INTRODUCTION
Trypanosoma brucei gambiense causes sleeping sickness, also known as human African trypanosomiasis (HAT), in west and central Africa; it is quite distinct from T. b. rhodesiense which causes East African sleeping sickness. Although these two parasites are morphologically indistinguishable sub-species of T. brucei s.l., they differ in host profile, clinical presentation and geographical distribution (Welburn et al. Reference Welburn, Fevre, Coleman, Odiit and Maudlin2001). The two sleeping sicknesses demand different methods of disease control, including different case-finding strategies and alternative chemotherapies in human patients (Brun et al. Reference Brun, Blum, Chappuis and Burri2010). In addition, control of T. b. rhodesiense, but not T. b. gambiense, demands interventions to reduce the prevalence of infection in the animal reservoir (Welburn et al. Reference Welburn, Fevre, Coleman, Odiit and Maudlin2001, Reference Welburn, Coleman, Maudlin, Fevre, Odiit and Eisler2006). The differences between T. b. gambiense and T. b. rhodesiense sleeping sickness, the implications for disease control and repercussions, should their foci ever merge, have been discussed elsewhere (Welburn et al. Reference Welburn, Fevre, Coleman, Odiit and Maudlin2001; Picozzi et al. Reference Picozzi, Fevre, Odiit, Carrington, Eisler, Maudlin and Welburn2005).
Classically T. b. gambiense is understood to cause chronic disease in humans (Checchi et al. Reference Checchi, Filipe, Barrett and Chandramohan2008). Although domestic pigs are known reservoirs of infection (Simo et al. Reference Simo, Asonganyi, Nkinin, Njiokou and Herder2006), their role in disease transmission remains unquantified and the disease is principally considered to be anthroponotic (Welburn et al. Reference Welburn, Fevre, Coleman, Odiit and Maudlin2001). Infection occurs when, in the course of obtaining a further blood meal, a previously infected tsetse fly injects saliva into a human host together with the parasites. Parasites become patent in the blood within one to two weeks and the early, haemolymphatic stage of the disease begins. At this stage signs and symptoms are poorly defined. After a period from several months to two years, parasites traverse the blood-brain barrier, initiating the second, meningoencephalitic stage of disease when more specific signs and neurological symptoms become apparent, and coma and death are considered to be inevitable (Checchi et al. Reference Checchi, Filipe, Barrett and Chandramohan2008).
However, this picture is not as clear as it first appears. In their review Checchi et al. (Reference Checchi, Filipe, Barrett and Chandramohan2008) describe four possible outcomes which might be described as trypanotolerant: (1) early spontaneous resolution of stage 1, (2) chronic asymptomatic, or very mildly symptomatic, carriage in stage 1, without progression to stage 2, (3) progression to stage 2 followed by early spontaneous resolution and (4) chronic asymptomatic, or very mildly symptomatic carriage in stage 2. After reviewing the evidence they conclude, somewhat cautiously, that there is some evidence for spontaneous cure from stage 1, but not from stage 2, and there is no evidence that chronic carriage occurs at either stage. However, the evidence for this view is limited, particularly by the sensitivity of current diagnostic tools.
Diagnosing T. b. gambiense
Serological screening and/or clinical signs are used to identify suspect cases, which are then confirmed upon direct visualization of parasites in the blood, lymph or cerebrospinal fluid, by microscopy. When parasites are detected the stage of the disease must then be determined according to WHO criteria, which include several CSF markers – parasite detection, lymphocyte count and alterations in the total protein concentration. Active case finding has been a successful component of effective disease control since Eugene Jamot established it in the 1920s. Jamot instigated the mobile medical surveillance team, detecting cases according to clinical signs and treating with the organo-arsenic compound, tryparsamide (Welburn et al. Reference Welburn, Maudlin, Simarro and Schaechter2009). To this day, active surveillance continues to be used, but screening is now performed using the Card Agglutination Test for Trypanosomiasis (CATT) as a means of detecting anti-trypanosome antibodies.
