Published online by Cambridge University Press: 04 March 2004
An area close to the Qinghai-Tibet plateau region and subject to intensive deforestation contains a large focus of human alveolar echinococcosis while sporadic human cases occur in the Doubs region of eastern France. The current review analyses and compares epidemiological and ecological results obtained in both regions. Analysis of rodent species assemblages within quantified rural landscapes in central China and eastern France shows a significant association between host species for the pathogenic helminth Echinococcus multilocularis, with prevalences of human alveolar echinococcosis and with land area under shrubland or grassland. This suggests that at the regional scale landscape can affect human disease distribution through interaction with small mammal communities and their population dynamics. Lidicker's ROMPA hypothesis helps to explain this association and provides a novel explanation of how landscape changes may result in increased risk of a rodent-borne zoonotic disease.
The life-cycle of Echinococcus multilocularis (Em) involves small mammal intermediate hosts and fox, Vulpes sp., (dog and cat) definitive hosts (Rausch, 1995). Humans become infected after ingesting tapeworm eggs (40 μm diameter) passed in the faeces of infected carnivores, probably through direct contact or via food contamination. This parasite is geographically widespread in the northern hemisphere (Schantz et al. 1995; Craig, Allan & Rogan, 1996) including Europe, where human alveolar echinococcosis (AE) could be considered as a potential emergent zoonotic disease especially in light of the increase in red fox populations (Lucius & Bilger, 1995; Romig, 2002).
More than 40 species of small mammals (mainly rodents and pikas) are known to be hosts for this parasite species with differences in susceptibility (Thompson & Lymbery, 1995). The main obstacles in transmission studies and modelling of E. multilocularis are the low prevalences (<1%) and the aggregation of the parasite in rodent intermediate host species (Roberts & Aubert, 1995). Also, the structure of populations of wild definitive hosts (primarily foxes) is very variable and often difficult to investigate. Furthermore, the long duration of asymptomatic development of the larval stage in humans (5–20 years) (Amman & Eckert, 1995; Craig et al. 1996) poses epidemiological problems. The prevalence alone in a particular rodent host species provides little information on the transmission pattern (e.g. one host species can be a life cycle cul-de-sac), and the low prevalences usually recorded prevent any comparisons between rodent species for statistical reasons. Prevalences in man are the cumulative results of events which may have occurred over many years prior to an active screening programme and, as a consequence, one cannot easily extrapolate any result obtained for animal hosts during such screenings carried out over a short time-span or within small spatial scales. With regard to its world distribution, it has been suggested that E. multilocularis is a parasite of cold climates, as the only stage of the tapeworm that occurs outside its mammalian hosts is the egg, which is known to be very sensitive to moderate heat and desiccation (Veit et al. 1995). However, these properties are common to other taeniids with global distributions, and cannot explain the specificity of the distribution of E. multilocularis by itself (Lucius & Bilger, 1995).
The world decline in forested areas of developing countries has serious consequences for wildlife populations and regional and global diversity (Kittredge, 1996). This is the case in China where the population has doubled since the 1950s with concomitant increased need for wood and farmland. On the contrary, in mid-altitude mountains of western Europe, forested areas have increased since the beginning of the century due to agriculture changes and land abandonment. In these regions (e.g. Northern Alps and Jura mountains, France), farmers specialised in milk production during the 1960s and converted ploughed fields into permanent grassland (Giraudoux et al. 1997). Landscape changes are known to affect both the population dynamics of wild mammals (Lidicker, 1995), and vector-borne disease transmission (Ashford, 1991; Molyneux, 1997; Delattre et al. 1998). However the transmission patterns and the processes involved may be various for different zoonotic diseases. In our case, the challenge is to determine which natural conditions make the whole host community capable of sustaining E. multilocularis life cycle.
