Published online by Cambridge University Press: 14 September 2004
Sexual size dimorphism (SSD) in mammals reveals the extent of sexual selection, which may in turn explain why males are often more infected by parasites than females and that parasites may contribute to the association between SSD and male-biased mortality. Here, we investigated the relationship between SSD in small mammals of Central Europe and the differences in sex infection by fleas. A comparative analysis was conducted for 10 species of rodents and insectivores. We found that males harbour higher flea species richness than females and that the abundance of fleas is higher in males than in females. This difference is not related to male-biased density. However, contrary to our hypothesis, we found that an increase in SSD is not related to an increase in male infection by fleas compared with female infection. We discuss our results in term of sex-differences in immunocompetence and/or sex-differences in behaviour.
Host males and females may differ in levels of parasitism. Differences between males and females were observed in helminth infections especially in birds and mammals, with parasite infections often higher in males than in females (Poulin, 1996; Zuk & McKean, 1996). Among others, Edler & Nilsson (1973), Matuschka et al. (1992), Soliman et al. (2001) observed, in the case of ectoparasites, that males were significantly more infected by ticks or mites than females. Such differences may arise due to differential exposures to parasitism or may result from differences in immunocompetence (Tinsley, 1989; Zuk, 1990; Zuk & McKean, 1996). Disentangling these two alternative hypotheses, not necessarily exclusive, is not an easy task. For example, males may have a large home range (e.g. Bujalska, 1993; Tew & McDonald, 1994) that will increase their chance of being exposed to parasites and pathogens. Males may also be engaged in sexual competition for access to females, which is under the control of hormones that may negatively affect the immune system (Møller, 1997).
In a recent study, Moore & Wilson (2002) showed a positive relationship between male-biased parasitism and the degree of sexual selection measured by the degree of sexual size dimorphism (SSD). Their results consistently support the hypothesis that parasites contribute to the relationship between SSD and male-biased mortality (Promislow, 1992). However, the comparative study of Moore & Wilson (2002) was not based on data collected from the same individuals i.e. parasite load and body weight.
Here, we investigate the relationship between ectoparasitism and sexual size dimorphism in small mammals. We also hypothesize that sexual size dimorphism reveals the extent of sexual selection. We test if the sex differences in ectoparasite load may be due to differences in sexual dimorphism, which may affect the immunocompetence (Møller, Sorci & Erritzøe, 1998; Møller, Christe & Lux, 1999). We predict a positive relationship between the extent of sexual size dimorphism in favour of males and a male-biased difference in ectoparasite loads. An increase in sexual dimorphism towards males would be linked with an increased difference in parasite loads in males, whereas an increase in sexual dimorphism towards females would be linked with an increased difference in parasite loads in females.
We used the data of Stanko et al. (2002) in order to explore the differences in flea infection in relation to host sex, taking into account the effect of host density. Using the data collected, it is possible to control for potential differences in exposure by taking into account the differences in host density in relation to the sex of the hosts.
Sampling of small mammals and parasitological investigations were carried out using the methods described by Stanko (1987a,b, 1988, 1994). In total, 10321 specimens of 24 species of small mammals were trapped from March 1983 until August 1997 in 4 geographical regions of Slovakia (Javorie mountains and Krupinská planina plain, Volovské vrchy mountains, East Slovakian Lowland) (see also Stanko et al. 2002). The traps (around 60000 in total) were deposited in the same areas following the same protocol. Each sample was trapped within a limited period (1 or 2 nights in a month) in the same place. The number of captures per trap and per night gives an estimate of host density. All mammals were sexed and weighed. Gravid females were weighed after removing embryos (only 6·2% of females were gravid).
We used the data concerning 10 species of small mammals, for which a sufficient number of populations were investigated (Table 1). These data were previously given in Stanko et al. (2002).

