INTRODUCTION
Complex parasite life-cycles continue to elicit wide interest focusing on processes underlying their evolution (Brown et al. 2001; Choisy et al. 2003; Parker et al. 2003) and epidemiological dynamics (e.g. Brooker et al. 2004; Galvani, 2005). One of the prerequisites for comprehensive understanding of these processes is that information is received from each step in the multiple host life-cycle. This may be particularly important in species with commercial or medical significance where dynamics at each step in the life-cycle must be explored for the design of effective preventative strategies. In many species of trematodes, much of the evidence comes from detailed studies on intermediate hosts but often relatively little is known about the infections in the definitive hosts or general life-history differences between related species (see Schleppe and Goater, 2004). One such group of species belongs to the genus Diplostomum, which infects the eyes of fish causing problems at fish farms and where patterns of infection in the avian definitive hosts are poorly known.
This study investigated the intestinal distribution and fecundity of 2 species, D. spathaceum (Rudolphi, 1819) and D. pseudospathaceum (Niewiadomska, 1984), at individual and population levels of their 2 definitive host species, herring gull (Larus argentatus) and common gull (L. canus). Both parasite species have similar complex life-cycles, which include snail, fish and bird hosts (Niewiadomska, 1986). Snails release high numbers of asexually produced cercariae (Karvonen et al. 2004a), which penetrate the fish second intermediate hosts and settle in the lens of the eye. While in the lens, parasites transform to metacercariae and cause cataracts (Shariff, Richards and Sommerville, 1980; Karvonen, Seppälä and Valtonen, 2004b), which may increase parasite transmission to avian definitive hosts (Seppälä, Karvonen and Valtonen, 2005). Diplostomids are also found in fish culture where they cause serious problems (Stables and Chappell, 1986; Field and Irwin, 1994). Despite of extensive literature on Diplostomum infections in intermediate hosts, fish in particular, very few data exist on the infections in definitive hosts as previous studies have focused mainly on parasite species diversity in gulls (Pemberton, 1963; Simková et al. 2003) and maintenance of the parasite life-cycle in the laboratory (Field, McKeown and Irwin, 1994). However, individual and population level patterns of infection in definitive hosts may have important practical implications for the control of Diplostomum infections.
The aim of the present work was first to examine the intestinal distribution and fecundity of the 2 Diplostomum species in their definitive hosts. We used both empirical field observations and infection experiments, which essentially represented different patterns of parasite exposure: gradual and single high-level, respectively. Second, we determined the distribution of the parasites on host population level and discussed the results in relation to parasite aggregation, prevention and transmission from fish intermediate hosts.
MATERIALS AND METHODS
Samples of wild gulls were obtained from a commercial fish farm where birds were shot by the farm staff (with permission from the local game district) during the open water period 2003. These samples included 9 herring gulls (L. argentatus) and 5 common gulls (L. canus). The intestine of each bird was frozen immediately after shooting to ensure the preservation of the parasites. Intestines were then brought to the laboratory, divided in 10 parts of equal size (i.e. each part represented 10% of the total length of the intestine; see Bush and Holmes, 1986) and dissected for parasites under a microscope. Diplostomum parasites were preserved in ethanol. Other parasite species were encountered only occasionally and they were excluded from subsequent analysis. A sample of worms (consisting of, on average, 41·6±5·8% (S.E.) of the total number of specimens) was haphazardly selected from each of the 10 parts of the intestine of each bird, and the worms were stained using Mayer's paracarmine and mounted in Canada balsam. Two species, D. spathaceum and D. pseudospathaceum, were encountered and they were identified to the species according to the description of Niewiadomska (1984). The proportion of each species in the samples was used to extrapolate the numbers in the 10 parts of the intestine of each bird. The number of developed eggs in the uterus of each stained parasite individual was also determined and used as an indirect measure of parasite fecundity (see Loker, 1983; Goater, Goss-Custard and Kennedy, 1995; Poulin, 1997). Although the number of uterine eggs in trematodes is known to correlate positively with egg production (Loker, 1983), our purpose was not to consider the total reproductive output of these parasites. Instead, we used the number of uterine eggs at one particular time as an instantaneous measure of parasite fecundity reflecting possible differences between the parasite species and the host species.
