INTRODUCTION
Chagas disease continues to represent an endemic health threat affecting an estimated 28 million people, living mostly in the large area of South and Central America with approximately 21 000 deaths per year. Chagas disease is a complex zoonosis, with a large number of vertebrate reservoirs and triatomine insects participating in the transmission chain, making disease eradication practically impossible (WHO, 2002, 2007; Moncayo and Silveira, Reference Moncayo and Silveira2009). Trypanosoma cruzi, the aetiological agent of Chagas disease (Chagas, Reference Chagas1909, Reference Chagas1911), has, in its life cycle, different morphological and functional forms that occur in its vertebrate and invertebrate hosts. The parasite presents replicative amastigotes and non-dividing bloodstream trypomastigotes in mammals, whereas the insect midgut shelters the development of dividing epimastigotes and infective metacyclic trypomastigotes which are deposited together with feces and urine on the vertebrate host (Garcia et al. Reference Garcia, Ratcliffe, Whitten, Gonzalez and Azambuja2007).
Several macromolecules involved in parasite internalization, transformation into amastigotes, intracellular multiplication and release of new infective forms in mammalian cells in vertebrate hosts have been characterized (Burleigh and Woolsey, Reference Burleigh and Woolsey2002; Tan and Andrews, Reference Tan and Andrews2002) but, there is only limited information concerning molecules modulating the life cycle of T. cruzi within the invertebrate hosts (Azambuja et al. Reference Azambuja, Ratcliffe and Garcia2005; Garcia et al. Reference Garcia, Genta, Azambuja and Schaub2010). Adhesion to perimicrovillar membranes (PMM) (Terra, Reference Terra1990) in the insect midgut is an essential step for parasite division since the blockade of PMM development by either endocrine manipulation or oral administration of antiserum raised against Rhodnius prolixus PMM and midgut tissue reduced the development of the parasite inside the vector (Gonzalez and Garcia, Reference Gonzalez and Garcia1992; Nogueira et al. Reference Nogueira, Gonzalez, Garcia, Mello and de Souza1997; Gonzalez et al. Reference Gonzalez, Nogueira, Feder, de Souza, Azambuja and Garcia1998, Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999, Reference Gonzalez, Hamedi, Albuquerque-Cunha, Nogueira, de Souza, Ratcliffe, Azambuja, Garcia and Mello2006). Glycoinositolphospholipids (Nogueira et al. Reference Nogueira, Gonzalez, Gomes, De Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007), hydrophobic proteins which bind to some glycopolypeptides with Mr of 13 to 97 kDa from R. prolixus PMM (Alves et al. Reference Alves, Albuquerque-Cunha, Melllo, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007) and calpain (Ennes-Vidal et al. Reference Ennes-Vidal, Menna-Barreto, Santos, Branquinha and d'Avila-Levy2011) are cell surface molecules involved in the adhesion of T. cruzi epimastigotes to the insect midgut epithelium.
Many pathogens use surface sulfated glycosaminoglycans (S-GAGs) as adhesion receptors (Tonnaer et al. Reference Tonnaer, Hafmans, van Kuppevelt, Sanders, Verwejj and Curfs2006; Dinglasan et al. Reference Dinglasan, Alaganan, Ghosh, Saito, van Kuppevelt and Jacobs-Lorena2007; Sinnis et al. Reference Sinnis, Coppi, Toida, Toyoda, Hinoshita-Toyoda, Xie, Kemp and Linhardt2007; Akhtar and Shukla, Reference Akhtar and Shukla2009). S-GAGs are large and linear complex carbohydrate molecules negatively charged and composed of disaccharide repeating units and include dermatan sulfate, keratan sulfate, heparin (Hep), heparan sulfate (HS) and chondroitin sulfate (CS) (Nader et al. Reference Nader, Lopes, Rocha, Santos and Dietrich2004; Volpi, Reference Volpi2006; Yamada and Sugahara, Reference Yamada and Sugahara2008; Nadanaka and Kitagawa, Reference Nadanaka and Kitagawa2008; Dreyfuss et al. Reference Dreyfuss, Regatieri, Jarrouge, Cavalheiro, Sampaio and Nader2009). Those composed of HS, but not of CS, mediate both attachment and invasion of mouse cardiomyocytes by T. cruzi trypomastigotes (Calvet et al. Reference Calvet, Toma, Souza, Meirelles and Pereira2003; Oliveira et al. Reference Oliveira, Alves, Calvet, Toma, Bouças, Nader, Côrtes, Krieger, Meirelles and Pereira2008; Bambino-Medeiros et al. Reference Bambino-Medeiros, Oliveira, Calvet, Vicente, Toma, Krieger, Meirelles and Pereira2011).