Epidemiological and ecological studies have been carried on since the 1980s in the Jura Mountains, France (Delattre, Pascal & Damange, 1985; Delattre et al. 1988; Delattre, Giraudoux & Quere, 1990; Giraudoux, 1991; Bresson-Hadni et al. 1994; Viel et al. 1999; Raoul, 2001), and in Southern Gansu, China (Craig et al. 1992; Giraudoux et al. 1998; Courant et al. 1999; Craig et al. 2000). The present paper analyses and compares results obtained in both regions and suggests that at the regional scale landscape can affect human disease distribution through interaction with small mammal communities and their population dynamics. Lidicker's ROMPA hypothesis, presented below, helps to explain this association and provides a novel explanation of how landscape changes may result in increased risk of a rodent borne zoonotic disease.
In China, studies were undertaken in the Zhang and Puma counties (34° 33N, 104° 34E) of South Gansu (Craig et al. 2000). The area (650 sq km) is characterized by small discrete villages (pop range 200–1500) in valleys and plateaux at approximately 2400–2600 m alt. These are populated almost exclusively by Han Chinese peasants involved in food and cash-crop production with extensive use of terraced farming on available slopes. A canine prevalence for E. multilocularis of 10% was recorded in 1991 (Craig et al. 1992), however rodent pest poisoning campaigns have now almost eliminated the dog in most localities in the study area. Fox hunters have also reported a significant reduction in red fox (Vulpes vulpes) numbers over the last 10–15 years.
A total of 3331 people in 31 villages were included (by self-selection) in a mass screening AE programme. Individuals (>5 years of age) were registered at a central point (school/dispensary/house) in each village, and their responses to a knowledge/attitudes/practices questionnaire were recorded. Every individual donated 4 drops of blood (from an ear lobe) onto Whatman No. 1 filter paper and was then given a liver scan by ultrasound (US) (portable, sonoline SX, Siemens). A 5 ml sample of venous blood was taken from any person with an abnormal liver US image, including microcalcifications. Advanced AE is usually pathognomonic on US images (Craig et al. 1996). Serum prepared from venous and filter paper blood samples was tested for anti-Echinococcus IgG antibodies using ELISA (Craig et al. 1992; Bresson-Hadni et al. 1994). One hundred and thirty five cases (prevalence 4·1%) of AE were confirmed by combined US-serological screening. In addition 21 cases exhibiting probable abortive AE (Bresson-Hadni et al. 1994) were detected, but have not been included in the data set. No further AE cases were confirmed in US-negative people as a result of filter paper blood results. Mean age of AE cases from Zhang and Puma Xian was 41 years (range 11–72 years).
Land use was studied on the basis of Chinese local government maps at 1[ratio ]100,000 scale drawn in 1989. This land use map showed the result of 15–20 years of deforestation carried out in the 70–80s, and it was confirmed by local authorities and peasants that areas with higher ratios of ploughed fields were the areas deforested first, and that the areas with lower ratio were deforested recently. The area of ploughed fields, of forest and of shrubland/scrubland were computed in sectors homogenous considering land use, and then expressed as a ratio of total land.
The Doubs department (46° 56N, 6° 21E; 5234 sq km) is located in eastern France at altitudes between 200–1450 m. It is divided into 29 cantons. However, due to changes in boundary definitions between the 1982 and 1990 censuses, yielding new ‘cantons’ partially overlapping some old ones, some were aggregated to obtain unequivocal and stable spatial units across years. Hence, 28 ‘cantons’ were used for this study. Data on populations were obtained from the French Office of Population Census (INSEE) for three census dates of 1975, 1980 and 1990. The population did not change greatly (2·98%) during this period and therefore the average population was used to estimate AE prevalences in each canton. The diagnosis and identification of human AE cases has been carried out since 1971 at the University Hospital of Besançon, which is the regional referral centre for this disease (Bresson-Hadni et al. 1994). Data were retrospectively compiled in 1988 and regularly updated. Data for the period 1971 to February 1997 were used and consisted of hospital records (total 83 patients, prevalence 1·74[ratio ]10,000) from the Hepatology and Digestive surgery departments.
Land use was studied on the basis of data from the 1988 General Agriculture Census (RGA) and the National Forest Census, both from the Ministry of Agriculture. The variable kept for this study was the ratio of permanent grassland to total land. It was shown that landscape has not changed significantly from the early 70s (Giraudoux et al. 1997). As human AE prevalences were far much lower in urban cantons these have been removed for further statistical analysis in order to point out the differences between rural cantons only (Giraudoux et al. 1996).
