INTRODUCTION
Circadian rhythms are endogenous physiological oscillations with a period of approximately 24 h (Calisi and Bentley, Reference Calisi and Bentley2009). These rhythms are widely found in free-living organisms from prokaryotic cyanobacteria to essentially all eukaryotes (Bünning, Reference Bünning1973). In free-living animals, daylight changes were proven to be a cue for setting the activity rhythms (Kumar et al. Reference Kumar, Singh and Rani2004). The adaptive value of such rhythms lies in the advantages for the organism in synchronizing its behavioural and physiological processes to periodically oscillating environmental factors (Sharma, Reference Sharma2003). Moreover, diurnal periodicity was even demonstrated for some of the endoparasites, experiencing the environmental changes mediated via their host. For instance, in various species of filariae, transmitted by different arthropode vectors, the concentration of microfilariae in the peripherial blood of their vertebrate hosts is reaching its maximum at different times of the day, according to the time the arthropod vectors are likely to bite (Asio et al. Reference Asio, Simonsen and Onapa2009). However, the adaptive reasons for synchronization of circadian rhythms in endoparasites with a direct life cycle, such as avian Isospora (Apicomplexa: Eimeriidae) species are still unclear (Dolnik, Reference Dolnik1999; Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009). These protozoan intracellular intestinal parasites are widespread among passerine birds (Svobodová, Reference Svobodová1994), with prevalence of infection reaching 100% in some populations (Schwalbach, Reference Schwalbach1960). Representing the parasites with direct life cycle, avian Isospora species require no vector for the spread of infection, and transmission occurs, if an appropriate host ingests sporulated oocysts (Long, Reference Long1982). To become infective, freshly excreted unsporulated oocysts need time to complete their development (sporulation), which takes, depending on the species and surrounding temperature, from 3 to 4 days to 18 h, and for some individual oocysts even less (Schwalbach, Reference Schwalbach1959; Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009).
Transmission is a key event during the parasite life cycle, and from an evolutionary perspective, it is generally assumed that any trait increasing the transmission success of the parasite would be selected for (e.g. Bush et al. Reference Bush, Fernandez, Esch and Seed2001; Combes, Reference Combes2001; Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009). The success of avian Isospora transmission depends on a combination of 2 factors: on the successful sporulation and survival of the oocysts on the one hand, and on the probability of being ingested by an appropriate host, on the other (Dolnik et al. Reference Dolnik, Dolnik and Bairlein2010).
From the temperate zone to the tropics, in different Isospora spp. from various families of passerine birds, oocyst output does not occur continually or stochastically during the day, but emergence of individual oocysts is highly synchronized, peaking in the afternoon (Boughton, Reference Boughton1933; Schwalbach, Reference Schwalbach1960; Grulet et al. Reference Grulet, Landau, Millet and Baccam1986; Dolnik, Reference Dolnik1999; Lindström et al. Reference Lindström, Dolnik, Yabsley, Hellgren, O'Connor, Pärn and Foufopoulos2009).
This periodicity was hypothesized to be an adaptation of the parasite to increase both (1) the success of the sporulation and survival of the oocysts (Dolnik, Reference Dolnik1999; Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009), and (2) the probability of being swallowed by a new host (Dolnik et al. Reference Dolnik, Dolnik and Bairlein2010). The first is possible because afternoon output enables the parasite to avoid lethal solar radiation and desiccation of its exogenous stages during the critical time of sporulation (Boughton, Reference Boughton1933; Schwalbach, Reference Schwalbach1960; Dolnik, Reference Dolnik1999). Vulnerability of unsporulated oocysts to desiccation and destruction via UV radiation has been experimentally demonstrated recently (e.g. Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009), although there are exceptions (Barré and Troncy, Reference Barré and Troncy1974). After completing their development, the now sporulated oocysts are resistant to sunlight and desiccation (Long, Reference Long1982; Belli et al. Reference Belli, Smith and Ferguson2006) and at this stage they serve the purpose of preservation and dispersion. The latter advantage for the transmission is possible due to the coincidence of oocyst output peak with the evening peak of bird feeding activity (Dolnik, Reference Dolnik1999), which allows the oocysts to be shed at the foraging grounds.