The occurrence of S-GAGs in internal organs of triatomine vectors of Chagas disease has previously been reported by Dietrich et al. (Reference Dietrich, Nader, Toma, Azambuja and Garcia1987). Their tissue distribution has also been determined by histochemical metachromatic staining (Costa-Filho et al. Reference Costa-Filho, Souza, Martins, dos Santos, Silva, Comaru, Moreira, Atella, Allodi, Nasciutti, Masuda and Silva2004). Regarding the digestive tract, CS and HS were the only S-GAGs detected in both Triatoma brasiliensis and Rhodnius prolixus (Souza et al. Reference Souza, Sarquis, Gomes, Moreira, Lima and Silva2004; Costa-Filho et al. Reference Costa-Filho, Souza, Martins, dos Santos, Silva, Comaru, Moreira, Atella, Allodi, Nasciutti, Masuda and Silva2004).
The aim of the present study was to investigate the possible participation of S-GAG components of the midgut of R. prolixus in both the in vivo protozoan development and the in vitro attachment of T. cruzi to the luminal surface of triatomine posterior midgut epithelium.
MATERIALS AND METHODS
Insect rearing and feeding procedures
Rhodnius prolixus (Hemiptera: Reduviidae) were reared and maintained in the laboratory at 28°C and relative humidity of 60–70%, as described by Azambuja and Garcia (Reference Azambuja, Garcia, Crampton, Beard and Louid1997). Randomly chosen fifth-instar male nymphs were identified, as previously described (Gillet, Reference Gillet1935; Lent and Juberg, Reference Lent and Juberg1969), starved for 30 days after the last ecdysis and then allowed to feed on citrated human blood using an artificial membrane apparatus (Garcia et al. Reference Garcia, Azambuja, Forster and Rembold1984).
Parasites
The Dm 28c clone of T. cruzi was grown at 28°C in a Brain Heart Infusion (DIFCO) culture medium, containing hemin and folic acid and supplemented with 20% heat-inactivated fetal calf serum (Garcia and Azambuja, Reference Azambuja, Garcia, Crampton, Beard and Louid1997). For both in vivo and in vitro experiments, parasites were collected during the exponential growth phase, washed 3 times by centrifugation at 3000 g in 0 15 m NaCl, 0 01 m phosphate-buffer, pH 7 2 (PBS) and immediately used (Carvalho-Moreira et al. Reference Carvalho-Moreira, Spata, Coura, Garcia, Azambuja, Gonzalez and Mello2003).
Chemicals and reagents
Hep, C 4-S, C 6-S, chondroitinase AC (Chase AC) (EC 4.2.2.5) from Arthrobacter aurescens and heparinase I (Hep I) (EC 4.2.2.7) from Flavobacterium heparinum were purchased from Sigma Chemical Co. (St Louis, MO, USA). Protamine chloridrate (Prot) (Roche) was kindly gifted by Dr Walmir Nelson Moreira (UFF).
Trypanosoma cruzi infection and parasite development assay
To conduct in vivo experiments, control insects were allowed to feed on a mixture of heat-inactivated citrated human blood and T. cruzi epimastigotes at a final concentration of 3×103 parasites/ml of blood (Garcia et al. Reference Garcia, Gonzalez, Azambuja, Baralle, Frainderaich, Torres and Flawia1995). For other groups, Hep, C 4-S or C 6-S GAGs were added immediately before feeding to the infected bloodmeal at a final concentration of 1 0 μg/ml. At different intervals post-feeding/infection, 8–10 insects were dissected, the whole digestive tract removed, homogenized in 1 ml of PBS, and the number of parasites quantified using a Neubauer haematocytometer under phase-contrast microscopy (Gonzalez et al. Reference Gonzalez, Nogueira, Mello, de Souza, Schaub, Azambuja and Garcia1999). Each experiment was repeated at least 3 times with groups of 40 insects.