CATT is a rapid, simple agglutination assay for T. b. gambiense-specific antibodies, which uses an antigenic reagent based on T. b. gambiense Variable Antigen Type LiTat 1.3 (Magnus et al. Reference Magnus, Van Meirvenne and Vervoort1978). During field surveys, the subjects are first screened using freshly collected heparinized whole blood. This is followed by further tests on blood, plasma or serum dilutions for whole blood-positive individuals. But CATT is problematic. Firstly, CATT cannot be used as an indication of cure since antibodies persist for up to three years post treatment (Paquet et al. Reference Paquet, Ancelle, Gastelluetchegorry, Castilla and Harndt1992; Lejon et al. Reference Lejon, Ngoyi, Boelaert and Buscher2010). Secondly, false negative CATT results have been observed in parasitologically positive cases in Cameroon and north western Uganda (Dukes et al. Reference Dukes, Gibson, Gashumba, Hudson, Bromidge, Kaukus, Asonganyi and Magnus1992; Enyaru et al. Reference Enyaru, Matovu, Akol, Sebikali, Kyambadde, Schmidt, Brun, Kaminsky, Ogwal and Kansiime1998), particularly where the LiTat 1.3 gene is absent in circulating strains of T. b. gambiense (Dukes et al. Reference Dukes, Gibson, Gashumba, Hudson, Bromidge, Kaukus, Asonganyi and Magnus1992; Kanmogne et al. Reference Kanmogne, Asonganyi and Gibson1996). CATT false negatives can also result from complement-mediated inhibition of the agglutination reaction, particularly at lower concentrations of sample (Pansaerts et al. Reference Pansaerts, Van Meirvenne, Magnus and Verhelst1998). Thirdly, CATT false positives can occur in patients with malaria and transient infection with non-human trypanosomes (Magnus et al. Reference Magnus, Van Meirvenne and Vervoort1978). Finally, it is extremely onerous and logistically difficult to monitor the high number of CATT-positive aparasitaemic individuals for two years post screening according to WHO guidelines (Magez and Radwanska, Reference Magez and Radwanska2009). Microscopic parasite detection is also difficult. The numbers of circulating T. b. gambiense can range from 10,000 ml−1 to less than 100 ml−1, which falls below the threshold of even the most sensitive methods (Brun et al. Reference Brun, Blum, Chappuis and Burri2010).
To improve the diagnostic sensitivity of parasitological diagnosis, several concentration techniques have been developed, of which the microhaematocrit centrifugation technique (HCT) and the mini anion exchange centrifugation technique (mAECT) are most commonly used. For HCT, centrifugation of the blood sample in a capillary tube concentrates the trypanosomes in the white blood cell layer, which can then be examined using a normal light microscope (Woo, Reference Woo1970). mAECT uses miniature anion exchange columns for the separation of trypanosomes from red blood cells prior to centrifugation (Lumsden et al. Reference Lumsden, Kimber, Evans and Doig1979) and is the most sensitive field-based method available for parasite detection in blood (Miezan et al. Reference Miezan, Meda, Doua and Cattand1994). HCT and mAECT can detect between 500 and 100 trypanosomes ml−1 blood respectively.
More sensitive parasite detection methods have been developed in the laboratory with several available PCR assays that detect and differentiate trypanosomes at the species and sub-species levels. For T. brucei s.l. PCR targeting a 177 bp repeat region can detect a DNA concentration equivalent to a single trypanosome (Moser et al. Reference Moser, Cook, Ochs, Bailey, McKane and Donelson1989). PCR-based identification of T. b. gambiense is also possible, using primers designed to amplify the single copy T. b. gambiense-specific glycoprotein (TgsGP) gene (Radwankska et al. Reference Radwanska, Claes, Magez, Magnus, Perez-Morga, Pays and Buscher2002; Picozzi et al. Reference Picozzi, Fevre, Odiit, Carrington, Eisler, Maudlin and Welburn2005) capable of detecting 10 trypanosomes per ml (Radwanska et al. Reference Radwanska, Claes, Magez, Magnus, Perez-Morga, Pays and Buscher2002).
