INTRODUCTION
Passerine bird species are frequently found to be infected with Coccidian parasites. In fact all passerines are likely to be potential hosts for at least 1 coccidian species (Svobodová, Reference Svobodová1994; Dolnik, Reference Dolnik2002a; Schrenzel et al. Reference Schrenzel, Maalouf, Gaffney, Tokarz, Keener, McClure, Griffey, McAloose and Rideout2005). Infection is transmitted via sporulated oocysts that are shed with faeces and then swallowed by the next host in contaminated food or water (Long, Reference Long1982). Host-specific foraging behaviour thus plays an important role in the transmission ecology of these parasites (Dolnik, Reference Dolnik2002a). Consequently, isosporan parasites of aerial feeding birds face exceptional transmission difficulties, usually resulting in low prevalence and low infection intensities compared to parasites of bird species with other foraging modes (Dolnik, Reference Dolnik2002a). However, this does not necessarily mean that parasite diversity is low in these host species (Pellerdy, Reference Pellerdy1974).
The Pied Flycatcher (Ficedula hypoleuca) is an aerial feeder or foliage gleaner, preying on various kinds of arthropods. This small migratory songbird breeds in tree holes and nest boxes across the Palaearctic Region, and winters in West- and Central-Africa south of the Saharan desert (Lundberg and Alatalo, Reference Lundberg and Alatalo1992). At least 3 species of coccidia have been described from Pied Flycatchers, and several additional unnamed species have been reported (Schwalbach, Reference Schwalbach1959; Cringoli and Quesada, Reference Cringoli and Quesada1990).
Descriptions of new isosporan species are traditionally based solely on oocyst morphology (e.g. Pellerdy, Reference Pellerdy1974; Upton et al. Reference Upton, Wilson, Norton and Greiner2001) and only recently has photomicrograph documentation become common (Duszynski, Reference Duszynski1999). Modern molecular methods enable a new level of documentation; morphological descriptions and photomicrographs are supported by molecular haplotypes determined from the same individual oocysts that were photographed, measured and described (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009). In this article, we describe a new species of Isospora Schneider, 1875 parasitizing Pied Flycatchers, based on oocyst morphology and confirmed by mitochondrial sequence data acquired from the same individual oocysts.
MATERIALS AND METHODS
Samples were collected between April and June 2006 in a breeding population of Pied Flycatchers near Itzehoe in northern Germany (54°01′ N; 9°33′ E). Adult birds where captured during incubation or while feeding their young, as part of a larger project investigating the population ecology of this species. Upon capture, each bird that had not been marked previously was provided with a uniquely numbered aluminium ring, measured, sampled and released. Nestlings were ringed, measured and sampled when 13 days old (hatching day=day 0). A small blood sample (10–100 μl) was collected from each bird by venepuncture of the brachial vein. Blood samples were kept at 4°C in the field and subsequently stored in 96% ethanol.
Faecal droplets, 1 pellet per bird, were collected immediately after shedding from 8 adult Pied Flycatchers that were trapped in the afternoon hours. Samples were kept in 2·5% aqueous potassium dichromate (K2Cr2O7) until processed. After flotation centrifugation in saturated NaCl solution for 5 min at 375 g, the surface layer was placed on slides and immediately examined under ×100 magnification to determine the presence and the number of oocysts. The whole slide was checked to avoid errors caused by oocyst clustering. Infection intensity was measured in o.p.d. (oocysts per defecation) according to the method described by Dolnik (Reference Dolnik2006). The detailed morphological structure of oocysts was studied under×1000 magnification with oil immersion.
To isolate individual oocysts for photographing and genetic analysis, we used the following method (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009). From a positive sample, a drop with oocysts was transferred to a new object glass using a sterile micropipette. Next it was serially rinsed through several 1 μl drops of ddH2O by transferring part of each drop to the next with microscopic control, until we achieved a single oocyst in a drop (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009). Each oocyst separated in this way was individually photographed and measured to the nearest 0·1 μm. Measurements are in micrometers, with range and number (n) of stages measured in parentheses.
Abbreviations used in the species descriptions are as suggested by Duszynski and Wilber (Reference Duszynski and Wilber1997). Oocyst characters include length (L), width (W), and their ranges and ratio (L/W); micropyle (M); residuum (OR); and polar granule (PG). Sporocyst characters include length (L), width (W), and their ranges and ratio (L/W); Stieda body (SB); sub-Stieda body (SSB); para-Stieda body (PSB); residuum (SR); sporozoites (SP); refractile bodies (RB); and nucleus (N) in SP.
