INTRODUCTION
The secondary sex ratio, expressed, for example, as the male to female ratio in the offspring, at the time of delivery is around an evolutionary stable value of 0·5 in most species. Several evolutionary explanations have been suggested for a significant deviation from 0·5. Theoretical models and data for the real population show that such a deviation can be caused, for example, by a difference in expensiveness (measured, for example, in amount of parental care) between male and female offspring (Fisher, Reference Fisher1958), local mate competition (Hamilton, Reference Hamilton1967) or local competition for other resources (Clark, Reference Clark1978). The Trivers-Willard hypothesis (Trivers and Willard, Reference Trivers and Willard1973) suggests that the female could also manipulate the probability of birth of male and female offspring depending on the ability to invest her resources to the offspring. Females in poor condition, which are unable to provide enough resources for their offspring, can increase their fitness by increasing the proportion of female offspring, as even low-quality females usually succeed in reproduction while low-quality males usually fail in generally stronger competition for mating partners between males. On the other hand, in species with matrilineal inheritance of resources (e.g. matrilineal inheritance of territories or a social position) the low-quality females could increase their fitness by increasing the proportion of male offspring (Altmann et al. Reference Altmann, Hausfater, Altmann and Clutton-Brock1988).
All of the above models assume that the aberrant secondary sex ratio is a result of adaptive evolution of the studied organisms. However, in some systems, the aberrant sex ratio is non-adaptive from the point of view of the studied organism. The deviation from the normal value in a studied species can be a product of so-called manipulative activity of parasitic organisms. Very strong effects are observed in the microorganisms that are transmitted vertically via oocytes (and not by spermatozoa) in many insects, crustaceans and helminths, e.g. in Wolbachia (Eubacteria) and Octosporea (Microsporidia) (Knight, Reference Knight2001; Dunn et al. Reference Dunn, Terry and Smith2001).
The influence of parasites on the sex ratio in the infected host could also increase the chance of survival and reproduction in the host organism. Resistance of female and male hosts to certain parasitic infections often differs. Therefore, the infection with some parasitic species, e.g. the tapeworm Taenia crassiceps, can lead to the hormonal feminization or masculinization (and thereby immunomodulation) of the organism of their infected host, which increases the chance that the parasite survives an immunological attack of the host's organism (Larralde et al. Reference Larralde, Morales, Terrazas, Govezensky and Romano1995).
Furthermore, the parasite could change the sex ratio of its host, which can enhance the ‘vector’ function of the host (Rozsa, Reference Rozsa2000), i.e. to enhance the host capacity to spread infection over long distances by increasing the proportion of the more migratory sex.
Toxoplasma gondii (Apicomplexa) is one of the most common parasitic protozoans in humans. There are 2 clinically different forms of post-natally acquired toxoplasmosis, depending on the stage of infection and host immunocompetence. One form is acute toxoplasmosis, which in immunocompetent subjects, spontaneously turns into the second form, i.e. latent toxoplasmosis. The latter is clinically asymptomatic, life-long infection characterized by the presence of anti-Toxoplasma antibodies in the blood and Toxoplasma bradyzoites in tissue cysts. Toxoplasma reproduces sexually only in its definitive host, i.e. any feline species. The intermediate host of Toxoplasma is any warm-blooded animal, acquiring the infection either pre-natally, by intrauterine transmission, or post-natally, by consumption of food or water contaminated with feline faeces containing the durable oocysts of the parasite (Beattie, Reference Beattie1982; Tenter et al. Reference Tenter, Heckeroth and Weiss2000) or by consumption of other intermediate hosts containing tissue cysts with Toxoplasma bradyzoites. Definitive hosts acquire the infection mostly by consumption of intermediate hosts containing tissue cysts with Toxoplasma bradyzoites.