Molecular DNA detection methods are significantly more sensitive than microscopy but PCR is too technologically demanding to be of any practical use as a point-of-care diagnostic in the African setting, so the search for more pragmatic methods continues. Recently two new isothermal nucleic acid amplification test methodologies have been developed and applied to sleeping sickness diagnosis: loop-mediated isothermal amplification (LAMP) (Kuboki et al. Reference Kuboki, Inoue, Sakurai, Di Cello, Grab, Suzuki, Sugimoto and Igarashi2003; Njiru et al. Reference Njiru, Mikosza, Matovu, Enyaru, Ouma, Kibona, Thompson and Ndung'u2008a, Reference Njiru, Mikosza, Armstrong, Enyaru, Ndung'u and Thompsonb; Matovu et al. Reference Matovu, Kuepfer, Boobo, Kibona and Burri2010a) and nucleic acid sequence-based amplification (NASBA) (Mugasa et al. Reference Mugasa, Schoone, Ekangu, Lubega, Kager and Schallig2008, Reference Mugasa, Laurent, Schoone, Kager, Lubega and Schallig2009; Matovu et al. Reference Matovu, Mugasa, Ekangu, Deborggraeve, Lubega, Laurent, Schoone, Schallig and Buscher2010b).
Recent cases of T. b. gambiense on the shores of Lake Albert, Uganda
Lake Albert is the second largest freshwater lake in Uganda, around 5300 km2 in area, with its western shoreline in the Democratic Republic of Congo. The Ugandan side is home to mainly itinerant communities of either local or foreign origin who are typically employed in the Lake Albert fishery, as well as camps re-housing thousands of refuges. Between June 2002 and October 2003, three Sudanese refugees living in a camp in Kyangwali Parish, Hoima District, were diagnosed and treated for T. b. gambiense sleeping sickness. These infections were generally thought by the medical authorities to have been acquired by the refugees in their country of origin; nevertheless they raised concerns that the T. b. gambiense focus from West Nile area was creeping southwards along the shores of Lake Albert.
Here we describe a series of studies performed around the shores of Lake Albert in Western Uganda with two main aims: (1) to identify the extent of T. b. gambiense on the shores of Lake Albert and (2) to evaluate PCR as a diagnostic tool with the potential to be a more sensitive detection method of current T. b. gambiense infections than traditional parasitological techniques.
MATERIALS AND METHODS
Preliminary surveys around Lake Albert
The first of our studies was performed in June 2007 originating from a parasitological survey of mothers and young children for intestinal schistosomiasis and malaria in Hoima District, from which 112 anonymised blood spots preserved on Whatman FTA cards (Whatman, UK) were analyzed by PCR.
Active screening, September 2008
Following the evidence from the preliminary PCR and CATT surveys, in September 2008 a WHO-funded mass screening initiative was undertaken on the Eastern shores of Lake Albert. Over ten days two Ugandan Ministry of Health teams screened 6207 individuals; 2794 in Butiaba Parish, Buliisa District and 3413 in Kyangwali Parish, Hoima District. After explaining the survey objectives to the local community, verbal informed consent was confirmed before individuals donated a finger-prick blood sample for CATT screening and were assessed for clinical signs of disease. A venous blood sample was then taken from all CATT whole blood-positive individuals for CATT tests upon serial plasma dilutions (1/4, 1/8 and 1/16), and for parasitological detection (microhaematocrit centrifugation on eight capillaries per individual). An aliquot of this venous blood was also applied to Whatman FTA cards and later used for PCR analysis. In addition, whole venous blood samples from age and sex matched CATT-negative controls were stored on Whatman FTA cards for comparative PCR analysis.