For both countries, differences between human groups were compared by univariate statistical test (χ2 with correction for continuity and permutation test between statistical units).
In both France and China, small mammals were sampled by using two techniques (i) standard trapping in every habitat of a landscape (Giraudoux et al. 1994, 1998), (ii) index methods and landscape transects (Giraudoux et al. 1994; Delattre et al. 1996; Giraudoux et al. 1997; Raoul et al. 2001a), and in China these studies were complemented by examination of rodents brought to us by local people (Giraudoux et al. 1998).
AE prevalences in humans were three times higher in the sectors with a higher ratio of shrubland/scrubland area to total land in southern Gansu, China (Fig. 1). Overall village AE prevalences varied from 0–16%. Fig. 2 shows that a similar figure was obtained in France with the ratio of permanent grassland to total land. Fig. 3 shows the distribution of small mammal assemblages in both landscapes. Species susceptible to Em were observed in every habitat.

Fig. 1. Human AE prevalence rates and land use in Zhang and Puma Xian area, China. Circles are villages, and their size is proportional to prevalence rates (average 4·1%). Black circles – prevalence rates significantly higher than the average (alpha[les ]0·05); white circles or s. – prevalence rates significantly lower; grey circles or n.s. – prevalence rates not significantly different from the average (data fitted with a Poisson distribution); Pv1, Pv2, Pv3, overall prevalence rate in the three landscape areas; n1, n2, n3, number of people screened. Prevalence rates appear to be highly variable within each landscape area, but were three times lower in sectors with lower ratio of scrub-, shrub-, grassland (χ2 with correction for continuity, P<0·0003; permutation test between villages, P=0·003; villages which were on the border between two areas were pooled conservatively, e.g. if a prevalence rate was higher than the average, data were pooled with the villages of the sector of lower prevalence rates, or conversely).

Fig. 2. Human AE prevalence rates and land use in the Doubs department, France. Circles in each canton are proportional to prevalence rates (average 1·74[ratio ]10,000). Black circles – prevalence rates significantly higher than the average (alpha[les ]0·05); white circles or s. – prevalence rates significantly lower; grey circles or n.s. – prevalence rates not significantly different from the average (data fitted with a Poisson distribution). Urban cantons (bold) were removed for further analysis. Even considering rural cantons only, human AE prevalence was highly variable within each land use area as in China. It is almost three times higher in areas with higher ratios of permanent grassland (see table, χ2 with correction for continuity, P<0·0001; permutation test between cantons, P=0·05).

Fig. 3. Distribution of the dominant species in each landscape element in the Zhang and Puma Xian area (China), and in the Doubs department (France). Bold species – potential hosts for Echinococcus multilocularis; non-bold name – species for which susceptibility to E. multilocularis is still unknown; bold, thin, dotted lines indicate the relative population density of each species between habitats. In France, potential and actual good physiological hosts (developing alveolar cystic masses with protoscoleces) are present in every habitat. A similar pattern was recorded in China in every key-habitat, even though the susceptibility of some hosts remains variable (e.g. Mus musculus) or unknown (e.g. Cricetulus longicaudatus) and the presence in forest of other very susceptible arvicolids likely.
The influence of landscape on small mammal distributions and the transmission of pathogens is not a new concept (Pawlovski, 1964) and has been highlighted for the vector-borne protozoa Leishmania spp. (Rioux, Dereure & Perières, 1990), for Rickettsia spp. (Trape et al. 1996), plague (Golvan & Rioux 1961), Schistosoma spp. (Combes, Leger & Golvan 1975), and more recently hantavirus (Weigler, 1995; Barclay & Rubinstein, 1997; Lundkvist et al. 1997). In each case, there was a single reservoir host species, linked to particular habitats. For instance, Leishmania major transmission in Morocco was increased in oases in the vicinity of waste ground where both the rodent reservoir host, Meriones shawi, and the insect-vector, Phlebotomus papatasi, reached high densities, and also by wet periods which caused increased plant resources and subsequent increase in the populations of Meriones (Rioux et al. 1990). Those studies established a link between habitats favourable to reservoir hosts, habitat resource quality for a particular species, and transmission.