We also exposed naïve gull chicks to simultaneous infection by D. spathaceum and D. pseudospathaceum. Five chicks of herring gull and 5 of common gull were obtained from nesting sites just prior to hatching, brought to the laboratory and placed into the hatchery. This ensured that chicks had not yet been exposed to parasites. After hatching, chicks were maintained for 14 days in the laboratory and fed with fish, previously frozen to kill any parasites. On day 14 post-hatching, each chick was given a dose of approximately 300 metacercariae (range 280–316) containing both parasite species (proportion of each species was not known, see below) and originating from the same fish farm where the wild gulls were shot. The exposure was done by administering several eye lenses of rainbow trout (Oncorhynchus mykiss), from which the number of metacercariae had been counted under a microscope, to each bird. Infection was then allowed to develop for 7 days, which is sufficient time for Diplostomum parasites to reach maturity (Chappell, Hardie and Secombes, 1994). After this, birds were euthanized using carbon dioxide. Based on the parasite distributions in the wild birds (Fig. 1), laboratory-infected birds were dissected by dividing the intestine in 2 parts of equal size; anterior and posterior (see details of the statistical analysis below). Haphazardly selected 24·4±0·6% of the individuals were then stained from each part, and parasites were identified and their uterine eggs counted as described above.

Fig. 1. Distribution of 2 Diplostomum species, D. spathaceum (open bars) and D. pseudospathaceum (filled bars), in the intestine of their definitive hosts. Each part represents 10% of the total length of the intestine. Bars (mean % of worms±S.E.) indicate combined data from 9 wild herring gulls and 5 wild common gulls shot from a commercial fish farm.
To simplify the statistical analysis, we combined parts 1–5 of the intestine of each wild bird as anterior part and parts 6–10 as posterior part. This was done because the distribution of the 2 parasite species resembled a 2-peak distribution between the anterior and posterior parts (Fig. 1) and our aim in this particular study was to determine larger-scale patterns in the species distribution. Percentages of parasite distribution between the 2 parts of intestine were calculated and analysed using paired-samples t-test on arcsine transformed data. When analysing differences in parasite fecundity between the parasites species, host species and data sets, mean parasite egg numbers for each bird were used for the analyses. This was done because numbers of eggs within individual worms were not considered independent although the mean egg numbers were not affected e.g. by parasite numbers (see the Results section). Parasite numbers and egg numbers were log transformed to meet the assumptions of parametric tests and the significance levels were Bonferroni corrected when needed.
RESULTS
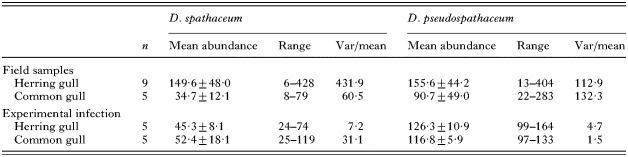
In wild gulls, the prevalence of infection was 100% for both parasite species (Table 1). Numbers of D. spathaceum in individual gulls ranged from 6 to 428 and those of D. pseudospathaceum from 13 to 404. Numbers of the 2 parasite species within individual hosts were positively correlated (Pearson correlation: r2=0·881, n=14, P<0·001), but the mean abundances did not differ between the gull species [t-test on log transformed data: t12=1·527, P=0·153 (D. spathaceum), t12=0·820, P=0·428 (D. pseudospathaceum), Table 1]. Both parasite species were highly aggregated at host population level as indicated by the variance to mean ratios (Table 1).