Toxoplasma is a classical model for the study of the so-called manipulation hypothesis (Hutchison et al. Reference Hutchison, Bradley, Cheyne, Welh and Hay1980a; Berdoy et al. Reference Berdoy, Webster and MacDonald1995). The manipulation hypothesis considers the ability of some parasites to modify the behaviour of their hosts to be an evolutionary adaptation (the product of a ‘blind’ natural selection) that can increase the efficiency of the transmission of the parasite to uninfected hosts. The behavioural changes observed in animal hosts with latent toxoplasmosis, e.g. learning defects (Witting, Reference Witting1979), impairment of motor performance and coordination (Hutchison et al. Reference Hutchison, Aitken and Wells1980b), reduced neophobia, increased trappability (Webster, Reference Webster1994), and decreased cat urine odour avoidance (Berdoy et al. Reference Berdoy, Webster and MacDonald2000), are usually interpreted in terms of the manipulative hypothesis. Intermediate hosts play the role of a biotic vector of toxoplasmosis under natural conditions as they spread the infection over long distances. Males and females of most rodent species differ considerably in home ranges (Brown, Reference Brown1969), exploratory activity (Frynta, Reference Frynta1994), risk of predation and, therefore, in their potential role in the life-cycle of Toxoplasma. As a rule, males are the more aggressive (e.g. Čiháková and Frynta, Reference Čiháková and Frynta1996; Frynta et al. Reference Frynta, Munclinger, Slábová, Volfová and Třeštíková2005) and consequently the more dispersing sex (e.g. Pocock et al. Reference Pocock, Hauffe and Searle2005). Therefore, we speculate that Toxoplasma could increase its transmission efficiency by changing the sex ratio of infected hosts to increase the proportion of more migratory (pre-natally infected) male offspring.
Recently, a pronounced increase in the secondary sex ratio was observed in children of women with latent toxoplasmosis, in particular in mothers with relatively high titres of anti-Toxoplasma antibodies (Kaňková et al. Reference Kaňková, Šulc, Nouzová, Fajfrlík, Frynta and Flegr2007). An observational study, however, cannot resolve whether the male-biased sex ratio is the effect of the infection or whether both the male-biased sex ratio and Toxoplasma infection are the outcome of a third factor, e.g. increased testosterone level (James, Reference James1986). To test the hypothesis that latent toxoplasmosis is the cause of the increased proportion of male offspring, we studied the secondary sex ratios (expressed here as the proportion of males in the litter, i.e. as the probability of birth of a male) in laboratory mice that had been experimentally infected with T. gondii. The study was performed under strictly controlled conditions to exclude the possibility for an unknown third factor to influence both the sex ratio and Toxoplasma infection risk.
MATERIALS AND METHODS
Experimental animals and experimental design
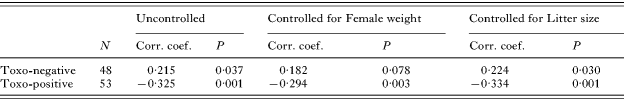
We performed 2 independent experiments of a similar design in 2004 and 2005 (Fig. 1). In Experiment 1 (performed in 2004), 70 BALB/c females and 10 C57Bl/10J males were used. One half of the females, aged 5–6 weeks (mean weight 17·5g), were orally infected with brain homogenate from mice infected with a relatively avirulent cystogenic strain HIF of T. gondii (Kodym et al. Reference Kodym, Blažek, Malý and Hrdá2002). To eliminate a possible effect of body weight, pairs of mice of the same weight were selected first, and then 1 mouse of each pair was included in the ‘infected’ group and the other was placed in the control group. Each mouse of the ‘infected’ group was given about 75 μl of brain homogenate containing approximately 10 tissue cysts by intubation. The controls were given the same amount of isotonic saline (0·8% NaCl). The course of the acute infection was monitored by visual inspection and regular measurement of body weight. The symptoms of acute toxoplasmosis (i.e. lethargy and ruffled fur) were observed on days 6–9. The females were maintained in groups of 5, for 7 weeks. One week before mating, they were placed into individual breeding boxes. The males were kept in individual boxes from the start of the experiment. The mating began after the end of acute toxoplasmosis, i.e. 2 months after the infection (Kodym et al. Reference Kodym, Blažek, Malý and Hrdá2002). The mice were regularly weighed (at least twice a week) during the entire experiment.
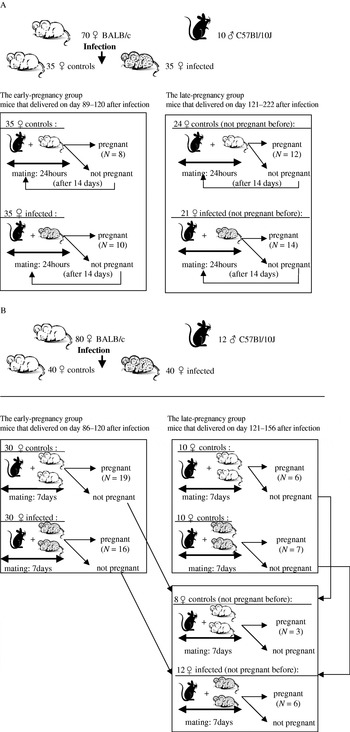
Fig. 1. Experimental set-up, Exp. 1 (A) and Exp. 2 (B).