Host organisms clearly are sensitive to spatial array of their optimal habitat patches in a landscape (Lidicker, 1995). One aspect of these landscape influences is their impact on the population dynamics of mammalian species. One way to conceptualise this with respect to arvicolid rodent multi-annual cycles is the ROMPA hypothesis (Lidicker, 1985, 1988, 2000; Ostfeld, 1992). ROMPA refers to the ratio of optimal to marginal patch areas and is most frequently expressed as the proportion of a landscape composed of optimal habitat for the target species. The ROMPA could influence the probability that arvicolid population densities would undergo multiannual cycles as a combined effect of dispersal and predation. If optimal habitats were scarce (low ROMPA), then the landscape matrix would serve as a large dispersal sink and population densities would be very stable and small. At very high ratios, rodent densities would also be stable but relatively large (vole dispersal occurs within optimal habitats and sink area, and predation is not enough to reduce population density). At intermediate ROMPA, multiannual rodent population cycles would be more likely (Lidicker, 1995; Hansson, 2002). Thus the average density of arvicolid populations would tend to be described by a non-linear function of the ROMPA. Furthermore, mammalian predator–prey relationships appear to change with the relative proportion of the most productive habitats in heterogeneous landscapes (Angelstam, Lindström & Widen, 1984; Oksanen, 1990; Hansson, 1995).
The present analysis provides the first indication, to our knowledge, that parasite transmission is dependent on interactions between the ratio of optimal to marginal patch area in a landscape (ROMPA) of rodent species and their population dynamics, in a context where potential and even actual hosts exist in every habitat of a given landscape. Moreover, the comparison between transmission studies in China and France shows that whatever the habitats and fauna are (the Doubs department and south Gansu do not share any dominant species and the optimal habitats of the focal potential intermediate host species are not the same), the patterns and likely the processes involved remain constant.
In France, empirical data support the importance of ROMPA to population dynamics on a regional scale for two grassland arvicolid species, Microtus arvalis and Arvicola terrestris. Frequent outbreaks occur where the ratio of permanent grassland exceeds a certain value (50% or 85% of farmland for M. arvalis and A. terrestris, respectively) (Delattre et al. 1992; Giraudoux et al. 1997). In such systems foxes (Vulpes vulpes) become specialists and feed almost exclusively on grassland rodents, their diet getting more diversified only during the population crashes of grassland rodents (Weber & Aubry, 1993; Giraudoux et al. 2002). Landscape composition therefore drives both the population dynamics of arvicolid species and the predator–prey relationships, and thus may influence any host–parasite interaction which depends on this relationship. In the Doubs department (France), those areas where arvicolid population outbreaks lasted longer were also those where the highest number of human AE cases were recorded (Viel et al. 1999). Prevalences of Em in foxes were similarly higher in this area (Giraudoux, 1991; Raoul et al. 1999; Raoul, 2001; Raoul et al. 2001b). It has been shown that the major cause of increase in the pluriannual fluctuations of density of Arvicola terrestris populations was the increase in permanent grassland from the late 1950s to the 1970s (Giraudoux et al. 1997). In Europe, Pesson & Carbiener (1989) in Alsace (France) and Tackmann et al. (1998) in Brandenburg, Germany, also established a link between grassland and higher prevalences of Em in foxes.
The study area of south Gansu showed human AE prevalences 100 times higher than those from the Doubs department, which is considered a high endemicity area in Europe. Beyond this difference the same distribution pattern was recorded, corresponding to land use differences. However, suitable potential (Thompson & Lymbery, 1995) or actual intermediate hosts did exist in every habitat of those landscapes in France (Houin, Deniau & Liance, 1980; Petavy, Deblock & Gilot, 1984; Delattre et al. 1988, 1990; Giraudoux et al. 1994) as well as in China (Giraudoux et al. 1998) (Fig. 3). This means that, considering human cases as markers of transmission intensity, this intensity depends either on the area of some habitats or on the density of hosts in these habitats, or both.
