INTRODUCTION
Host-sterilizing parasites are thought to have evolved in response to the tradeoff between maximum parasite reproduction and host longevity (Baudoin, Reference Baudoin1975; Ebert and Hamilton, Reference Ebert and Hamilton1996; Lafferty and Kuris, Reference Lafferty and Kuris2009). By exploiting non-vital organs and physiological processes, parasites can maximize host tissue and energy use while minimizing parasite-induced host mortality (Obrebski, Reference Obrebski1975). In most cases the differences in reproductive output between parasitized and non-parasitized individuals can be readily compared with egg counts and dissections. However, sterilizing parasites can not only impact reproductive output but also alter host longevity. Host longevity is a more subtle property of fitness that can largely influence the host-parasite relationship. Based on laboratory studies, the effects of sterilizing parasites on host longevity range from significantly increasing host lifespan (see Hurd et al. Reference Hurd, Warr and Polward2001) to significantly decreasing host lifespan (see Jaenike et al. Reference Jaenike, Benway and Stevens1995). Therefore, for a given relationship between a sterilizing parasite and its host, comparing reproductive rates between parasitized and non-parasitized hosts is not enough. It is important to determine which components of host fitness are affected by parasitism, especially when assessing host and parasite population dynamics.
At the population level, mathematical models have shown that the effects of parasitism on the host (e.g. parasitic sterilization, reduced longevity) are important determinants of host abundance in natural systems (Anderson and May, Reference Anderson and May1978; May and Anderson, Reference Anderson and May1978). Parasite-induced reductions in host fecundity and longevity reduce both the mean net reproduction rate in host populations. However, a parasite that is capable of reducing both fecundity and longevity can result in unstable population dynamics (May and Anderson, Reference Anderson and May1978). In past studies, the effect of Paraiotonchium autumnale (Nickle) (Tylenchida: Iotonchiidae) parasitism on natural face fly (Musca autumnalis DeGeer [Diptera: Muscidae]) populations has been evaluated based solely on the proportion of oviposition prevented by parasitic sterilization. For example, Krafsur et al. (Reference Krafsur, Church, Elvin and Ernst1983) calculated modest reductions of 2·4–6·3% in net reproductive rate based on prevalence of parasitized flies in central Iowa, USA. However, this may be an underestimate if P. autumnale parasitism also alters face fly longevity.
An important detail to consider when comparing effects of a sterilizing parasite on its host is to assess the effects of parasitism separately by sex. Parasitism can affect male and female hosts in very different ways, which translates to different fitness costs (Poulin, Reference Poulin1996). For example, in the case of P. autumnale and face flies, parasitism results in parasitic sterilization among female flies but not among male flies (Nappi, Reference Nappi1973). Male face flies are considered dead-end hosts. It is possible that parasitism may be more lethal to one sex than the other especially if one sex is not a ‘natural host’ (Poulin, Reference Poulin1996; Druilhe et al. Reference Druilhe, Hagan and Rook2002).
Several studies have characterized the behavioural and physiological differences between parasitized and non-parasitized face flies (Stoffolano, Reference Stoffolano1967; Stoffolano and Streams, Reference Stoffolano and Streams1971; Nappi, Reference Nappi1973; Kaya et al. Reference Kaya, Moon and Witt1979; Chirico, Reference Chirico1990, Reference Chirico1994), but few have specifically focused on differences in longevity. A previously published longevity study reported that parasitization did not affect face fly lifespan, but no statistical analysis was done (Robinson and Combs, Reference Robinson and Combs1976). The sheer number of nematodes that is usually present in the abdomen of a single fly would suggest that survival would be affected. A study by Geden (Reference Geden1997) detected significantly decreased lifespan among female house flies (Musca domestica Linnaeus) (Diptera: Muscidae) parasitized with a nematode in the genus Paraiotonchium. Here we hypothesized that parasitized face flies would have a reduced lifespan compared to non-parasitized individuals. The main objectives of this laboratory study were to determine if P. autumnale alters face fly longevity and determine if there are differential effects of parasitism based on host sex.