Experimental work on temperate zone passerines has shown that the peak of Isospora oocyst production depends on daylight periodicity and occurs during the second half of the day (Boughton, Reference Boughton1933; Dolnik, Reference Dolnik1999; Wild, Reference Wild2003). Furthermore, experiments on House Sparrows showed that when the light regime was reversed (by swapping the dark and light periods), not only host activity rhythms changed but also the peak of Isospora oocyst output, being adjusted to the altered light conditions (Boughton, Reference Boughton1933). Also, experiments involving food provision of sparrows during either the first or the second part of the day only, revealed that host temporal pattern of foraging does not affect the temporal emergence of oocysts in the birds' faeces (Boughton, Reference Boughton1933). Experimental conditions with constant dim light and food ad libitum destroy not only the circadian activity rhythms of the host, but also the rhythms of oocyst output in House Sparrows (Wild, Reference Wild2003). However, if then the food availability is restricted to a particular time of the day, the oocyst output happens at the same time of the day (Boughton, Reference Boughton1933; Wild, Reference Wild2003).
On the other hand, Isospora parasites are recorded in passerine birds breeding in the Arctic (>66°33′ above the Polar Circle). During the Arctic summer, there are 24 h of continuous daylight for several months while there is no light at all during the Arctic winter. The lack of light-dark alternation poses unique challenges to the circadian systems of both the hosts and their parasites.
The aim of this study was to investigate how the polar day affects the diurnal periodicity of isosporan parasites from natural polar inhabitants. By suggesting that in the wild, synchronization of oocyst output is under positive selection throughout the latitudes, we hypothesized that in arctic inhabitants under natural conditions, the synchronized rhythm of oocyst output is kept under continuous light of the polar day in summer.
MATERIALS AND METHODS
Our model host species, the Snow Bunting (Plectrophenax nivalis nivalis), is the northernmost breeding passerine bird in the world (Cramp and Perrins, Reference Cramp and Perrins1994), and the only passerine breeding annually and in large numbers in the High Arctic conditions of Svalbard. This small bird typically arrives at the breeding grounds on Svalbard in the end of March–April, and leaves in August–September, to spend the winter in temperate areas (Cramp and Perrins, Reference Cramp and Perrins1994). By this pattern, the Snow Bunting spends over half of the year in a temperate zone with a light-dark rhythm, but during the reproductive season, this bird experiences continuous daylight.
Isospora plectrophenaxia Dolnik and Loonen Reference Dolnik and Loonen2007 was chosen as the model parasite species. This intracellular intestinal parasite has been described from Snow Buntings and is known to have successful transmission on Svalbard (Dolnik and Loonen, Reference Dolnik and Loonen2007), thus allowing investigation of its circadian rhythm at the breeding site of its host.
The samples were collected in Ny-Ålesund, Svalbard (78°55′N; 11°55′E) in July 2006, 2007 and 2009. Due to the location of the study site north of the polar circle, there is continuous sunlight during the period from 19 April until 23 August. In total, 250 fresh faecal samples, homogenously distributed through different times of the day (0–24 h), were collected from 37 nestlings, 10 juvenile and 4 adult Snow Buntings. Fresh faecal samples of nestlings were collected from 38 nestlings from 11 nests; 14 nestlings were sampled once, 24 nestlings were sampled repeatedly, at least twice, at different time of the day. Additionally, samples from fledged juveniles and adult birds were collected by the following method. Juvenile and adult birds from the village population often rest alone on wooden covers of pipes, ca. 1 m broad, which are numerous and stretch along and across the village. Binocular observations of resting birds determined the exact moment and the spot of defecation, and the fresh faecal droplets were collected from the wooden pipe covers immediately after shedding. By this method, an additional 3 adults and 8 juvenile Snow Buntings were sampled.