In vitro interaction between R. prolixus PMM and epimastigotes
After washing in PBS, epimastigotes were resuspended in fresh BHI to a density of 2 5×107 cells/ml. Samples of an interaction medium composed of 200 μl of this parasite suspension together with posterior midguts, freshly dissected and washed from insects 10 days after non-infectious feeding, were placed into Eppendorf microtubes (Alves et al. Reference Alves, Albuquerque-Cunha, Melllo, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007) and incubated for 30 min at 25°C. Some experiments were performed with parasites previously incubated (30 min, 25°C) in PBS with Hep, C 4-S, or C 6-S at different final concentrations (0 01, 0 1 or 1 0 μg/ml of interaction medium). Parasites were also incubated in the same conditions with protamine chloridrate (250–1000 UI/ml). For other experiments, posterior midguts were previously incubated with different enzymes to remove specific GAGs (Garcia-Abreu et al. Reference Garcia-Abreu, Mendes, Onofre, Freitas, Silva, Moura Neto and Cavalcante2000) or, alternatively, with only the reaction medium without the specific enzyme (non-treated control). For these enzyme experiments, incubation occurred for 2 h at 25°C either in 100 mm sodium acetate and 10 mm calcium acetate, pH 7 0 containing Hep I (1 5 units/50 μl) for the removal of HS or in 50 mm Tris-HCl, pH 8 0, containing 5 mm EDTA and 15 mm sodium acetate with chondroitinase AC (0 25 units/50 μl) for the removal of CS, or in reaction medium alone. The treated-posterior midguts were then washed in fresh BHI and immediately added to the interaction medium. After incubation (30 min, 25°C) with the parasites, all midgut preparations were spread onto glass slides to count the number of attached parasites. A Zeiss microscope with reticulated ocular, equipped with a video microscopy camera, was used for counting parasites attached to 100 randomly chosen epithelial cells in 10 different fields of each midgut preparation. For each experimental group, 10 insect midguts were used (Nogueira et al. Reference Nogueira, Gonzalez, Gomes, De Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007).
Localization of anionic groups
To localize exposed anionic sites, posterior midguts obtained from insects 10 days after non-infectious feeding were previously incubated for 2 h at 25°C with either Hep I (1 5 units/50 μl) or Chase AC (0 25 units/50 μl). Following the work of Houk et al. (Reference Houk, Hardy and Chiles1986), fragments of these treated-posterior midguts and also from non-treated midguts (control) were collected, washed in PBS, incubated for 30 min in 50 mm ammonium chloride to block free aldehyde groups and then for 15 min in a solution containing colloidal iron hydroxide particles in PBS buffer, pH 1 8 (Gasic et al. Reference Gasic, Berwick and Sorrentino1968). Subsequently, the fragments were washed twice in PBS, fixed with 2 5% glutaraldehyde in 0 1 m sodium-cacodylate buffer, pH 7 2, for 2 h at room temperature, washed in 0 1 m cacodylate buffer, pH 7 2, and post-fixed with 1% osmium tetroxide in 0 1 m cacodylate buffer, pH 7 2 for 1 h at room temperature in the dark. The tissue fragments were then dehydrated in acetone, infiltrated and embedded in Epoxy resin and polymerized at 60°C for 3 days. Approximately 50 thin sections of each group were analysed. These thin sections, each one containing 5 to 10 different cells depending on the magnification used, were examined in a Zeiss 900 transmission electron microscope. The results were only considered when almost 100% of the insects in each experimental group displayed the same posterior midgut ultrastructural arrangement. (Nogueira et al. Reference Nogueira, Gonzalez, Garcia, Mello and de Souza1997; Gonzalez et al. Reference Gonzalez, Nogueira, Feder, de Souza, Azambuja and Garcia1998; Albuquerque Cunha et al. Reference Albuquerque-Cunha, Gonzalez, Garcia, Melllo, Azambuja, Almeida, de Souza and Nogueira2009).
Data analysis
Significance of the results was analysed using ANOVA and Turkey's test according to Stats Direct Statistical Software, version 2.2.7 for Windows 98 (Armitage et al. Reference Armitage, Berry, Matthews and Armitage2002). The difference between treated and control insects was considered to be not statistically significant when P>0 05. Probability levels are specified in the text.
RESULTS
Influence of S-GAGs on epimastigote attachment to midgut epithelium
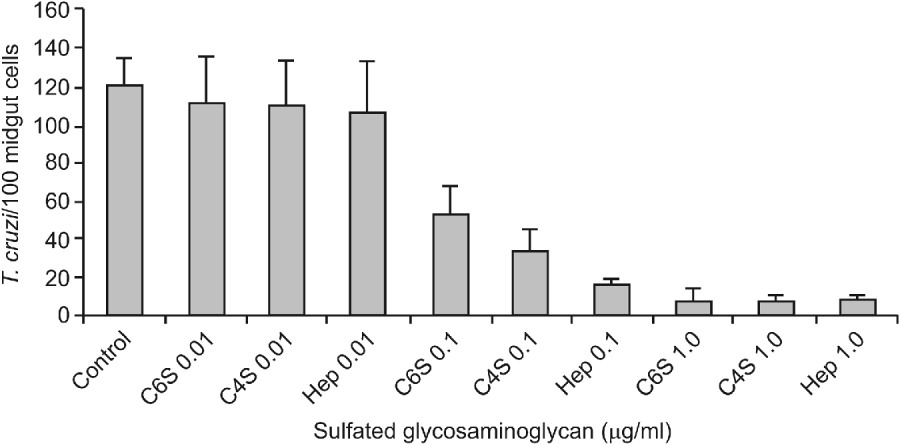
Previous incubation of the parasites with all the S-GAGs tested partially inhibited the attachment of T. cruzi to posterior epithelial midgut cells in the interaction medium (Fig. 1). In the preparations obtained from the control parasites, about 120 7±13 3 epimastigotes attached per 100 midgut cells were observed. Similar adhesion rates were obtained when flagellates were previously incubated with 0 01 μg/ml of Hep, C 4-S or C 6-S (P>0 05). In contrast, attachment of only 15 3±3 3 and 7 6±2 7 parasites per 100 cells of the midgut epithelium were recorded when the flagellates were incubated with either 0 1 or 1 0 μg/ml of Hep (P<0 0001), respectively. In the same way, parasite incubation with either 0 1 or 1 0 μg/ml of C 4-S reduced the T. cruzi attachment to 32 6±12 8 and 7 3±2 6 epimastigotes per 100 midgut cells (P<0 0001), respectively. Similar results were observed after parasite incubation with 0 1 or 1 0 μg/ml of C 6-S.