MATERIALS AND METHODS
The impact of P. autumnale on the longevity of M. autumnalis adults was evaluated in the laboratory between May and September in 2009 and 2010. Musca autumnalis eggs and larvae were obtained from field-collected cattle dung pats from the University of California Sierra Foothill Research and Extension Center in Browns Valley, California. Flies that eclosed from pupae collected from these dung pats were the subjects of this study.
Natural history of the host-parasite relationship
Paraiotonchium autumnale is a host-specific nematode parasite of the face fly, that causes parasitic sterilization in female flies (Nickle, Reference Nickle1967; Stoffolano and Streams, Reference Stoffolano and Streams1971). This parasite's life cycle alternates between gamogenetic and parthenogenetic generations and is linked with its host's life cycle (Stoffolano, Reference Stoffolano1970). Initial invasion of hosts takes place in cow dung by mated gamogenetic female nematodes. Once inside the host's haemocoel, the gamogenetic female continues to mature as the face fly larva goes on to complete its life cycle. Following the eclosion of the adult fly, the gamogenetic female produces a generation of parthenogenetic female nematodes. These parthenogenetic females produce the second generation of sexually reproducing male and female nematodes. In female face flies, these nematodes invade the ovaries. While healthy female flies oviposit on dung pats, parasitized female face flies deposit male and female 4th-stage juvenile nematodes (‘nemaposit’) (Kaya et al. Reference Kaya, Moon and Witt1979; Kaya and Moon, Reference Kaya and Moon1980). The nematodes moult and mate in the dung. The mated female nematode is the infective stage that then searches for a face fly larva host.
Dung collection
Dung pats were collected from pastures where we observed female M. autumnalis activity on fresh dung pats and respiratory stalks of eggs were visible in the dung. The collection of dung pats based on these two criteria allowed for increased likelihood of obtaining an abundance of fly puparia (Teskey, Reference Teskey1969). A spade was used to collect an intact individual dung pat which was placed into a Sterilite® 5·7-litre plastic shoebox (34·6 cm L × 21·0 cm W × 12·4 cm H) (Sterilite Corporation, Townsend, MA, USA). Each shoebox was covered with a mesh lid and transported in a cooler to the laboratory. Ten to 15 dung pats were collected monthly.
Collection of puparia
Upon returning to the laboratory, ca. 1 kg of dry coarse grit sand (0·05–1 mm particle size) was added to each shoebox between the dung pat and the edges of the shoebox. The sand provided a medium for M. autumnalis pupariation. The shoeboxes were kept at ambient temperature (23 °C) and 30 ± 2% relative humidity (RH). On average, the larvae emerged from the dung pat and initiated pupariation within 7 days of collection. Two days following pupariation, a household stainless steel strainer (22·9 cm diameter) was used to separate the puparia from the sand. The puparia from dung pats collected on the same day were stored individually in 29·6 ml Solo® soufflé portion cups (Solo Cup Company, Highland Park, IL, USA). The puparia were kept in a 25 °C incubator (23 ± 2% RH) with a photoperiod of 16 h light: 8 h dark, and checked daily for eclosion.