Sexual size dimorphism was assessed for each species using the following formula:
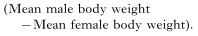
Fleas were removed and identified from the small mammals examined for parasites. For each population (with at least 30 individuals) of each species of mammals it was then possible to compute the following variables according to each sex. (1) Total parasite species richness: the total number of flea species found on a given host species. (2) Abundance: the mean number of ectoparasites found in a population of a given host species. (3) Prevalence: the percentage of hosts found to be parasitized in a population of a given host species. The above-mentioned flea infection parameters are given in Table 1 as mean values per population.
Sex differences in ectoparasite load or ectoparasite species richness was assessed as follows:

We first analysed the effect of host species and sex on the flea species richness and the abundance and prevalence of fleas, controlling for host density using MANOVA.
We secondly performed the analysis using the method of the phylogenetically independent contrasts (Felsenstein, 1985). We used a working phylogenetic tree of the hosts obtained from various sources (see Stanko et al. 2002). We used CAIC 2.0 (Purvis & Rambaut, 1995). Branch lengths were assigned to one. Nine independent contrasts were obtained. Regressions on independent contrasts were forced through the origin (Garland, Harvey & Ives, 1992).
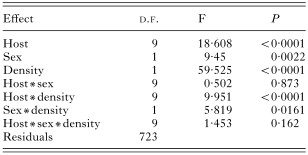
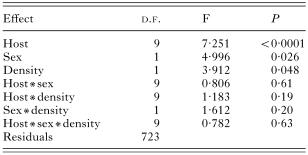
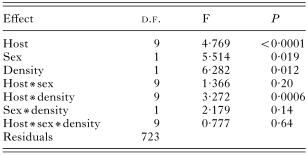
We found a significant influence of the host sex on the species richness, abundance and prevalence of fleas (Tables 2, 3 and 4). No interactions between host species, sex and density significantly influenced species richness (Table 2), prevalence (Table 3) and abundance (Table 4) of fleas.
There was a significant effect of sex on flea species richness (P=0·007), with males significantly more infected than females (post hoc tests <0·05) except for Apodemus sylvaticus and Sorex minutus (post hoc tests >0·05) (Fig. 1).
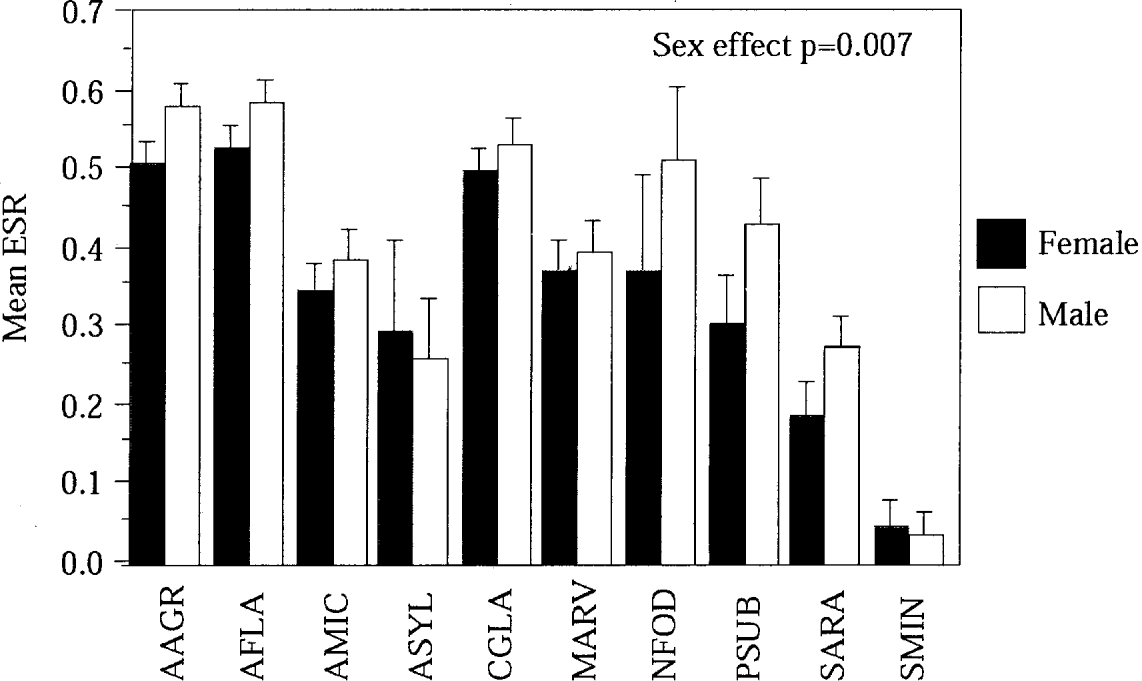
Fig. 1. Sex effect on flea species richness (FSR) in small mammals (error bars=standard deviation).