All the samples were collected individually and stored in separate vials with a 2·5% aqueous solution of potassium dichromate K2Cr2O7 at room temperature until processed. Each sample was labelled with date, time of the day, and age of the bird. For nestlings, individual colour ring combinations were also noted, to allow individual attribution. In the laboratory, oocysts were separated from faeces by flotation in saturated NaCl solution for 5 min at 375 g, and the surface layer was removed using a 5-mm diameter loop, deposited on a slide, and immediately examined under ×100 magnification to determine the presence of the oocysts (for details, see Dolnik, Reference Dolnik2006); for species determination, ×1000 magnification with oil immersion was used.
The Atmospheric Observatory of the AWIPEV Base in Ny-Ålesund kindly provided data on the daily pattern of UV radiation on a clear July day at our study site, with the permission to use them for this publication.
Data were analysed using PASW statistics 18 and Oriana 2.0 for circular statistics. In the first approach, all faecal samples were treated as independent samples, because due to the life cycle of single parasite individuals, there is no reason to expect that variation between host individuals would explain variation in the parasite prevalence in different faecal samples. Each sample was assigned to the next even hour of the day. For each even hour (12 per 24 hours), mean prevalence (% of positive faecal samples) was calculated. Samples attributed to the next even hour were homogenously distributed among these 2-h intervals during the day (chi2=12·848, D.F.=11, P=0·303, n=250). Therefore, it was possible to test the time pattern of oocyst output by the Rayleigh test.
To control for the effects of repeated sampling of some individuals, as well as year- and nest-effects, in the second analysis, a general mixed model was performed. In the model, the oocyst output (0=oocysts absent; 1=oocysts present) was the response variable, time of the day (2-h intervals) was a fixed factor, while host individual, date and nest were random factors.
RESULTS
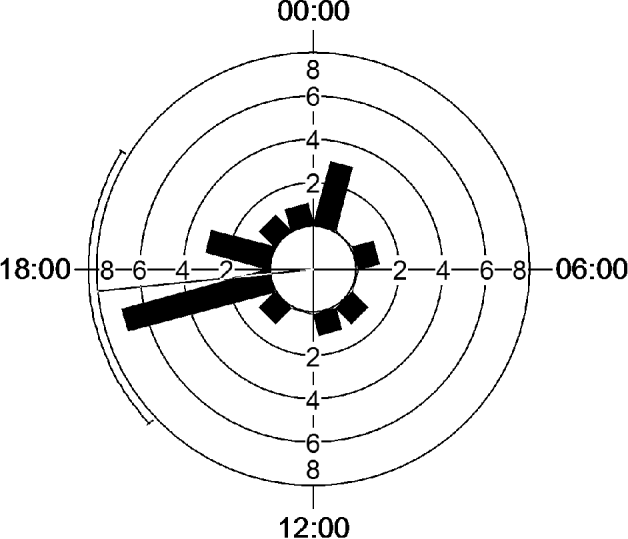
All oocysts belonged to the species Isospora plectrophenaxia. Oocysts were found in faeces of 13 individual birds, in 19 of the 250 examined samples. Positive oocyst samples (n=19) peaked at 4 p.m. (about 37% of all positive) with a prevalence of 33% ±10·5% S.E.M. (Resulting vector: direction μ=17:37, length r=0·46, concentration 1·034, circular variance 0·54, circular standard deviation 71·4°, Rayleigh Z=4·02, P=0·016) (Figs 1 and 2).

Fig. 1. Diurnal pattern of Isospora plectrophenaxia oocyst appearance in faecal samples of free-ranging Snow Buntings on Svalbard under continuous sunlight. Bars represent vectors of positive faecal samples with length=number of positive samples within 2-h intervals, and direction=time of the day; the line indicates direction of resulting mean vector±circular standard error.