Longevity experiment
Mating is essential for parasitized female flies to nemaposit, and because we do not know the consequences that could arise from females withholding their nematode loads, we caged male and female flies together to allow for mating and the parasite life cycle to proceed normally. Additionally, parasitized male face flies successfully mate with and inseminate parasitized and non-parasitized females, and therefore all flies in our study had a chance to mate, and we assumed there was no bias in terms of the costly process of mating (Nappi, Reference Nappi1973). Within 24 h of eclosion, 20 male and 20 female flies from the same emergence date were released into steel-mesh, sleeved cages (30·5 cm L × 30·5 cm W × 30·5 cm H). Each cage was placed 4·2 cm from a clamp-on aluminum lamp with a shade (21·6 cm diameter) with a 60 W incandescent light bulb. The photoperiod was set to 16 h light: 8 h dark which maintained the temperature of the cages between 27 °C and 29 °C and 29 ± 2% RH. Water in a 236·6 ml, 4·9 cm × 9 cm (height × diameter) hi-plas® polyethylene cup (Highland Plastics Inc. Mira Loma, CA, USA) with a moist cotton wick, 5 sugar cubes, and ca. 10·5 g of powdered skim milk in a 60 ml Petri dish were provided and replenished as needed. Slices of fresh beef liver (ca. 30 g) were hung with a paper clip inside the cages and were replaced weekly. Two hundred g of fresh cattle dung free of fly larvae was provided every 2 days in a 177 ml Solo® double poly-paper cup to allow for oviposition. There were 9 replicate cages in 2009 and 7 replicate cages in 2010.
When the flies were placed into the cages, the infection status of each fly was unknown. There is no evidence of the transfer of nematodes from parasitized to non-parasitized individuals during mating, and therefore we were not concerned about fluctuating levels of parasitism throughout the study (Nappi, Reference Nappi1973). The cages were checked daily and dead flies were collected and kept in a freezer (−17 °C) until they could be dissected and examined for the presence of P. autumnale. Flies were dissected in water, sex was determined, and nematode presence (i.e., parasitism) was determined using a dissecting microscope at 25× with transmitted light. For parasitized individuals, the status of parasite development within the fly was graded according to Kaya and Moon (Reference Kaya and Moon1978) (Table 1).
Table 1. Parasitism class and corresponding stages of Paraiotonchium autumnale present in Musca autumnalis

Re-analysis of the data of Robinson and Combs (Reference Robinson and Combs1976)
Data values from the figures published by Robinson and Combs (Reference Robinson and Combs1976) were retrieved with graph digitizer software, DigitizeIt (Bormann, Reference Bormann2010). The figures were imported as JPG formats into the software. For each figure, a list of coordinates was created based on a defined and calibrated axis. Coordinate values were in the format of number of survivors over time. The values for the longest-lived flies were reported in the paper, and these values were used to indicate the end of the study time. The difference in the numbers of survivors between each day was calculated to obtain the number of dead flies collected per day. A daily count of the number of flies at risk and the number of flies that died was established. Kaplan-Meier survival curves were plotted in the statistical program JMP® 9.0 (SAS 2010). Log-rank tests were done to compare survivorship curves between parasitized and non-parasitized female and male face flies (Fig. 2).

Fig. 2. Projected Kaplan-Meier survival curves for parasitized and non-parasitized face flies, Musca autumnalis, re-analysed with published results from Robinson and Combs (Reference Robinson and Combs1976). (A) Female face flies. (B) Male face flies.
Data analysis
All statistical analyses were carried out in JMP® 9.0 (SAS, 2010). Kaplan-Meier curves were generated, and the log-rank test, (P = 0·05) was used to assess the homogeneity of the survival data from 2009 and 2010. There were no censored individuals. Data for the California flies were found to be homogeneous within both non-parasitized female (χ2 = 0·5894, log-rank = 0·4426) and male (χ2 = 0·9625, log-rank = 0·3270) flies. Homogeneity was found within parasitized male flies (χ2 = 3·2913, log-rank = 0·0696), but highly significant heterogeneity was found within parasitized female flies (χ2 = 8·8465, log-rank = 0·0029). The data were analysed separately by year for analysis since homogeneity was found only within non-parasitized groups. Subsequent analysis of the longevity data was done with additional log-rank tests based on plotted Kaplan-Meier curves. Flies were grouped by year and infection status and analysed separately by sex.