We found a significant negative relationship between the sex difference in flea abundance and host SSD (Fig. 2A). Also we found a negative but non-significant relationship between the sex difference in flea species richness (male infection to female infection) and host SSD (Fig. 2B).

Fig. 2. (A) Relationship between the ratio of flea abundance in males to flea abundance in females and the ratio of male to female body size (the relationship is significant using a Bonferonni correction). (B) Relationship between the ratio of flea species richness in males to flea species richness in females and the ratio of male to female body size. (C) Relationship between contrasts in ratio of flea abundance in males to flea abundance in females and contrasts in ratio of male to female body size (the relationship is not significant when removing the outlier). (D) Relationship between contrasts in ratio of flea species richness in males to flea species richness in females and contrasts in ratio of male to female body size (the relationship is not significant when removing the outlier).
Using the independent contrasts, we found similar and significant negative relationships between the sex difference in infection (either in terms of abundance or species richness) and SSD (Fig. 2C and D). A negative relationship between differences in prevalence of infection and SSD was also found (R=0·77; P=0·0088). However, these relationships were not significant when outliers are removed.
Mammal species exhibiting a sexual size dimorphism in favour of males have males less infected than females in terms of abundance and species richness, controlling for potential biases such as host density and phylogenetic effects.
The small mammals we studied for flea infection clearly show differences in sexual size dimorphism with species showing SSD in favour of males (such as A. flavicollis or S. minutus) and other species showing SSD in favour of females (such as N. fodiens or S. araneus).
In many animal groups, where the female is larger than the male, it has been postulated that females are larger to produce more progeny (Lewin, 1988; Shine, 1989; Charnov, 1993). SSD is also interpreted as the product of sexual selection, resulting from competition among males to access females (Andersson, 1994). Sexual selection frequently favours large-sized males and/or exaggeration of some characters, i.e. secondary sexual characters (McLain, 1993; Andersson, 1994). SSD is associated with a male-biased mortality (Promislow, 1992).
Numerous comparative studies (see Poulin, 1998; Morand & Poulin, 1998; Poulin & Morand, 2000; Morand, 2000) have shown that parasite load and parasite species richness may correlate with several host features. Large-bodied hosts, living in high density over a large geographical range, encounter more parasites, and more ectoparasites. Hence, if differences between sexes in these features are noted, differences in parasite loads may simply reflect sex differences in exposure.
Stanko et al. (2002) found that mammal body size does not influence the level of parasitism, whereas host density does influence flea species richness. Although a lack of, or negative relationships were found between abundance and host density, this is not in agreement with simple epidemiological hypotheses.
Taking into account potential confounding effects such as host phylogenetic relationships and host density, we found that the level of SSD (male to female) is negatively correlated with the male-biased infection in fleas. This negative relationship, when controlling for potential phylogenetic artefacts, is only supported by a single point, i.e. one contrast. Moreover, we found no effect of host density on parasite loads between males and females, suggesting that males and females do not differ in exposure to parasites for this factor. We should emphasize that, contrary to the data collected by Moore & Wilson (2002), we measured body weight and level of parasite infection for the same individuals. Two non-exclusive hypotheses may explain our results, the first is linked with sex differences in immunocompetence, and the second is linked with sex difference in behaviour.