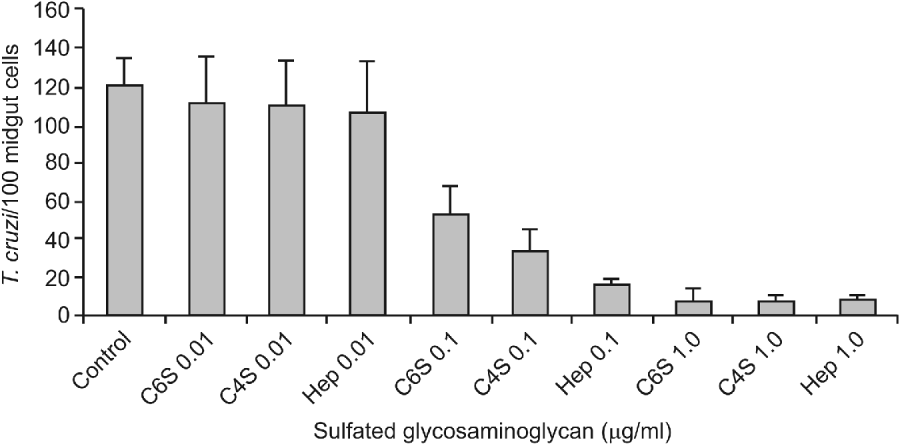
Fig. 1. Effect of sulfated glycosaminoglycans (S-GAGs) on in vitro attachment of Trypanosoma cruzi Dm 28c clone to posterior midgut epithelium obtained from male fifth instar larvae Rhodnius prolixus 10 days after the bloodmeal. Epimastigotes were previously incubated with different concentrations of S-GAGs (0 01, 0 1 and 1 0 μg/ml of BHI medium containing 250×105/ml), washed and added to the interaction medium. Adhered epimastigotes were counted per 100 epithelial cells in 10 different fields of each midgut preparation using a Zeiss microscope. Each group represents means±s.e. of parasites attached in 10 midguts. Non-treated (Control), Chondroitin 6-S (C6S), Chondroitin 4-S (C4S) and Heparin (Hep). Non-treated (control) groups were performed without S-GAGs in the same pre-incubation conditions.
Influence of protamine chloridrate and S-GAG-specific degradative enzymes on epimastigote attachment to midgut epithelium
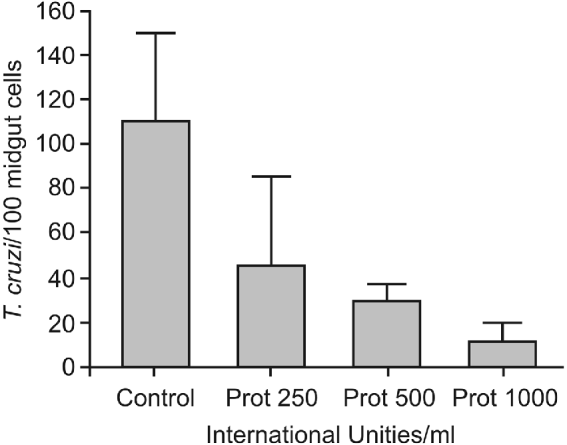
While the control group exhibited an adhesion rate of 109 7±39 8 epimastigotes/100 midgut epithelium cells (Fig. 2), a partial reduction of the adhesion rates to 45 7±39 5 (P<0 01), 30 9±6 8 (P<0 001) and 11 7±8 3 (P<0 0001) were observed when the posterior midguts were previously incubated with 250, 500 and 1000 UI Prot/ml of interaction medium, respectively.