In the south Gansu area where the mass-screening for human AE was undertaken, two rodent species dominated the intermediate stages of the deforestation gradient, Microtus limnophilus a vole, and Cricetulus longicaudatus a hamster (Giraudoux et al. 1998). M. limnophilus and M. oeconomus are sibling species which can be differentiated only by karyotype (Malygin, Orlov & Yatsenko, 1990; Courant et al. 1999). M. oeconomus is the host ‘par excellence’ of E. multilocularis in Alaska and Siberia where this vole can sustain very high population densities (Rausch, 1995). Nothing is known so far about the susceptibility of C. longicaudatus to E. multilocularis, though Cricetulus kamensis, a close species, has been found naturally infected on the Tibetan plateau of Sichuan, China (Raoul et al., unpublished) and Cricetulus griseus, can be used to maintain isolates of E. multilocularis (Sakamoto, Ishii & Kobayashi, 1996). Laboratory studies have proved that M. limnophilus is highly susceptible with production of fertile cystic lesions (Zhou, 2001) as every species of the genus Microtus (Rausch, 1995). The population dynamics of forest rodent species is known to be much more stable than that of species in deforested areas (Hsia, 1958; Hsia & Chu, 1963) or grasslands (Giraudoux et al. 1994). Deforestation in south Gansu in the last 30 years has led to a significant extension of shrubland, scrubland and grasslands with resultant increase of the optimal habitats for M. limnophilus and C. longicaudatus. It can therefore be expected that the percentage of optimal habitat (ROMPA) became critical for those species and triggered outbreaks. As a matter of fact both M. limnophilus and C. longicaudatus were reported as rodent pests from the grasslands of south Gansu in the early 1980s (Chen, Yao & Liao, 1982). Interviews with farmers witness that this period also coincided with high densities of foxes (Vulpes vulpes) and high numbers of domestic dogs in villages. All such conditions would be very favourable for transmission of E. multilocularis with resultant high prevalences appearing 10–15 years later in humans.
Euzet and Combes have proposed the concept of meeting and compatibility filters (or screens) to symbolise the mechanisms responsible for the formation of host ranges and sustainable transmission of a parasite (Euzet & Combes, 1980; Holmes, 1986; Combes, 2001). As long as physiologically susceptible hosts for Em do exist in virtually every habitat of any landscape, studies in transmission should focus on the meeting screen. The understanding of the ways a parasite can overcome the meeting screen involves at least four fields of ecology: community ecology, population dynamics, ethology and ecophysiology. Fig. 4 shows the two tops of the gradient of each factor known to impact Em transmission in wildlife in Western Europe (for more details see (Giraudoux et al. 2002)); the left-hand top is considered unfavourable to transmission and the right-hand favourable. (1) Landscape composition at regional scale impacts population dynamic patterns of intermediate host species and the structure of predator communities; (2) parasite egg survival (e.g. desiccation) and the age structure of host populations vary seasonally and interannually (variation in population age structure has consequences on the percentage of infected prey available for definitive hosts and on the adult worm burden of the definitive host population); (3) the population density of intermediate hosts varies both seasonally and inter-annually; (4) intermediate host populations may be more or less protected by their habitat characteristics and behaviour and thus more or less accessible to predation by foxes and domestic carnivores; (5) the population densities of definitive hosts vary in space and time; (6) dietary specialisation of foxes on focal species of intermediate hosts is correlated with more intensive transmission; (7) at local scale, egg survival is higher in wetter habitats; and (8) at local scale, prevalences of Em increases in areas where definitive host faeces density (subsequently Em egg density) is larger. Every factor acts at different spatial and temporal scales and may be antagonistic or synergistic to others. Undoubtedly for practical and methodological reasons most of those factors cannot be investigated at relevant scales and precision. It is thus unlikely to come by this way to a deterministic model of transmission integrating each of them and the environment variability at several scales, to which should be added hard data on the compatibility screen (host immunity, etc.). The current addressable question is to determine which global environmental variables may be generally linked to variations in transmission, and from this to determine which ecological processes may be mainly involved in a given system by using comparative and experimental methods of community ecology and epidemiology.

Fig. 4. Range of factors influencing Echinococcus multilocularis transmission in Western Europe (see text for comments).