After photographing, 22 sporulated oocysts (1–6 oocysts per individual bird) were individually collected in separate vials and frozen at −80°C. DNA of each individual oocyst was extracted by adjusted Chelex extraction (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009). Molecular analysis of oocysts was performed individually using a nested PCR method to amplify a 250 bp long fragment of the alpha subunit of the mitochondrial cytochrome oxidase gene (COI) of the parasite using external primers COX tenella F4/R and internal primers COX tenella F2/R2 (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009). Positive controls with genomic DNA from Isospora sp. oocysts from Blackcap Sylvia atricapilla, and negative no-template controls were included in each PCR reaction.
Total DNA from blood was extracted using a standard phenol-chloroform method (Sambrook et al. Reference Sambrook, Fritsch and Maniatis2002), and 2 μl of diluted genomic DNA (25 ng/μl) was used as template in the same PCR protocol as for the oocysts. The success of PCR amplifications was checked on 2% agarose gels and positive samples were sequenced with BigDye (®Applied Biosystems, Foster City, CA, USA) terminator cycle sequencing kit. Sequences were edited and aligned using the software BioEdit (Hall, Reference Hall1999). We used ‘Basic Local Alignment Search Tool’ (Blast) to compare obtained sequence fragments with known parasite sequences (http://www.ncbi.nlm.nih.gov/blast).
RESULTS
Isosporan oocysts were found in faeces from 6/8 (75%) adult Pied Flycatchers. All infections detected were monospecific and intensity of infection varied between individuals from 1 to 500 oocysts per defecation. A comparison revealed several morphological differences between the oocysts from this species and those of previously described Isosporan species found in Ficedula- and Muscicapa-Flycatchers, as described below. From 22 single oocysts, we obtained 14 (64%) high quality COI sequences.
None of the blood samples of the adult birds showed positive PCR reactions. However, blood samples of nestlings from nests with infected parents showed positive PCR reactions, which resulted in high quality sequence. We found infections in 3 out of 12 (prevalence 25%) nestlings belonging to 1 out of 3 nests. We obtained the same Isospora haplotype (iFICEHYP1, GenBank No. FJ269363) from both oocysts of adult Pied Flycatchers and blood samples from the nestlings. When comparing iFICEHYP1 to lineages iSAT1-iSAT6 from Blackcaps (Dolnik et al. Reference Dolnik, Palinauskas and Bensch2009) we found sequence divergences between 2·5 and 4·5%. We observed only minor variation in oocyst size and morphology among isolates from the flycatchers. For morphological comparison and species description we deliberately took measurements only of the 14 oocysts that were individually sequenced and proved to belong to the same haplotype iFICEHYP1.
DESCRIPTION
Isospora hypoleucae sp. n. ( Figs 1and 2)
Description of sporulated oocyst: The spherical oocysts have a brownish smooth bi-layered 1·3 thick wall, M absent. L×W (n=14), 19·4×19·3 (17·5–22·8×17·5–22·8) L/W ratio, 1·01 (1·00–1·05), OR absent. Several small PGs ~0·9×1·3 often cluster into 2–3 dumbbell-shaped formations.

Fig. 1. Composite line drawing of a sporulated oocyst of Isospora hypoleucae sp. n. PG – polar granule; SB – Stieda body; SR – sporocyst residuum; SP – sporozoites; RB – sporozoite refractile bodies; N – nucleus.
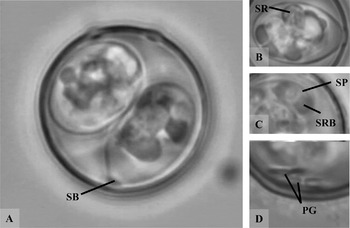
Fig. 2. Photomicrograph of sporulated oocysts of Isospora hypoleucae sp. n. A – round oocyst, note the absence of SSB; B – sporocyst, note compact SR; C – sporozoite; D – two dumbbell-shaped PGs. Abbreviations as in Fig. 1.
Description of sporocyst and sporozoites: Sporocysts are slightly elongated, rounded at the end opposite to SB, L×W (n=14), 15·3×9·2 (13·8–16·1×8·5–10·3), L/W ratio, 1·66 (1·56–1·79). SB present, with prominent knob-like cap; SSB and PSB absent. Sporocysts contain small compact SR and 4 SP that are usually rolled up into balls. Each SP has 2 RB and N.
Taxonomic summary
Type host: Ficedula hypoleuca (Pallas 1764), Pied Flycatcher
Type locality: (54°01′ N; 9°33′ E), North-West Germany.