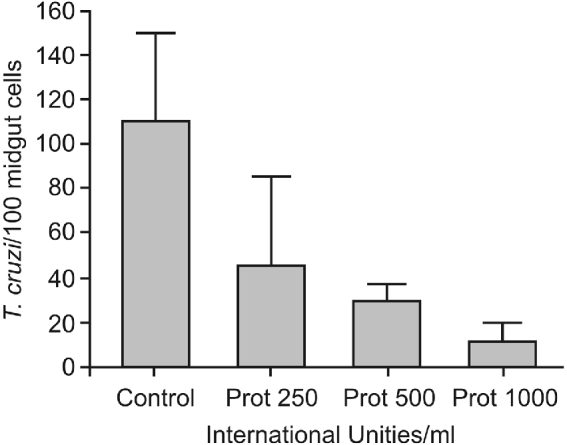
Fig. 2. Effect of protamine treatment on in vitro attachment of Trypanosoma cruzi Dm 28c clone to posterior midgut epithelium obtained from male fifth instar larvae Rhodnius prolixus 10 days after the bloodmeal. Midguts were previously incubated with different concentrations of protamine chloridrate, washed, and then added to the interaction medium containing the flagellates (250×105/ml). Adhered epimastigotes were counted per 100 epithelial cells in 10 different fields of each midgut preparation using a Zeiss microscope. Each group represents means±s.e. of parasites attached in 10 midguts. Non-treated (Control) and protamine (Prot).
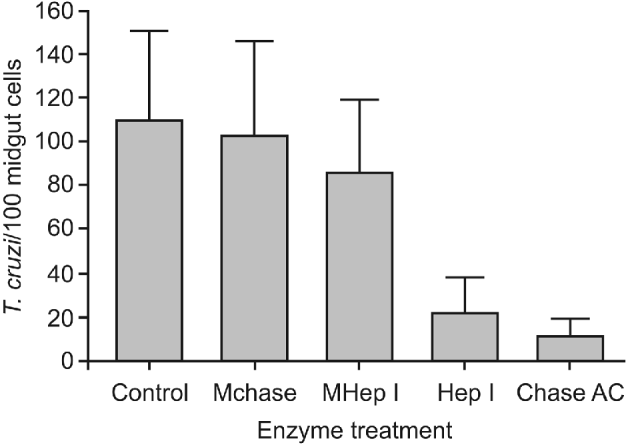
After midgut incubation with either chondroitinase AC (Chase AC) or heparinase I (Hep I), the adhesion rates decreased to 21 1±18 3 and 11 3±8 5 epimastigotes/100 midgut epithelium cells (P<0 001), respectively (Fig. 3). No statistical difference was detected among the control group and the groups incubated with reaction medium without the respective specific enzymes (P>0 05).
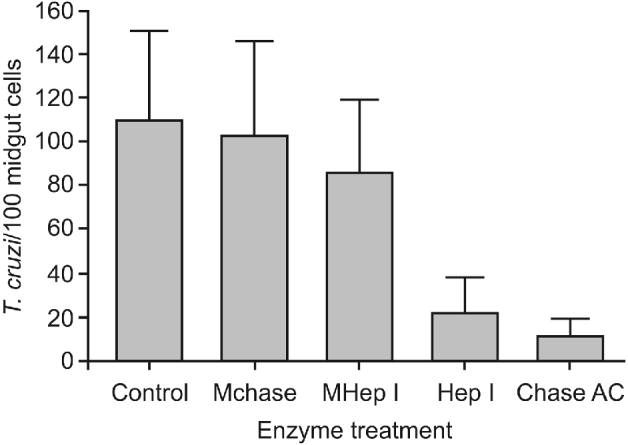
Fig. 3. Effect of sulfated glycosaminoglycans (S-GAGs) removal on in vitro attachment of Trypanosoma cruzi Dm 28c clone to posterior midgut epithelium obtained from male fifth instar larvae Rhodnius prolixus 10 days after the bloodmeal. Midguts were previously incubated with different enzymes – to selectively remove S-GAGs – at a final concentration of 1 5 units/50 μl (for heparinase I) and 0 25 units/50 μl (for chondroitinase AC), washed, and then added to the interaction medium containing the flagellates (250×105/ml). Adhered epimastigotes were counted per 100 epithelial cells in 10 different fields of each midgut preparation using a Zeiss microscope. Each group represents means±s.e. of parasites attached in 10 midguts. Non-treated (Control), medium for Chase AC without the enzyme (Mchase AC), medium for Hep I without the enzyme (MHep I), chondroitinase AC (Chase AC) and heparinase I (Hep I).