On a regional scale, landscape and host community approaches may provide a framework for a global understanding of the ecology of transmission in animals (Giraudoux et al. 2002) (Fig. 5). It should lead to spatial models using global variables (e.g. quantified landscape, human activity, population dynamic patterns of focal hosts, etc.) actually controlling ‘top down’ the nested hierarchy of ecological and anthropogenic factors and processes that lead to variations in parasite transmission intensity in space and time.

Fig. 5. Global variables and model of Echinoccocus multilocularis transmission. A. behaviour, animal behaviour; H. behaviour, human behaviour.
The ecology of potential small mammal intermediate hosts, which includes landscape quantification and consideration of ROMPA may be an important approach in the study of transmission of rodent-borne zoonoses at regional scale such as alveolar echinococcosis. This should enable prediction of zoonotic disease occurrence, which may be expected to increase as landscape alterations (e.g. deforestation in China or agricultural changes in France) lead to higher ROMPA for potentially cycling rodent host species. It also paves the way to the use of remote sensing and geographical information system for risk modelling of alveolar echinococcosis (Danson et al. 2002 and Danson et al. in this supplement).
Financial support from the European Union (STD TS3-CT94-0270), the French-Chinese programme of Advanced Research and the French Ministry of Foreign Affairs (PRA-95-1) and the US National Institutes of Health and National Science Foundation (TWO1565-02). Thanks to Claude Combes for the early design of figure 4. Technical assistance for land use analysis in Gansu from Renaud Scheifler.

Fig. 1. Human AE prevalence rates and land use in Zhang and Puma Xian area, China. Circles are villages, and their size is proportional to prevalence rates (average 4·1%). Black circles – prevalence rates significantly higher than the average (alpha[les ]0·05); white circles or s. – prevalence rates significantly lower; grey circles or n.s. – prevalence rates not significantly different from the average (data fitted with a Poisson distribution); Pv1, Pv2, Pv3, overall prevalence rate in the three landscape areas; n1, n2, n3, number of people screened. Prevalence rates appear to be highly variable within each landscape area, but were three times lower in sectors with lower ratio of scrub-, shrub-, grassland (χ2 with correction for continuity, P<0·0003; permutation test between villages, P=0·003; villages which were on the border between two areas were pooled conservatively, e.g. if a prevalence rate was higher than the average, data were pooled with the villages of the sector of lower prevalence rates, or conversely).
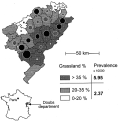
Fig. 2. Human AE prevalence rates and land use in the Doubs department, France. Circles in each canton are proportional to prevalence rates (average 1·74[ratio ]10,000). Black circles – prevalence rates significantly higher than the average (alpha[les ]0·05); white circles or s. – prevalence rates significantly lower; grey circles or n.s. – prevalence rates not significantly different from the average (data fitted with a Poisson distribution). Urban cantons (bold) were removed for further analysis. Even considering rural cantons only, human AE prevalence was highly variable within each land use area as in China. It is almost three times higher in areas with higher ratios of permanent grassland (see table, χ2 with correction for continuity, P<0·0001; permutation test between cantons, P=0·05).

Fig. 3. Distribution of the dominant species in each landscape element in the Zhang and Puma Xian area (China), and in the Doubs department (France). Bold species – potential hosts for Echinococcus multilocularis; non-bold name – species for which susceptibility to E. multilocularis is still unknown; bold, thin, dotted lines indicate the relative population density of each species between habitats. In France, potential and actual good physiological hosts (developing alveolar cystic masses with protoscoleces) are present in every habitat. A similar pattern was recorded in China in every key-habitat, even though the susceptibility of some hosts remains variable (e.g. Mus musculus) or unknown (e.g. Cricetulus longicaudatus) and the presence in forest of other very susceptible arvicolids likely.
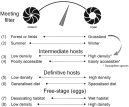
Fig. 4. Range of factors influencing Echinococcus multilocularis transmission in Western Europe (see text for comments).
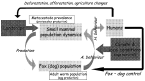
Fig. 5. Global variables and model of Echinoccocus multilocularis transmission. A. behaviour, animal behaviour; H. behaviour, human behaviour.