary leishmaniasis patients, and the existence of shared epitopes between this parasite protein and potato apyrase was confirmed. Our results also suggest that L. (L.) amazonensis amastigote forms expresses the same ATP diphosphohydrolase isoform found in the promastigote stage, but this hypothesis should be more extensively explored.
From the analysis of the disease progression and of the time-course of appearance of specific antibodies against the shared epitopes in amastigote-infected mice, several features must be pointed out. Twenty days after infection, BALB/c mice infected by L. (L.) amazonensis amastigote forms presented at the site of inoculation discreet inflammatory infiltrates that increased progressively during the course of the infection, and at 90 days post-infection the dermis presented necrotic tissue, as already demonstrated by histopathological studies (Abreu-Silva et al. Reference Abreu-Silva, Calabrese, Cupolilo, Cardoso, Souza and Gonçalves Da Costa2004). IgG2a antibody levels were significantly elevated in early stages of infection and later the reactivity this antibody subtype returned to background, while IgG1 antibody levels increased at 90 days post-infection, coinciding with the more active stage of the disease. Given that the IgG2a and IgG1 isotypes are associated with the Th1- and Th2-type immune responses, respectively, the present data suggest that the ATP diphosphohydrolase and potato apyrase shared epitopes are able to stimulate a cellular immune response. The role of the host genetic background, virulence factors of Leishmania strains and immunological response, especially the Th1/Th2 pattern of immunity, in the outcome of Leishmania infection have been extensively studied and implicated in susceptibility or resistance to infection (Soong et al. Reference Soong, Chang, Sun, Longley, Ruddle, Flavell and McMahon-Pratt1997; Jones et al. Reference Jones, Buxbaum and Scott2000). It has been shown that BALB/c mice are highly susceptible to L. (L.) amazonensis, and that this susceptibility is related to the Th2 response (Abreu-Silva et al. Reference Abreu-Silva, Calabrese, Tedesco, Mortara and Gonçalves Da Costa2003, Reference Abreu-Silva, Calabrese, Cupolilo, Cardoso, Souza and Gonçalves Da Costa2004; Gonçalves da Costa, Reference Gonçalves Da Costa and Coura2005). The present study is the first observation of L. (L.) amazonensis ATP diphosphohydrolase antigenicity, and the results here demonstrated motivate further investigations.
It has been demonstrated that ATP diphosphohydrolases from T. gondii (Asai et al. Reference Asai, Mizuno, Kojima, Takeuchi, Kobayashi and Suzuki1992) and S. mansoni (Faria-Pinto et al. Reference Faria-Pinto, Meirelles, Lenzi, Mota, Penido, Coelho and Vasconcelos2004) are antigenic proteins. Furthermore, as demonstrated here for L. (L.) amazonensis ATP diphosphohydrolase, S. mansoni ATP diphosphohydrolase and potato apyrase also share conserved epitopes (Faria-Pinto et al. Reference Faria-Pinto, Meirelles, Lenzi, Mota, Penido, Coelho and Vasconcelos2004). The life-cycles of these parasites are very different in mammalian hosts, and many Leishmania antigens have been identified as members of conserved protein families but they elicit specific immune responses, possibly due to the pathways by which parasite antigens are processed and presented to effectors cells from the host immune system (Requena et al. Reference Requena, Alonso and Soto2000). Analyses of gene banks using known sequences of these enzymes did not reveal any putative L. (L.) amazonensis homologues for ATP diphosphohydrolase. Thus, the study of the immune response elicited by parasite ATP diphosphohydrolase native or recombinant forms in different parasitic diseases will be interesting for evaluation of antigenicity and to determine whether the shared epitopes between parasites and vegetable proteins are immunodominant.
The high purity of the potato apyrase was previously demonstrated by SDS-PAGE, Coomassie blue staining and Western blots (Faria-Pinto et al. Reference Faria-Pinto, Meirelles, Lenzi, Mota, Penido, Coelho and Vasconcelos2004), and also in this work, assuring its significance and reproducibility in immunological assays. We recently reported the absence of cross-immunoreactivity between mammalian ATP diphosphohydrolases and potato apyrase, suggesting that autoantibodies are not induced during potato apyrase immunization (Faria-Pinto et al. Reference Faria-Pinto, Meirelles, Lenzi, Mota, Penido, Coelho and Vasconcelos2006). We still consider that further analysis of the potato apyrase, either in its native form or suitable synthetic peptide derivatives, could implement its use in current control methods, such as diagnosis, protective immunogenicity and/or immunotherapy.
This work was supported in part by grants from the Fundação Oswaldo Cruz (FAPES) and Instituto Oswaldo Cruz, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). E. S. Coimbra, C. S. F. Souza and A. Abreu-Silva were recipients of Doctoral Fellowships from the CNPq. F. A. Rezende-Soares, B. L. S. Costa and N. L. L. Giarola were recipients of Ms and IC Fellowships from UFJF, PIBIC/CNPq or BIC/UFJF. S. C. Gonçalves da Costa is a Senior Fellow supported by CNPq (Proc. N 305388/2005-3).