Histochemical localization of anionic sites in the posterior midgut of R. prolixus
Posterior midgut preparations obtained from control group insects showed a typical posterior columnar midgut epithelium with homogeneously distributed microvilli covered by plexiform PMM connecting the microvilli to each other and projecting into the midgut lumen (Fig. 4A). Numerous anionic sites were detected in the luminal surface of PMM when the midgut tissue was previously incubated with colloidal iron. Some labelling was observed at both the bases of microvilli and microvillar membranes and between microvilli, but no electron-dense reaction was detected in the cytoplasm of this experimental group (Fig. 4B). Similar results were obtained in the posterior midguts pre-treated only with the reaction medium (without the enzymes) for either Chase AC or Hep I enzymes (not shown). However, no labelling at PMM or microvilli was observed after previous treatment with chondroitinase AC, but in this group intense deposition of reaction products was noted in the cytoplasm at the basis of microvilli (Fig. 4C). Only sparse regions of labelling were present at PMM and microvilli of posterior midgut cells previously treated with Hep I enzyme. No cytoplasmic reaction was detected in this experimental group (Fig. 4D).

Fig. 4. Transmission electron microscopy of posterior midgut epithelium of male fifth instar larvae Rhodnius prolixus 10 days after feeding. (A) Control. The apical regions of these posterior midgut epithelial cells (mc) show numerous and homogeneous distributed microvilli (star) surrounded by luminal perimicrovillar membranes (black arrow). (B) After a single incubation with colloidal iron, the apical regions of these cells show perimicrovillar membranes (black arrow) with intense labelling of anionic sites detected in its luminal surface (white arrow). Microvilli (star). (C) After incubation with chondroitinase AC following incubation with colloidal iron, no labelling was observed either in perimicrovillar membranes (black arrow) or in microvilli (star) but, several anionic sites were detected in line at the apical cytoplasm near to the basis of microvilli (white arrow). (D) After incubation with heparinase I following incubation with colloidal iron, only few anionic sites were exposed at perimicrovillar membranes (white arrow) while no labelling was observed at apical cytoplasm or lumen (black arrow). Microvilli (star).
In vivo experiments
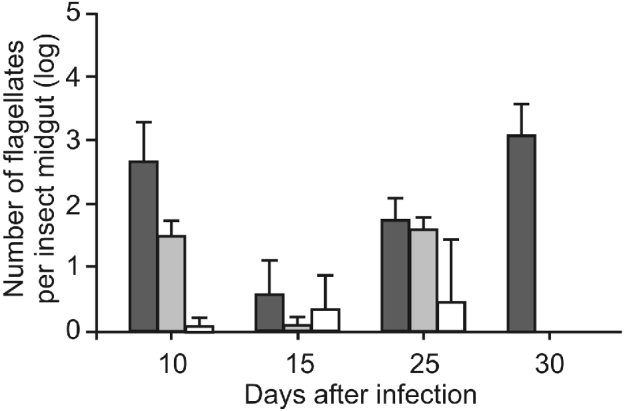
Control insects displayed high infection levels from 66 6±6 4×102 flagellates per insect midgut at 10 days after infection to 8 3±5 0×103 flagellates per insect midgut at 30 days after infection (Fig. 5). In contrast, all other experimental groups (treated with different GAGs) exhibited significant lower levels of T. cruzi infection. Hep-treated insects showed significantly reduced infection levels of 50±23 4 (P<0 001), 10±10 0 (P<0 0001) and 58 3±20 0 (P>0 01) protozoans per insect midgut at days 10, 15 and 25 after infection, respectively. In the same period, C 4-S-treated insects also displayed significantly reduced infection levels of 10±10 (P<0 0001), 33 3±51 6 (P<0 01) and 41 6±20 (P<0 001) flagellates per midgut. No parasites were observed at 30 days of infection in both Hep and C 4-S-treated insects. Flagellates were never detected in C 6-S-treated insects throughout the whole experimental period.

Fig. 5. Effect of sulfated glycosaminoglycans (S-GAGs) on in vivo development of Trypanosoma cruzi Dm 28c clone in the midgut of male fifth instar larvae Rhodnius prolixus after infection. Insects were fed on citrated and complement-inactivated human blood containing 3×103 flagellates/ml. Each S-GAG was added to the bloodmeal at a dose of 1 0 μg/ml. Non-treated (black), heparin (grey), chondroitin 4-S (white). Parasites were not detected in the chondroitin 6-S group. Each group represents means±s.d. of flagellates in 10 insect midguts.