RESULTS
Kaplan-Meier analysis by year revealed a non-significant difference in longevity between healthy and parasitized flies for female (χ2 = 0·5876, log-rank = 0·4434) and male (χ2 = 1·8358, log-rank = 0·1754) flies in 2009 (Fig. 1A, C). In the 2009 dataset, the median lifespan for non-parasitized female flies was 29 days (CI = 22–33 days) and 26 days (CI = 15–32 days) for parasitized flies. For the 2009 male flies, the median lifespan was 40·5 days (CI = 28–45 days) for non-parasitized individuals, and a median time of 18·5 days (CI = 13–25 days) for parasitized individuals. Significant differences were found between parasitized and non-parasitized female (χ2 = 10·4765, log-rank = 0·0012) and male (χ2 = 15·0849, log-rank = 0·0001) flies in 2010 (Fig. 1B, D). In the 2010 dataset, the median time to death for non-parasitized female flies was 20 days (CI = 13–29 days) and 15 days (CI = 8–19 days) for parasitized females. The 2010 median survival time for non-parasitized male flies was 33 days (CI = 19–39 days) and 15 days (CI = 3–23 days) for parasitized males.

Fig. 1. Kaplan-Meier survival curves for parasitized and non-parasitized face flies. (A) Female face flies from 2009. (B) Female face flies from 2010. (C) Male face flies from 2009. (D) Male face flies from 2010.
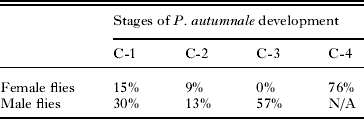
The intensity of nematode parasitism was not quantified, but the first generation gamogenetic female burden usually ranged from 1 to 3 females present in female face flies and a single first generation gamogenetic female nematode was present in male face flies. Overall, 24% (CI = 15% − 35%) of parasitized female flies died prior to infection level C-4. For parasitized male face flies, 43% (CI = 32% − 55%) of male flies died prior to infection level C-3. No deaths were recorded during the C-3 stage for parasitized female flies (Table 2).
Table 2. Proportion of deaths associated with specific stages of parasite Paraiotonchium autumnale development (Table 1) (n = 67 for female face flies and n = 69 for male flies.)
Re-analysis of the data of Robinson and Combs (Reference Robinson and Combs1976)
The log-rank tests revealed no significant differences in longevity among parasitized female (χ2 = 0·2489, log-rank = 0·6178) (Fig. 2A) and male (χ2 = 0·0944, log-rank = 0·7586) (Fig. 2B) flies compared to their non-parasitized counterparts. The median lifespan was 63 days (CI (95%) = 58–70 days) for non-parasitized females while parasitized females had a median lifespan of 74 days (CI = 65–84 days). The median lifespan for non-parasitized males was 74 days (CI = 74–79 days) while parasitized male flies had a median lifespan of 80 days (CI = 69–84 days).
DISCUSSION
While this study is the first to report decreased longevity among P. autumnale-parasitized face flies, this study is not the first to report decreased longevity among hosts parasitized by nematodes in the genus Paraiotonchium. Paraiotonchium muscadomesticae Coler and Nguyen (Tylenchida: Iotonchiidae) has a similar biology to that of P. autumnale. This nematode, however, parasitizes houseflies. Geden (Reference Geden1997) reported that P. muscadomesticae-parasitized female houseflies live half as long as non-parasitized female flies.
Of the 67 parasitized female flies in this study, 76% harboured a C-4 class parasitism at death. Paraiotonchium autumnale parasitization shortens the lifespan of its host; however, this outcome may have little significance towards overall parasite reproductive and transmission success. Kaya et al. (Reference Kaya, Moon and Witt1979) stated that female face flies that harbour C-4 class parasitism, change their behaviour and become dung seekers. It was reported that the parthenogenetic female nematodes continually produce second-generation gamogenetic stages so the ovaries of female flies are continuously distended. This state of the ovaries apparently provides females with the perception of a continual presence of eggs. Thus, these parasitized flies continuously seek dung to nemaposit rather than to oviposit. Host death during C-4 may be of little consequence to the parasite as long as the next generation of parasitizing nematodes is released.