In this experiment, a male mated in the morning with a female and then the pair was kept together for 24 h. The next morning, the male was mated to another female and the pair was again kept together for 24 h. Half of the males mated with a Toxoplasma-infected female on day 1 and with a control female on day 2, while the other half of the males mated with a control female on day 1 and with an infected female on day 2.
Experiment 2 (performed in 2005) differed from Exp. 1 in the age of the animals (about 2 weeks older at the start of the experiment), and in the design of mating, see below. Eighty females and 12 males were used in Exp. 2. In this experiment 2 Toxoplasma-infected or 2 control females were kept together with a male for 7 days to increase the probability of fertilization. The same male was mated to another pair of either Toxoplasma-infected or control mice after a span of 3 days. Half of the males mated with Toxoplasma-infected females first and with control mice later while the other half of the males mated with control and infected females in reverse order. The mice were mated within a single mating period 8–19 weeks after infection, with the equal numbers of Toxoplasma-infected and control mice being mated.
As a higher sex ratio (0·71) was observed (Kaňková et al. Reference Kaňková, Šulc, Nouzová, Fajfrlík, Frynta and Flegr2007) in children of women with relatively high titres of anti-Toxoplasma antibodies (i.e. with relatively recent infection) while the sex ratio in women with low titres (with old or weak infection) was lower than in Toxoplasma-free women, the mice were separated into 2 groups according to the start of gestation (early-pregnant and late-pregnant mice) after the data collection in both experiments.
In Exp. 1, the early pregnancy group consisted of 18 mice that delivered between days 89 and 120 after infection. The late pregnancy group included 26 mice that delivered between days 121 and 222 after infection. In Exp. 2, the early pregnancy group comprised 35 mice that delivered between days 86 and 120 while the later pregnancy group involved 22 mice that delivered between days 121 and 156 after infection.
The pregnant mice were weighed every third day. One week before the expected delivery, the females were provided with specially enriched food and nest building material. At the time of expected delivery, they were checked several times a day and the number of newborn males and females (estimated from anogenital distance and abdomen profile) was recorded as soon as possible and confirmed the next 21 days. The dead offspring (23 and 33 for Exps 1 and 2, respectively) were preserved in 80% ethanol for PCR analysis. The DNA from the preserved dead mice and those that died soon after birth was isolated using a High Pure PCR Template Preparation kit, and the sex of each animal was determined by PCR amplification of a 202bp fragment of the SRY-HMG box of the Sry gene located on Y-chromosome and a 447/445bp region of the Zfy-Zfx genes was amplified as a positive control of PCR (Aasen and Medrano, Reference Aasen and Medrano1990; Bryja and Konečný, Reference Bryja and Konečný2003).
Infection in all infected mice was confirmed by the complement fixation test (CFT; Ondriska et al. Reference Ondriska, Čatár and Vozarová2003) for detection of specific serum antibodies and altogether 7 mice from both experiments with undetected or very low specific antibodies were withdrawn from the analysis.