Follow-up survey, 2009
To search for parasites using mAECT, follow-up was undertaken in April 2009, in which CATT whole blood-positive suspect cases from the September 2008 mass screening were re-visited. CATT was performed and venous blood was taken in order to search for parasites by the HCT and the more sensitive mAECT method, as well as for remote PCR analysis.
PCR analysis
DNA was eluted from the Whatman FTA cards and all samples were subject to the TBR PCR (Moser et al. Reference Moser, Cook, Ochs, Bailey, McKane and Donelson1989) (which identifies all members of the sub-genus Trypanozoon) in triplicate. This is a well established PCR protocol, with 10,000 targets per genome. The TgsGP PCR, which is specific for T. b. gambiense with a single copy gene target was performed according to the nested protocol developed by Picozzi et al. (Reference Picozzi, Fevre, Odiit, Carrington, Eisler, Maudlin and Welburn2005), in quintuplicate for samples presenting strong positive signal in TBR PCR.

Fig. 1. Map of Uganda, highlighting the sampling areas of Butiaba and Kyangwali, along the shores of Lake Albert, as well as the previously identified foci of T. b. rhodesiense and T. b.gambiense.
RESULTS
Preliminary PCR survey from Hoima District
PCR analysis showed that 23·2% (26/112; 95% confidence interval 15·4–31·0%) individuals tested positive for T. brucei s.l., of those 26 individuals five tested positive for T. b. gambiense. No samples tested positive for T. b. rhodesiense. Although T. b. gambiense was detected in only five of the human samples, it is likely that many if not all of the T. brucei s.l. positive individuals were infected with T. b. gambiense.
Active screening in 2008
The results of the CATT screening are shown in Table 1. In Kyangwali Parish 2·1% of those screened were positive by CATT on whole blood, and more than a quarter of these remained positive at the 1/16 dilution. However, far fewer suspect cases were detected in Butiaba Parish, and parasites could not be confirmed in any of the cases using the microhaematocrit centrifugation technique. Neither did any of the CATT-positive suspect cases display any of the classical clinical signs.
Table 1. Results of the September 2008 mass screening by CATT
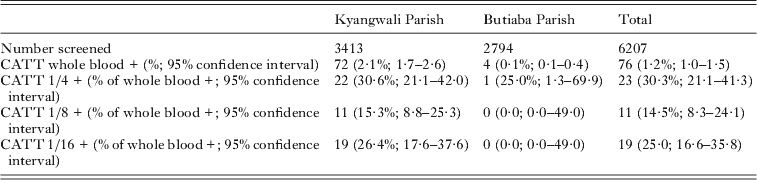
Samples for PCR analysis were taken from all four CATT whole blood-positive suspect cases in Butiaba, and for 68/72 CATT whole blood-positive suspect cases in Kyangwali. A sample from one additional clinically suspected case was taken in Butiaba. Seventy two additional matched control and 48 random control samples were also taken. Of the 73 suspected case blood samples analyzed by TBR PCR, 31 generated positive signals (42·47%; 95% confidence interval 31·78–53·91%). The parasite sub-species was confirmed as T. b. gambiense in 1/7 strongly T. brucei s.l.-positive samples. In addition 17/120 CATT-negative controls also generated a positive signal for T. brucei s.l. by the TBR PCR (14·17%; 95% confidence interval 8·94–21·60%). Considering age, 36·26% (95% confidence interval 21·91% – 44·68%) of those 12 years or younger were PCR positive. A smaller proportion of those over 12 years were PCR positive (21·37%; 95% confidence interval 15·18–29·20%). This difference is not statistically significant at the 95% level of confidence. The distribution of PCR positivity with age is summarised in Table 2.
Table 2. PCR positivity and age at initial screening

Follow-up 2009
In total 43/76 CATT whole blood-positive suspect cases were followed up, as well as nine of the 73 CATT negative controls. Again no parasites were detected in any of these individuals, by either HCT or mAECT. Considerable differences were seen in the CATT status of these individuals from September 2008 to April 2009 as shown in Table 3.