A previous study by Robinson and Combs (Reference Robinson and Combs1976) concluded that parasitism by P. autumnale does not affect the longevity of adult face flies based on a laboratory study in which flies were provided sugar and water as the diet. A Kaplan-Meier survival analysis with values extracted from their published figures confirms this conclusion with non-significant log-rank test values. Notably, however, the median lifespans for non-parasitized and parasitized face flies are 2–3 times longer than the values reported in our study. There are numerous factors that may have contributed to these different results. One possible explanation for this outcome is the difference in diet provided to the flies during the study. Kaya and Moon (Reference Kaya and Moon1980) stated that dietary protein is not only necessary for ovarian development but also necessary for P. autumnale development. Robinson and Combs (Reference Robinson and Combs1976) did not provide a protein supply for the flies in their study. In Kaya and Moon's (Reference Kaya and Moon1980) study, protein-deprived parasitized female face flies harboured parasitism classes from C-1 to C-3 and rarely C-4, and ovarian development and the parasite life cycle resumed after addition of protein. Previously, Turner and Hair (Reference Turner and Hair1967) reported longer adult longevity and zero reproductive output for non-parasitized flies deprived of dietary protein. The female flies in Robinson and Combs's (Reference Robinson and Combs1976) study may have had extended lifespans due to the suspension of reproductive development, which is an energetically expensive process. This suspension of reproductive development may have halted parasite development and subsequent pathogenic activity, reducing the negative effects of being parasitized. The 1976 conclusion was based on starved hosts, and may have underestimated the impact of P. autumnale on face fly longevity. Provided adequate nutrients, decreased longevity is a more plausible outcome of P. autumnale-parasitized face flies. However, it is unclear if dietary protein would influence physiological processes and parasite development among male face flies in a similar fashion. It appears that host starvation can be linked to parasite-induced host death, but further investigations are needed to determine whether or not host diet plays a significant role in P. autumnale-induced face fly death.
By analysing female and male flies separately we have discovered that, based on the results from our 2010 study, the decrease in lifespan was much more pronounced in male face flies than female flies. Male face flies are considered dead-end hosts since there is no natural exit for P. autumnale to escape (Stoffolano, Reference Stoffolano1970; Nappi, Reference Nappi1973). A possible explanation for the pronounced effect in male face flies may be that the P. autumnale parasites cause substantial internal damage or interrupt vital biological processes since the nematodes are not localized within the host. Nappi (Reference Nappi1973) reported that less than 50% of female flies oviposit when mated with a parasitized male, suggesting that sperm production also may be affected; it was also reported that the testes are not invaded by P. autumnale, but heavily parasitized males often lacked accessory glands. Here we have shown how the sex of the host and the role it has in the parasite's life can largely influence the magnitude of host damage.
Female and male flies are parasitized at equal frequencies (Kaya and Moon, Reference Kaya and Moon1980; Chirico, Reference Chirico1990). Are male face flies truly dead-end hosts for P. autumnale? Nemaposition by female face flies is not a requirement for P. autumnale to be parasitic to face fly larvae. Second-generation gamogenetic nematodes extracted from male flies are equally capable of parasitizing hosts as nematodes from female flies (Stoffolano, Reference Stoffolano1970). Although male flies are not frequent dung visitors, it is not uncommon to see male flies on dung pats in the field or laboratory (Nappi, Reference Nappi1973; Kaya et al. Reference Kaya, Moon and Witt1979). Since nematodes were found in the head of male face flies, Nappi (Reference Nappi1973) postulated that nematodes could possibly exit from the mouthparts while the flies are on the dung. However, there was no evidence that the nematodes could exit from the mouthparts of males. An alternative possibility is that nematodes are capable of escaping from dead hosts. Preliminary tests with agar revealed that P. autumnale could escape from dead face flies if the substrate is moist enough to cause the disintegration of the cadavers (unpublished observations).