All gull chicks became infected following the experimental exposure in the laboratory and the mean percentage of parasite establishment was 58·4±3·0 in herring gulls and 56·7±6·7 in common gulls (t-test on arcsine transformed data: t8=0·18, P=0·86). Mean abundances did not differ between the gull species [t-test on log transformed data: t8=−0·014, P=0·989 (D. spathaceum), t8=0·706, P=0·500 (D. pseudospathaceum), Table 1].
In wild gulls, the percentage of D. pseudospathaceum encountered from the anterior part of the intestine was higher compared to D. spathaceum whereas the opposite was true for the posterior part [paired-samples t-test on arcsine transformed data: t8=7·594, P<0·001 (herring gulls), t4=7·570, P<0·01 (common gulls), Fig. 2]. The pattern of infection was also similar for the experimental infection where D. pseudospathaceum had a higher percentage in the anterior part [paired-samples t-test on arcsine transformed data: t4=5·848, P<0·001 (herring gulls), t4=4·634, P<0·01 (common gulls), Fig. 2]. Furthermore, the degree of parasite divergence between the anterior and posterior part was not affected by the number of individuals of the other species either in D. spathaceum (Pearson correlation: r2=−0·282, n=24, P=0·182) or D. pseudospathaceum (r2=−0·363, n=24, P=0·081).

Fig. 2. Distribution of 2 Diplostomum species, D. spathaceum (open bars) and D. pseudospathaceum (filled bars), in the anterior and posterior parts of the intestine of their definitive hosts: wild herring gulls (A), wild common gulls (B), experimentally infected herring gulls (C) and experimentally infected common gulls (D). Bars indicate mean % of worms±S.E. Sample sizes are given in Table 1.
Mean fecundity of D. spathaceum was not affected by the number of conspecifics (Pearson correlation: r2=−0·139, n=24, P=0·518) or the number of D. pseudospathaceum in the intestine (r2=0·094, n=24, P=0·662). The same was true for mean fecundity of D. pseudospathaceum (r2=0·048, n=24, P=0·824 and r2=0·020, n=24, P=0·927, respectively). The patterns were also similar when analysed separately for both wild and experimentally infected gull species. Thus, effect of worm burden on parasite fecundity was not considered in subsequent analyses. However, mean parasite fecundity within individual hosts was strongly correlated between the parasite species in both wild gulls (r2=0·818, n=14, P<0·001) and experimentally infected gulls (r2=0·678, n=10, P<0·05). In the latter case, parasite fecundity did not correlate with the percentage of parasite establishment (r2=−0·194, n=10, P=0·592).
Fecundity was higher in D. spathaceum compared to D. pseudospathaceum in wild common gulls (paired-samples t-test on log transformed data: t4=11·332, P<0·001), but no such difference was observed in wild herring gulls (t8=−1·334, P=0·219), experimentally infected herring gulls (t4=1·939, P=0·124) or experimentally infected common gulls (t4=2·207, P=0·092; Fig. 3). Furthermore, parasite fecundity did not differ between the gull species [ANOVA on log transformed data: F3,20=0·992, P=0·331 (D. spathaceum), F3,20=1·722, P=0·204 (D. pseudospathaceum), Fig. 3] or between wild and experimentally infected gulls [F3,20=0·179, P=0·677 (D. spathaceum), F3,20=1·116, P=0·303 (D. pseudospathaceum), Fig. 3].

Fig. 3. Mean number of uterine eggs of 2 Diplostomum species, D. spathaceum (open bars) and D. pseudospathaceum (filled bars), in the anterior and posterior parts of the intestine of their definitive hosts: wild herring gulls (A), wild common gulls (B), experimentally infected herring gulls (C) and experimentally infected common gulls (D). Bars indicate mean±S.E. Sample sizes are given in Table 1.