Table 3. Comparison of CATT WB status at initial screening and follow up
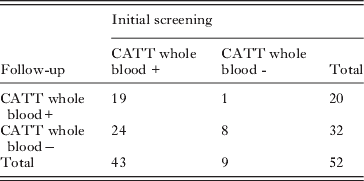
Overall the CATT (whole blood) status was unchanged for 27 individuals, but differed for 25. Here κ=0·165 (95% confidence interval=−0·071–0·401). This kappa value represents poor agreement, and since the confidence interval spans zero, agreement is not significantly more than might be expected by chance alone (Altman, Reference Altman1991). Furthermore, 16 of 43 CATT whole blood positives were positive at 1/8 dilution at the initial screening. However, at follow-up, none of the CATT 1/8 positives were confirmed.
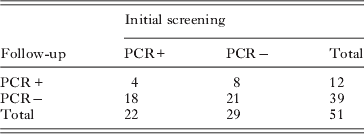
Considerable change was also seen in the PCR status of the cases and controls over time, as shown in Table 4. Overall PCR status was unchanged for 25 individuals but differed for 26 individuals.There was no agreement between the PCR status of these individuals at the two time points (κ=−0·100; 95% confidence interval=−0·395–0·196).
Table 4. Comparison of PCR status between initial screening and follow-up
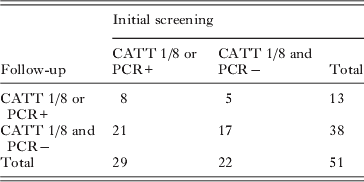
Finally we considered the CATT 1/8 and PCR results together, to generate a new case definition: anyone positive by either PCR or by CATT at a plasma dilution of 1/8 or above. Again we see considerable change over time, with almost no agreement (κ=0·045; 95% confidence interval=−0·212–0·302) from the initial screening to follow up (Table 5).
Table 5. Changes in case status, as defined by PCR and/or CATT positivity at 1/8 plasma dilution, or above
DISCUSSION
The present work has shown that T. b. gambiense parasite DNA can be detected in clinically unaffected, microscopically aparasitaemic children and adults on the Eastern shores of Lake Albert. However, PCR results did not agree with CATT seropositivity and, moreover, CATT positivity and PCR positivity were both unstable over time which may be manifestations of differential parasite DNA turnover in the human host as well as evolving immunological responses.
This is not the first study to identify discrepancies between PCR and CATT, or between PCR and parasitological tests. In 2000, Kyambadde et al. compared PCR to CATT and HCT in 35 individuals in Uganda (of which 33 were CATT positive). The PCR reaction used was a modification of the original TBR PCR (Masiga et al. Reference Masiga, Smyth, Hayes, Bromidge and Gibson1992) that also detects all T. brucei s.l. It generated 13 false negative results compared to CATT and six false positive results compared to HCT. However, no CATT-negative PCR-positive cases were observed (which is unsurprising given that only two CATT-negative cases were included in the sample) and no HCT positive, PCR-negative cases were observed. One explanation may be that CATT is non-specific, and PCR is more sensitive than HCT.
Garcia et al. (Reference Garcia, Jamonneau, Magnus, Laveissiere, Lejon, N'Guessan, N'Dri, Meirvenne and Buscher2000) also used CATT and TBR PCR during a longitudinal follow-up survey of 77 seropositive parasitologically unconfirmed individuals in Côte d'Ivoire. In line with our observations, CATT seropositivity was heterogeneous; some individuals remained seropositive over time, while others changed status. Furthermore they found, strangely, that PCR positives were more frequent among individuals who did not show consistently strong CATT positive results over time. Garcia et al. (Reference Garcia, Jamonneau, Magnus, Laveissiere, Lejon, N'Guessan, N'Dri, Meirvenne and Buscher2000) concluded that the TBR PCR might be cross reacting with T. b. brucei DNA which could persist after abortive inoculation by tsetse into these human hosts.