Increasing evidence suggests that environmental heterogeneity and pathogen diversity can maintain high levels of host genetic variation for immune traits (Lazzaro and Little, Reference Lazzaro and Little2009). Abiotic factors such as temperature and nutrition levels may heavily influence host susceptibility to parasites and parasite activity (Wolinska and King, Reference Wolinska and King2009). Furthermore, there is evidence that exposure to higher temperatures can reduce susceptibility to low levels (Blanford et al. Reference Blanford, Pugh and Pell2003). Condition-dependent host immunity and pathogen activity may explain the different results between the 2009 and 2010 flies. The temperatures for the collecting dates in 2009 were between 27 °C and 37 °C while in 2010, the temperatures were between 19 °C and 25 °C, with one day being 34 °C. The flies present in field during the 2009 collecting days may have been exposed to stressful environmental conditions that influenced susceptibility, manifested by longer lifespans of parasitized flies from this year. Additionally, the total percentage parasitism was 13% for females and 16% for males in 2009 compared to 31% for females and 29% for males in 2010. The lower percentage of parasitized flies in the 2009 cohort may also support the existence of variable immunity influenced by environmental factors. Furthermore, nutrition levels of the hosts may also have influenced the outcomes of our study. Another factor that may influence the longevity of flies is the quality of cattle dung in which the face fly larvae feed. Greenham (Reference Greenham1972) effectively showed how the seasonal changes in the quantity and quality of forage affects the composition and formation of cattle dung, which can ultimately influence the biology of bush flies (Musca vetustissima) in Australia. The data indicated that the difference in the quality of dung affects survival and reproduction of coprophagous insects. Possibly, the variability of the cattle dung may have had an effect on the longevity of face flies by giving certain cohorts a nutritional advantage. In general it would be interesting if environmental temperatures and nutritional quality can influence the relationship between sterilizing parasites and their hosts.
We have demonstrated that it is possible for P. autumnale parasitism to significantly reduce the M. autumnalis lifespan in the laboratory setting. However, given the different outcomes between the years, the impact of a sterilizing parasite on its host longevity may depend upon environmental as well as physiological factors. Whether such decreases in face fly lifespan can be measured in the field settings remains to be demonstrated. A possible way to measure P. autumnale impact on face fly longevity in natural systems is to characterize the age structure of parasitized and non-parasitized flies present in the field. Age structure of face flies can be characterized by measuring the pterin content and gonotrophic status of field-collected adult flies (Moon and Kaya, Reference Moon and Kaya1981; Krafsur et al. Reference Krafsur, Moon and Kaya1985, Reference Krafsur, Moon and Kim1995; Moon and Krafsur, Reference Moon and Krafsur1995; Butler et al. Reference Butler, Moon, Hinkle, Millar, McElfresh and Mullens2009). If prevalence of parasitism decreases with host age, then it is possible that parasitism reduces host longevity in natural systems. If significant reduction in host longevity due to P. autumnale is measured in the field, the overall impact of P. autumnale on face fly populations may have been underestimated.
For a given host-parasite relationship it is important to analyse the effect of the parasites separately by sex because the cost of parasitism may be different for conspecific males and females. Lastly, the interplay between environmental conditions and host susceptibility must be considered together to make forecasts about the impact of parasites, both on individual hosts and on host populations.
ACKNOWLEDGEMENTS
Comments and suggestions from Dr Bradley A. Mullens improved methods and drafts of this manuscript. Dr Jerome Brown provided additional statistical analysis advice. Thank you to Andrew Ross, Soledad Villamil, Alexander Dedmon, and Alexander Ko, of the Lewis Laboratory for their assistance in maintaining this project. Thank you to the staff at the University of California Sierra Foothill Research and Extension Center and the staff at the University of California, Davis Beef Barn Facility. A special thank you to Dustin K. Flavell, Dan M. Myers, Chris A. Feddersen, and Jerry Johnson for granting permission to use these facilities.
FINANCIAL SUPPORT
Funding for this project was provided by the McBeth Memorial Scholarship and the Jastro Shields Research Scholarship Award.