Fig. 2. Prevalence (%) of the Snow Bunting faecal samples containing Isospora plectrophenaxia oocysts at different day times on Svalbard under continuous sunlight in the wild in 2-h intervals (± S.E.M.) (bars), and solar UV radiation (UV300-700 nm) at the study site on a clear day in July (line).
Time (2-h interval) was the significant factor in a general mixed model to explain the variance in oocyst output state (F=2·516, D.F.=11, P<0·005). Host individual, nest or sampling date were not associated with oocyst output.
On a clear day, the UV radiation reaches its maximum at noon, which in July on Spitsbergen can be up to 20 W/m2 for UV300-700 nm UVR, differing 5 times from that at midnight (Fig. 2).
DISCUSSION
The presence of diurnal periodicity and synchronism of oocyst output in the Arctic summer is especially interesting, because neither its mechanisms, nor adaptive reasons are clear. However, based on literature data available until now, some possible explanations can be discussed.
Except in the Polar Regions, daylight is the main external time cue (i.e. Zeitgeber) for synchronization of circadian rhythms in living organisms (Kumar et al. Reference Kumar, Singh and Rani2004). In the wild, a 24-h period of light-dark cycle synchronizes the rhythm of melatonin secretion in pineal organ of the bird, which in turn coordinates the circadian rhythm of bird's activity. These cyclic changes of melatonin concentrations in the host's blood are used by avian Isospora for oocyst output synchronization (Wild, Reference Wild2003; Brandmeier, Reference Brandlmeier2006). However, the very slight increase of melatonin around midnight, shown for Lapland Longspurs at 68th latitude in Alaska indoors (Hau et al. Reference Hau, Romero, Brawn and Van't Hof2002) can be expected to be even lower in free-ranging Snow Buntings at the 78th latitude. Indeed, in the experiments on Snow Buntings at Spitsbergen, it was confirmed that the slight differences of light intensity between ‘day’ and ‘night’ is not effective as the Zeitgeber for the birds (Krüll et al. Reference Krüll, Demmelmeyer and Remmert1985). Low or more or less constant melatonin leads to a destruction of the bird's own circadian rhythm under experimental conditions (Gwinner et al. Reference Gwinner, Hau and Heigl1997) and in the wild (Cockerm, Reference Cockrem1991; Reierth et al. Reference Reierth, Van't Hof and Stokkan1999) and is not enough to synchronize oocyst output rhythms of Isospora (Brandmeier, Reference Brandlmeier2006).
Therefore, Isospora parasites of birds in the High Arctic must be more sensitive to the slight changes in melatonin concentrations than their congeners from temperate zones, if we assume that they still use it as a Zeitgeber under polar day conditions. Otherwise, if host's melatonin is not available as a Zeitgeber for Isospora parasites, the oocyst output can be synchronized with an alternative Zeitgeber, namely the host's foraging activity (Wild, Reference Wild2003; Brandmeier, Reference Brandlmeier2006). However, this alternative Zeitgeber is also not available for I. plectrophenaxia on Svalbard, as foraging activity of Snow Buntings seems to be randomly distributed throughout the 24 h, except for a 2-h break around midnight (Haarhaus, Reference Haarhaus1968; Loonen, personal observations). In the absence of this alternative Zeitgeber, Isospora of experimental House Sparrows are not able to synchronize their rhythms any more, and the oocyst output loses its diurnal periodical pattern (Wild, Reference Wild2003; Brandmeier, Reference Brandlmeier2006). On the contrary, although also lacking a possibility to use the host's melatonin or foraging activity rhythm to synchronize their circadian rhythmic, Isospora parasites of Snow Buntings on Svalbard nevertheless synchronize their life cycle, with a peak of oocyst output during the afternoon hours. This might be triggered by (1) endogenous rhythmicity in any other physiological parameter of the bird, e.g. body temperature (Willmer et al. 2000), or (2) have exogenous reason, e.g. midnight ‘lull’ in foraging activity of the host, due to diurnal variation in food availability, which in its turn can be caused by ambient temperature (Erikstad, Reference Erikstad1989). Whatever is playing the role of Zeitgeber for Isospora of Snow Buntings, this factor either does not exist in experimental House Sparrows under continuous light, or for some reason does not play the role of trigger for oocyst output from House Sparrows. Therefore, further research is needed to reveal which alternative Zeitgeber is used by Isospora parasites in the Polar Regions.