Penchenier et al. (Reference Penchenier, Simo, Grebaut, Nkinin, Laveissiere and Herder2000) applied the TBR PCR to 1858 blood samples from the Fontem focus in Cameroon. The samples were also subject to CATT and parasitological detection by the quantitative buffy coat (QBC) technique. Only one patient (CATT+, QBC+) was not PCR positive. Fifty of 1432 serological suspects (CATT +, QBC−) were PCR positive. When these were subject to further more sensitive parasitological detection by KIVI (Kit for In Vitro Isolation of trypanosomes) an excess of PCR positives remained. Three of 222 endemic controls (CATT−, QBC−) were also PCR positive (1·35%; 95% confidence interval 0·27–4·08%). Similarly in Côte d'Ivoire, Koffi et al. (Reference Koffi, Solano, Denizot, Courtin, Garcia, Lejon, Buscher, Cuny and Jamonneau2006) observed four PCR positives among 73 CATT negative controls (5·48%; 95% confidence interval 1·74–13·67%). Both these previous observations are less than the 14·17% (95% confidence interval 8·94–21·60) of CATT negatives noted to be PCR positive in our study. False negative CATT reactions have been observed where the circulating strains of T. b. gambiense lack LiTat 1.3 (Dukes et al. Reference Dukes, Gibson, Gashumba, Hudson, Bromidge, Kaukus, Asonganyi and Magnus1992; Kanmogne et al. Reference Kanmogne, Asonganyi and Gibson1996). However, in north-western Uganda PCR positive results were seen for CATT-negative individuals carrying LiTat 1.3 positive parasites (Enyaru et al. Reference Enyaru, Matovu, Akol, Sebikali, Kyambadde, Schmidt, Brun, Kaminsky, Ogwal and Kansiime1998). It may be concluded that CATT is insensitive, as well as poorly specific; alternatively some parasites might possess LiTat 1.3 without expressing it, or only express it late in infection. False negative CATT results may also be attributed to complement mediated inhibition of the agglutination reaction (Pansaerts et al. Reference Pansaerts, Van Meirvenne, Magnus and Verhelst1998).
In the present study we also observed that PCR status changed considerably over time for the 51 patients followed up after 6 months. This is in sharp contrast to Penchenier et al. (Reference Penchenier, Simo, Grebaut, Nkinin, Laveissiere and Herder2000) who saw no change in the PCR status of 22 PCR-positive and 33 PCR-negative individuals over three months. The unreliability of PCR has been noted by Koffi et al. (Reference Koffi, Solano, Denizot, Courtin, Garcia, Lejon, Buscher, Cuny and Jamonneau2006) who observed a disproportionate number of doubtful PCR results for aparasitaemic serological suspects. Koffi et al. (Reference Koffi, Solano, Denizot, Courtin, Garcia, Lejon, Buscher, Cuny and Jamonneau2006) suggested that there may exist a “long lasting human reservoir that may contribute to the maintenance or periodic resurgences of HAT in endemic foci” implying that these serological suspects harbour infection at a level too low to be picked up by even the most sensitive conventional parasitological detection techniques, and indeed so low, as to be at the limit of detection for PCR (leading to inconsistent results). This hypothesis would fit with our own observations.
However, we cannot discount the possibility that we are detecting persistent DNA from lysed T. b. brucei parasites. From an alternative methodological perspective, FTA card samples are non-homogeneous and the filter paper matrix results in local parasite lysis and focal fixation of parasite DNA. Hence samples with low parasitaemia will likely not return a positive signal for every punch (Cox et al. Reference Cox, Tosas, Tilley, Picozzi, Coleman, Hide and Welburn2010). More repeat screenings of the CATT-positive samples may have given a higher proportion of PCR positive samples, and might have improved the consistency of the PCR results over time. Deborggraeve and Buscher (Reference Deborggraeve and Buscher2010) suggest that T. brucei nucleic acids might be a marker for infection, but not disease, as has been observed for a PCR based Leishmania diagnostic (Deborggraeve et al. Reference Deborggraeve, Boelaert, Rijal, Doncker, Dujardin, Herdewijn and Buscher2008). They further suggest that T. brucei DNA may be incorporated into the human genome, giving rise to false positive PCR results. With regards CATT, it is noteworthy that these communities are hyper-parasitised by malaria and Schistosoma mansoni. Together with HIV, these diseases have unquantified effects on responsiveness to CATT, which detects anti-trypanosomal antibodies.