DISCUSSION
This study explored the intestinal distribution and fecundity of 2 economically important parasite species, Diplostomum spathaceum and D. pseudospathaceum, in 2 definitive host species. Results from wild hosts and those infected in the laboratory indicated that the parasite species occupied different locations in the intestine. In general, site segregation in parasites may be caused by factors such as negative interspecific interactions (reviewed by Poulin, 2001). However, our results indicate that the parasite species did not affect the numbers, fecundity or site selection of each other, which is consistent with the idea of non-interactive community structure. Specialization of parasite species to a more restricted habitat may also ensure effective mate finding and reproduction, or help to avoid reproductive encounters with wrong species (Rohde, 1977, 1979; Morand et al. 2002; Bagge et al. 2005). However, in our data, parasite fecundity did not correlate with the number of conspecifics at least in the present infection intensities. In general, the detailed nature of these interactions would have to be untangled in experimental single-species infections, which are currently impossible to perform, for example, because the morphological characteristics of the larval stages overlap between the species (Niewiadomska, 1986).
The distribution of parasites in host populations has important implications for parasite population dynamics and epidemiological preventative strategies (e.g. Hudson et al. 2002). Parasites typically exhibit an aggregated distribution (Shaw and Dobson, 1995), where few hosts harbour the majority of the parasite population and thus maintain a large proportion of the overall parasite transmission. In our data on wild gulls, both Diplostomum species were highly aggregated. They were also concentrated to the same host individuals as indicated by the positive correlations between the numbers of individuals. This may reflect individual differences between hosts in exposure to infected fish or general predisposition to parasite infection. Diplostomids are typically aggregated also in their fish intermediate hosts (Pennycuick, 1971; Sweeting, 1974; Burrough, 1978; Karvonen et al. 2004c) and, although not studied here, it is likely that D. spathaceum and D. pseudospathaceum tend to accumulate to the same fish individuals from which the aggregation transfers to definitive hosts. This would seem reasonable because of similarity in the life-histories between the species and their ability to occur in the same fish individuals as suggested by our experimental infections of gulls.
Another essential factor for parasite population dynamics is the variation in parasite fecundity between host individuals and species. As noted above, both parasite species were aggregated in the host populations, but parasite numbers did not affect parasite fecundity. However, fecundity was strongly correlated between the parasite species, which suggests that parasites did better in some bird individuals than in others in terms of reproduction. Indirect evidence from experimental infections suggests that gulls acquire some resistance against D. spathaceum (Chappell et al. 1994) and the variation in parasite reproduction between hosts may be related to the ability of hosts to resist the infection. Interestingly, mean egg numbers did not differ between the wild gulls that presumably had acquired resistance, and naïve, experimentally infected gulls, suggesting that host resistance does not affect parasite reproduction. No correlation was observed either between parasite fecundity and percentage of parasite establishment in experimentally infected hosts although the latter could also reflect variation in host resistance. This does not, however, exclude the possibility that parasite establishment is decreased in subsequent infections, but this could not be analysed from the present data. At the level of host species, parasite establishment and fecundity did not differ between the host species suggesting that both gull species are equally suitable hosts for the parasites. In general, diplostomids are quite unspecific while infecting the definitive hosts (Chappell et al. 1994), but it is likely that the relative importance of the potential host species for the parasite population dynamics is different and also varies between populations.
To conclude, D. spathaceum and D. pseudospathaceum occupied different parts of the intestine but showed roughly equal fecundity in their definitive hosts. Segregation in the place of infection may be caused, for instance, by competition, but these aspects require further work. Parasite aggregation at host population level and their higher fecundity in certain host individuals suggest that some hosts are probably mainly responsible for maintenance of the parasite populations. Diplostomum parasites have received considerable attention in the literature because of their economical importance to fisheries, but studies at the level of definitive hosts are scarce. The present results on parasite distribution in host individuals and populations provide important implications for preventative protocols based on predictions from epidemiological modelling where one of the main objectives is to identify the key proportion of host individuals for the maintenance of the parasite life-cycle.
We thank Miia Savolainen for help with data collection and Jukka Jokela for comments on the manuscript. The study was supported by the Finnish Cultural Foundation, the Kone Foundation, the Chinese Scholarship Committee (CSC) and the Academy of Finland.