Our results may indicate that the differences in male to female infection may be due to differences in immunocompetence, which is defined as the ability or capacity to develop an immune response (i.e., antibody production and/or cell-mediated immunity) following exposure to antigen. Host immune defence may be related to host life-history because of the relationships between sex hormone production, immunocompetence and sexual selection (Zuk, 1996; Møller, 1997; Christe, Arlettaz & Vogel, 2000). It has been suggested that sex hormones associated with reproduction and stress affect the relationships between behaviour and immune function (Folstad & Karter, 1992; Barnard & Bhenke, 2001). Males engage in high sexual competition and are supposed to invest largely in testosterone production with an immunosuppression side-effect, i.e. the immuno-handicap hypothesis (Folstad & Karter, 1992). The sexual difference in immunocompetence may be explained by proximate mechanisms, i.e. hormones mediating immunity, or by variation in reproductive success between the sexes. Laboratory experiments on rodents have demonstrated that males are more susceptible to infection than females (Klein, Gamble & Nelson, 1997). Klein (2000) reviewed studies showing that males are more susceptible to infections caused by helminths, arthropods, fungi, bacteria and viruses. Several studies emphasize that the alteration of sex hormone production may contribute to the increased infection in males compared to females (Klein, 2000). Males of many species are more aggressive than females and this aggressive behaviour is under the influence of the levels of circulating sex hormones. Hence, high testosterone concentrations increase both aggressiveness and infection susceptibility (Møller, 1994).
Male-biased infection may also be related to sex differences in behaviour. Males often have a larger home range than females, which may increase their exposure to parasitism. Moreover, Hughes & Randolph (2001) showed that rodents with a higher level of testosterone have increased locomotion activity and reduced innate and acquired resistance to tick feeding. Sex differences in ectoparasitism may also arise due to different time allocation to grooming activities. Data on home range, grooming activity, levels of circulating hormones and levels of immunocompetence are unfortunately lacking for most of the small mammals studied.
In conclusion, we found that males are more infected by parasites than females but, because of the lack of a positive relationship between SSD and male-biased infection, we cannot conclude that male biased mortality, which may be associated with SSD, is related to ectoparasite loads.
This paper has been supported by grant no. 2/2017/22 from the Slovak Grant Agency VEGA and by the ‘Institut Français de la Biodiversité’. We thank Boris Krasnov for comments on an earlier draft. We thank two anonymous referees for their helpful comments.

Table 1. Data and mammal species and their ectoparasite fleas (sources are given in Stanko et al. 2002)
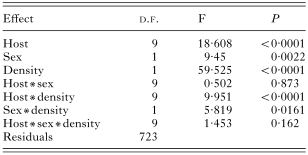
Table 2. Results of a MANOVA on species richness of fleas (in log)
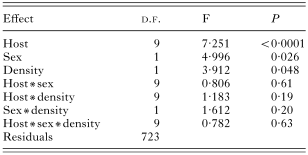
Table 3. Results of a MANOVA on prevalence of fleas (in arcsinus)
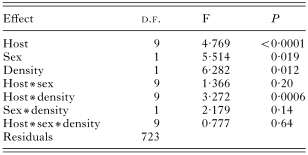
Table 4. Results of a MANOVA on abundance of fleas (in log)

Fig. 1. Sex effect on flea species richness (FSR) in small mammals (error bars=standard deviation).
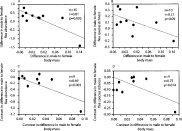
Fig. 2. (A) Relationship between the ratio of flea abundance in males to flea abundance in females and the ratio of male to female body size (the relationship is significant using a Bonferonni correction). (B) Relationship between the ratio of flea species richness in males to flea species richness in females and the ratio of male to female body size. (C) Relationship between contrasts in ratio of flea abundance in males to flea abundance in females and contrasts in ratio of male to female body size (the relationship is not significant when removing the outlier). (D) Relationship between contrasts in ratio of flea species richness in males to flea species richness in females and contrasts in ratio of male to female body size (the relationship is not significant when removing the outlier).