Resolution of these diagnostic ambiguities might lie within the new developments in isothermal nucleic acid amplification that are being promoted for their potential as field-friendly diagnostic tools. These advances include LAMP (Kuboki et al. Reference Kuboki, Inoue, Sakurai, Di Cello, Grab, Suzuki, Sugimoto and Igarashi2003; Njiru et al. Reference Njiru, Mikosza, Matovu, Enyaru, Ouma, Kibona, Thompson and Ndung'u2008a) and NASBA (Mugasa et al. Reference Mugasa, Laurent, Schoone, Kager, Lubega and Schallig2009) assays for Trypanozoon as well as specific LAMP for T. b. rhodesiense (Njiru et al. Reference Njiru, Mikosza, Armstrong, Enyaru, Ndung'u and Thompson2008b). As with the TBR PCR, LAMP and NASBA assays for Trypanozoon need to be treated with caution, since they may detect DNA from abortive T. b. brucei inoculation. Yet, again, these assays target pan-Trypanozoon multicopy targets, while multicopy T. b. gambiense and T. b. rhodesiense diagnostic indicator genes remain unavailable. Single copy target genes always offer inherently low sensitivity for detection. Before these assays can be applied as diagnostic tools it is imperative that the clinical and epidemiological significance of a positive signal is properly understood. At present this is not the case but simpler, possibly more sensitive tools, LAMP and NASBA may help to improve research and disease control in this area.
Elsewhere, isolated cases of congenital transmission of sleeping sickness have been observed (Rocha et al. Reference Rocha, Martins, Gama, Brandae and Atougia2004) but the risk of vertical transmission remains unknown (Lindner and Priotto, Reference Lindner and Priotto2010). However, given the high proportion of under 12 s positive for T. brucei s.l. parasites according to TBR PCR, we ask whether congenital transmission should be considered as part of the local epidemiological dynamic in Western Uganda. This is particularly important given the focus put on tsetse control for disease suppression. Would congenital transmission be able to perpetuate a silent reservoir of infection in the absence of tsetse?
After 100 years of sleeping sickness research, DNA-based diagnostics are becoming ever more sensitive and practical. However, ironically, we remain relatively ignorant about disease progression for T. b. gambiense sleeping sickness and the factors which determine endemic and epidemic disease. Thus we are unfortunately confined to undertake reactive action alone against sleeping sickness, waiting to respond to disease outbreaks in affected communities in which infections have progressed to being clinically critical and requiring treatment with damaging toxic drugs. Until we are able to undertake long-term longitudinal studies, to screen and clinically examine the sleeping sickness and health status of people living in silent foci for sleeping sickness and relate the presence of DNA to parasite to infection status and subsequent disease, we will continue to speculate as to what is a real infection and what should be treated. It would be a real tragedy, for children and adults, if we are indeed missing very early infections, which could potentially be easily identified and safely treated.
ACKNOWLEDGEMENTS
We are grateful for ethical approval from the Uganda Ministry of Health and the Ugandan Ministry of Health T. b. gambiense screening teams who performed the mass-screening and to Tanoé Miézan who performed the mAECT.
FINANCIAL SUPPORT
We thank the UK Department for International Development (DFID) RNRRS and Research into Use Programmes for the benefit of developing countries (SCW; BvW; NAW, SLW, ASK) the World Health Organization (SCW; BvW, NAB, ASK), and the Wellcome Trust (SCW) for support for this study. SLW was supported by an MRC PhD studentship (www.mrc.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.