Prevalence: In faeces: 6/8 (75%) adults (4/5 females and 2/3 males). In blood: 0/8 adults, 3/12 juveniles of infected females.
Sporulation time: Unknown, oocysts were completely sporulated.
Pre-patent and patent periods: Unknown, parasite appears in blood at least on day 13.
Site of infection: Unknown. Oocysts recovered from faeces, haplotypes detected in blood.
Material deposited: Photosyntype (see Duszynski, Reference Duszynski1999) of sporulated oocyst deposited in the US National Parasite Collection No. 101305. Haplotype iFICEHYP1 is deposited in GenBank (Accession number FJ269363).
Etymology: The name is derived from the specific epitaph of the scientific name of the type host, Ficedula hypoleuca.
DISCUSSION
Narrow host specificity of avian Isospora was demonstrated by cross-transmission experiments, particularly for Isospora species of passerine birds (Černá, Reference Černá1973; Box, Reference Box1981; Dolnik, Reference Dolnik2002b). Levine (Reference Levine1982a) proposed that “a coccidian species may be transmissible from one species to another in the same genus, but not from one genus to another in the same family until otherwise demonstrated.” Molecular data later showed that these parasites are predominantly host specific at the species level and only occasionally undergo lateral transfer (Schrenzel et al. Reference Schrenzel, Maalouf, Gaffney, Tokarz, Keener, McClure, Griffey, McAloose and Rideout2005).
We compared the morphological characteristics of Isospora hypoleucae sporulated oocysts with other Isospora spp. previously described from 2 closely related Flycatcher genera, Ficedula and Muscicapa.
Three Isospora species have been described previously from Ficedula and Muscicapa spp. (Table 1): Isospora ficedulaeSchwalbach Reference Schwalbach1959 and I. landauae Cringoli and Quesada Reference Cringoli and Quesada1990 are described from the Pied Flycatcher, and I. parvae Chatterjee and Choudhury 1976 from the Redbreasted Flycatcher Muscicapa parva. Further, Schwalbach (Reference Schwalbach1959) reported the presence of I. phoenicuri Schwalbach Reference Schwalbach1959 and I. wurmbachiSchwalbach Reference Schwalbach1959 in Pied Flycatchers. Host specificity for Isospora spp. is now thought to be narrower than in the time of Schwalbach's investigations, thus the 2 species he saw were probably not I. phoenicuri and I. wurmbachi, but other species that resemble them.
Table 1. Morphological characteristics of isosporan oocysts observed in various Flycatcher species

The absence of SSB distinguishes the new species from I. ficedulae and I. landauae, and from Isospora sp. non I. phoenicuri described by Schwalbach (Reference Schwalbach1959). From all Isospora species recorded from Flycatchers, 2 species lack SSB. These are I. parvae, found in the Red-breasted Flycatcher in West Bengal, and Isospora sp. non I. wurmbachi. I. parvae resembles the species described here in the size of its oocysts and form of SB (Table 1). However, the oocysts of I. hypoleucae can easily be distinguished from those of I. parvae by the absence of M, which is a distinct characteristic of I. parvae. I. hypoleucae can be distinguished from Isospora sp. non I. wurmbachi the by the smaller size of the oocysts, form and number of PGs, prominent SB and compact SR (Table 1).
Based on these characteristics, we consider the species described here to be a new coccidian. Presence of the COI haplotype in the blood of nestlings suggests that the new species can form blood stages, and in this case follows the Isospora serini-like life cycle (Box, Reference Box1975, Reference Box1977). Some authors place such blood-inhabiting coccidia into genus Atoxoplasma (e.g. Levine Reference Levine1982b), yet recent studies consider them rather as a facultative variation of the life cycle within the Isospora genus (e.g. Upton et al. Reference Upton, Wilson, Norton and Greiner2001; Schrenzel et al. Reference Schrenzel, Maalouf, Gaffney, Tokarz, Keener, McClure, Griffey, McAloose and Rideout2005). It is interesting that despite the fact that adult birds were shedding oocysts, no blood stages of I. hypoleucae were detected in these birds, but only in 13-day-old nestlings. The explanation of this age-dependent difference remains unclear; future investigation, preferably employing molecular tools, is required to clarify the situation.
We would like to thank Sönke Martens for the opportunity to work in this area and for his help during the field season, and Leslie Turner for improving the English in the last version of the paper. We also thank the two anonymous reviewers for their valuable comments on the manuscript. This study was financially supported by a grant from the Swedish Institute, Visby Programme (for O. V. D.). The experiments comply with the current laws of Germany.