DISCUSSION
Throughout its life cycle in both vertebrate and invertebrate hosts, T. cruzi undergoes adhesion to specific host tissues which is crucial for development in different host tissues since it triggers a variety of interaction events such as cell internalization, replication and transformation to infective stages (Burleigh and Woolsey, Reference Burleigh and Woolsey2002; Tan and Andrews, Reference Tan and Andrews2002). Previous observations concerning the dynamics of parasite interaction with its triatomine vector showed that the insect midgut (particularly the PMM in the posterior midgut and the hydrophobic rectal cuticle) contains components exposed on the surface that mediate epimastigote attachment followed by parasite multiplication in the posterior midgut and metacyclogenesis in the rectum (Garcia et al. Reference Garcia, Ratcliffe, Whitten, Gonzalez and Azambuja2007; Schaub, Reference Schaub2009). Furthermore, research analysing the parasite tropism and chemotaxis to insect midgut tissues established that molecules inhibiting the parasite attachment to insect midgut surfaces in vitro also often efficiently block the in vivo development of T. cruzi in its invertebrate host (Alves et al. Reference Alves, Albuquerque-Cunha, Melllo, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007; Nogueira et al. Reference Nogueira, Gonzalez, Gomes, De Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007), and may constitute key factors in avoiding parasite transmission by its vectors. Since S-GAGs are synthesized by triatomines (Garcia et al. Reference Garcia, Azambuja, Nader and Dietrich1986; Dietrich et al. Reference Dietrich, Nader, Toma, Azambuja and Garcia1987) and are present in the digestive tract of T. brasiliensis and R. prolixus (Costa-Filho et al. Reference Costa-Filho, Souza, Martins, dos Santos, Silva, Comaru, Moreira, Atella, Allodi, Nasciutti, Masuda and Silva2004; Souza et al. Reference Souza, Sarquis, Gomes, Moreira, Lima and Silva2004), it is possible that these molecules may be important for the attachment of T. cruzi to the midgut epithelium of R. prolixus nymphs and posterior development of the protozoan in the vector. Our data support this idea since previous incubation of T. cruzi with S-GAGs before contact with R. prolixus posterior midguts in the interaction medium inhibited the attachment of epimastigotes to the triatomine luminal surface of midgut epithelium in vitro in a dose-dependent manner. In addition, there was a severe reduction of the flagellate population in the digestive tract of R. prolixus when nymphs were infected with epimastigotes of T. cruzi and simultaneously orally treated with very low doses of 1 0 μg of Hep, C 4-S or C 6-S GAGs per ml of the infective bloodmeal.
Polypeptides of 13 to 97 kDa from R. prolixus PMM, some of them related to N-acetylglucosamine and N-acetylgalactosamine, have been shown to bind to either hydrophobic proteins or glycoinositolphospholipids (GIPLs) on the surface of epimastigotes (Gonzalez et al. Reference Gonzalez, Hamedi, Albuquerque-Cunha, Nogueira, de Souza, Ratcliffe, Azambuja, Garcia and Mello2006; Alves et al. Reference Alves, Albuquerque-Cunha, Melllo, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007; Nogueira et al. Reference Nogueira, Gonzalez, Gomes, De Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007; Albuquerque-Cunha et al. Reference Albuquerque-Cunha, Gonzalez, Garcia, Melllo, Azambuja, Almeida, de Souza and Nogueira2009). Similarly, S-GAGs have disaccharide repeating units composed of hexosamine (D-glucosamine or D-galactosamine) and either hexuronic acid (D-glucuronic or L-iduronic acid) or galactose and are covalently bound to a protein core forming a structure known as a proteoglycan (Didraga et al. Reference Didraga, Barroso and Bischoff2006; Taylor and Gallo, Reference Taylor and Gallo2006; Gandhi and Mancera, Reference Gandhi and Mancera2008).
Calvet and co-workers (Reference Calvet, Toma, Souza, Meirelles and Pereira2003) have examined the role of S-GAGs in the attachment of T. cruzi trypomastigotes to mouse cardiomyocytes and demonstrated that HS, but not CS mediate not only the attachment but also the invasion process. Subsequently, the same group reported that T. cruzi trypomastigotes have heparin-binding proteins, which specifically bind to HS (Oliveira et al. Reference Oliveira, Alves, Calvet, Toma, Bouças, Nader, Côrtes, Krieger, Meirelles and Pereira2008). Bacteria, viruses and protozoa also often utilize HS and/or CS as receptors for adherence as with pneumococcal binding to mucosal epithelial cells (Tonnaer et al. Reference Tonnaer, Hafmans, van Kuppevelt, Sanders, Verwejj and Curfs2006) and human papillomavirus and herpex simplex virus for attachment and invasion to host cell surfaces (reviewed by Akhtar and Shukla, Reference Akhtar and Shukla2009; Sapp and Bienkowska-Haba, Reference Sapp and Bienkowska-Haba2009).