Possible adaptive reasons of afternoon oocyst output also remain unclear. The discrepancy between the results of the present study on the parasites of High Arctic inhabitants in the wild and those of birds in captivity (Boughton, Reference Boughton1933; Wild, Reference Wild2003; Brandmeier, Reference Brandlmeier2006) suggests the adaptive significance of synchronizing the circadian rhythms in continuous daylight conditions. The absence of a foraging activity pattern of Snow Buntings gives the afternoon appearance of the oocysts no advantages of being shed at the feeding grounds. The most reasonable explanation is adaptation of exogenous stages to changes in UV-radiation. The sun's irradiance, though being lowest in the Arctic, still varies significantly throughout the day, in clear summer days increasing 5 times at noon compared to midnight. Humidity as another abiotic factor which has been suggested as an adaptive reason for afternoon oocyst output of Isospora species in temperate zones (Dolnik, Reference Dolnik1999; Martinaud et al. Reference Martinaud, Billaudelle and Moreau2009) can hardly be of significant importance for I. plectrophenaxia on Svalbard, because it remains over 70% throughout the day there.
An alternative explanation can be that the adaptive reason is linked to endogenous stages of the parasite's life cycle. First, it can be that the circadian rhythm of I. plectrophenaxia evolved on the winter grounds of the host in the temperate zone, and continues at the host's breeding grounds. However, in this case the question with the alternative Zeitgeber remains open. Second, it might be that synchronic re-colonization of new cells during merogony has advantages in the fight against the host's immune system. This is indirectly supported by the data of Grulet et al. (Reference Grulet, Landau, Millet and Baccam1986), showing that all stages of the Isospora life cycle in the House Sparrow are strictly synchronized. The third possible advantage of synchronic oocyst output might be that it is crucial for the parasite to reach a high concentration of oocysts in a droplet of faeces, to enable the infection of the next host. Indeed, the dose-dependent effect of coccidian infection is known for both domestic and wild animals (Long, Reference Long1982; Dolnik, Reference Dolnik2002). If so, for the parasite, even in the High Arctic, massive synchronized oocyst release, enabling higher oocysts concentration in faecal droplet, might take over the advantage of being equally distributed over time and space.
Whatever the proximate functions and ultimate reasons are, we suggest that the ability of successful invoking of an alternative Zeitgeber by ineffectiveness of the ‘classical’ ones is kept under positive selection pressure in the wild. This, in its turn, proves that maintaining synchronization of circadian rhythms of individual Isospora parasites within their passerine host remains crucial for the success and survival of these parasites above the Polar Circle. The reasons for the persistence of the synchronized temporal pattern in oocyst output under the High Arctic midnight sun, as well as the Zeitgeber for its synchronizing, remain to be investigated in detail.
ACKNOWLEDGEMENTS
We express gratitude to Janwillem Loonen, Oebele Dijk and Yvan Satgé for their great assistance and support during the field seasons on Svalbard. We are very grateful to Siegrid Debatin from AWI-Potsdam for weather-station data, and to Franz Bairlein and Anton Antonov for their comments on the manuscript. Permission to ring and handle Snow Bunting nestlings was given by the Stavanger Museum, the Governor of Svalbard and the Norwegian Animal Research Authority (project number 07/22333).
FINANCIAL SUPPORT
The work was supported by the Netherlands Organisation for Scientific Research (NWO) (grant number 851.40.071).