In mosquitoes, HS binds to the circumsporozoite protein of Plasmodium, and S-GAG is presumably engaged in the infection and transmission of the Plasmodium parasite (Sinnis et al. Reference Sinnis, Coppi, Toida, Toyoda, Hinoshita-Toyoda, Xie, Kemp and Linhardt2007) and a mosquito CS has been shown to interact in vivo with Plasmodium falciparum during invasion of the midgut (Dinglasan et al. Reference Dinglasan, Alaganan, Ghosh, Saito, van Kuppevelt and Jacobs-Lorena2007).
Previously it has also been shown that incubation of T. cruzi epimastigotes with sialic acid and mannose inhibited the attachment of flagellates to the midgut epithelium of R. prolixus. This suggests the involvement of both negatively charged specific carbohydrates in the midgut and carbohydrate binding proteins on the T. cruzi surface in the attachment process (Nogueira et al. Reference Nogueira, Gonzalez, Gomes, De Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007; Alves et al. Reference Alves, Albuquerque-Cunha, Melllo, Garcia, Nogueira, Bourguingnon, de Souza, Azambuja and Gonzalez2007). Moreover, carbohydrate residues have been suggested as important molecules in the development of T. cruzi in vitro and in the insect vector (Pereira et al. Reference Pereira, Andrade and Ribeiro1981; Tyler and Engman, Reference Tyler and Engman2000, Reference Tyler and Engman2001; Bonay et al. Reference Bonay, Molina and Fresno2001; Bourguignon et al. Reference Bourguignon, Mello, Santos, Gonzalez and Souto-Pádron2006). In the present investigation, the previous incubation of R. prolixus posterior midguts with Prot, Hep I or Chase AC enzymes before contact with T. cruzi in the interaction medium also inhibited the attachment of epimastigotes to the triatomine luminal surface of the midgut epithelium. The ultrastructural analysis of the posterior midgut demonstrated that, in contrast with control insects, anionic sites on the luminal surface of PMM were rarely detected following treatment with Hep I or Chase AC enzymes, which efficiently remove negatively charged HS or CS, respectively. It is important to mention that T. cruzi morphology and motility were not affected after treatment with either GAGs or enzymes (Chase AC and Hep I) during both in vivo and in vitro experiments (data not shown). Similar results on T. cruzi viability and longevity were obtained after exposure to HS by Calvet et al. (Reference Calvet, Toma, Souza, Meirelles and Pereira2003) and Bambino-Medeiros et al. (Reference Bambino-Medeiros, Oliveira, Calvet, Vicente, Toma, Krieger, Meirelles and Pereira2011).
So, our present results demonstrate that HS and CS present on the luminal surface of R. prolixus midgut are involved in T. cruzi epimastigote binding. The high negative charge of both S-GAGs might act as a non-specific step for T. cruzi adhesion to the luminal midgut and reduce the level of these negative binding sites on the luminal midgut since enzymatic removal of either HS or CS inhibits the attachment of the parasites. Similarly, the pre-incubation of midgut preparations with protamine – a cationic polypeptide that can bind to negatively charged Hep and neutralize its antithrombin-mediated anticoagulant properties (Ni Ainle et al. Reference Ni Ainle, Preston, Jenkis, Nel, Johnson, Smith, White, Fallon and O'Donnell2009). This reduces the level of negative binding sites available on the midgut, probably by binding to S-GAGs, resulting in the inhibition of T. cruzi attachment. Curiously, T. cruzi trypomastigotes selectively bind to HS GAGs, as demonstrated in in vitro binding assays with mouse cardiomyocytes (Calvet et al. Reference Calvet, Toma, Souza, Meirelles and Pereira2003), whereas T. cruzi epimastigotes show a less selective profile by binding to both HS and CS GAG. This may suggest the presence of different GAG-binding sites between the two forms of the parasite. These observations also indicate that S-GAGs are one of the determinants of parasite infection in the insect vector and that the recognition mechanisms involved might depend upon the physical-chemical nature of both GAGs in the insect midgut and carbohydrate binding proteins on the surface of T. cruzi.
ACKNOWLEDGEMENTS
We thank Dr Walmir Nelson Moreira from the pharmacy of Hospital Universitário Antonio Pedro (HUAP) of the Universidade Federal Fluminense (UFF) who kindly gave us the protamine chloridrate. We also thank Rodrigo Mexas, Heloisa Maria Nogueira Diniz and Genilton José Vieira (Laboratório de Análise e Reprodução de Imagens/Fiocruz) for the photography treatment, Professor Norman Ratciliffe (University of Swansea, UK) for the English revision and the anonymous referee for scientific suggestions.
FINANCIAL SUPPORT
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Fundação Oswaldo Cruz (FIOCRUZ, PAPES). L. C. F. Silva, P. Azambuja and E. S. Garcia are CNPq senior research fellows. D. P. Castro is a researcher with a FAPERJ Post-doc fellowship. D. P. Mattos is a student with FAPERJ scientific initiation fellowship.