Statistical analysis
The mice that had delivered only dead offspring or had cannibalized all offspring were excluded from the analysis. The number of such mice was 4 among controls and 6 among infected mice. The general linear model (GLM) was used for statistical testing of the influence of ‘Toxo’ status (and Group) on weight of the females on the first day of mating and for estimation of effect size (Eta2) for all models. However, GLM could provide biased results with hierarchically structured sex ratio data (Krackow and Tkadlec, Reference Krackow and Tkadlec2001). Therefore, the effect of latent toxoplasmosis on the sex ratio (expressed as the proportion of males in all offspring) was analysed with a generalized linear model (GLMZ), with the number of males as a dependent variable with binomial distribution, and litter size as the binomial denominator (not a predictor), and female weight on the first day of mating as continuous predictor and Toxo status (Toxo-infected vs controls) and Group (earlier pregnancy vs later pregnancy) as categorical (binary) predictors. Parameters for GLMZ were estimated with the maximum likelihood method and their significance was evaluated by χ2 approximation computed as minus 2 times the difference in the likelihood between the full and reduced models (Myers et al. Reference Myers, Montgomery and Vining2002). The effect of interaction between Toxo status and Group on sex ratio was also analysed using the Generalized Linear Mixed Models (GLMM) method to control the influence of Male identity and cluster-specific effect of Female identity. (Fixed categorical factors Toxo status, Group, Toxo-Group interaction and Male identity, covariate weight of female, random factor Female identity). The cluster-specific effect for individual females was evaluated by maximization of the penalized log likelihood function (Fahrmeir and Tutz, Reference Fahrmeir and Tutz2002). Optima of the likelihood and penalized likelihood functions were obtained using the Matlab optimization toolbox. The effect of Toxo-Group interaction was evaluated on the same basis as in GLMZ by using the optimal values of Female identity cluster-specific effects. The influence of time from infection to birth on the secondary sex sratio was evaluated by GLM and GLMZ (with continuous variable time from infection to birth instead of a binary variable group as an independent factor) and by Kendall correlation and partial Kendall correlation (Sheskin, Reference Sheskin2003) with the secondary sex ratio as a dependent continuous variable and time from infection to birth (in days) and female weight on the first day of mating or litter size as independent variables. As the effect of the male on the sex ratio was non-significant (P>0·8, GLMZ), the male identity was not included in GLM and GLMZ models (but was included in GLMM models). The chi-square goodness of fit and Shapiro-Wilks tests were used for testing normality of the data. SPSS v.12 software was used for all statistical testing.
RESULTS
In Exp. 1, we analysed the sex ratios of 24 litters of Toxoplasma-infected and 20 litters of control mice. (Six mice died before the start of mating, 2 infected mice were Toxoplasma-negative in CFT, 10 mice did not get pregnant, 1 mouse died during pregnancy, 1 mouse aborted, and 6 mice ate their (probably stillborn) offspring). The earlier pregnancy group (mice that delivered on days 89–120 after infection) consisted of 10 infected and 8 control mice and the later pregnancy group (mice that delivered on days 121–222 after infection) included 14 infected and 12 control mice. The GLMZ analysis showed no significant main effect (Toxo: χ12=0·142, P=0·706; Female weight: χ12=0·104, P=0·747, Group: χ12=0·136, P=0·712). At the same time, the effect of the Toxo-Group interaction was highly significant (GLMZ: χ12=15·208, P<0·001; GLMM χ12=14·430, P<0·001; GLM partial Eta2=0·319), with early-pregnant mice having higher sex ratios than controls and late-pregnant mice having lower sex ratios than controls (Fig. 2). The analysis of the model with the dependent variable of Female weight on the first day of mating, and using the Toxo and Group as independent variables showed that infected mice had a lower mean weight than controls (22·62 g vs 23·73 g, GLM: F1,49=6·204, P=0·016, partial Eta squared=0·112). The females that ate their (probably stillborn) offspring were also included in this analysis.

Fig. 2. Differences in the secondary sex ratio between Toxoplasma-positive and Toxoplasma-negative mice. The left graph shows the early-pregnancy group and the right graph represents the late-pregnancy group (data from Exp. 1).
In Exp. 2, we analysed the sex ratios of 29 Toxoplasma-infected and 28 control mice. (Five infected mice were Toxoplasma-negative in CFT, 14 mice did not get pregnant, and 4 mice ate their, probably stillborn, offspring.) The earlier pregnancy group (mice that delivered on days 86–120 after infection) consisted of 16 infected and 19 control mice and the later pregnancy group (mice that delivered on days 121–156 after infection) comprised 13 infected and 9 control mice. As Fig. 3 shows, the early-pregnant mice had higher sex ratios than controls while the late-pregnant mice had lower sex ratios than controls. The analysis showed no significant main effect (GLMZ: Toxo: χ12=0·404, P=0·520, Female weight: χ12=0·453, P=0·500, Group: χ12=2·52, P=0·112) and a non-significant (significant in one-tailed GLMM and GLM tests (Rice and Gaines, Reference Rice and Gaines1994)) effect of the Toxo-Group interaction (GLMZ: χ12=1·946, P=0·163; GLMM: χ12=3·812, P=0·051; GLM partial Eta2=0·071). The analysis of the model with the dependent variable Female weight on the first day of mating and the Toxo status and Group as independent variables found the infected mice to have approximately the same weight as controls (23·84 g and 24·11 g, respectively, F1,59=0·889, P=0·349, partial Eta2=0·015).

Fig. 3. Differences in the secondary sex ratio between Toxoplasma-positive and Toxoplasma-negative mice. The left graph shows the early-pregnancy group and the right graph represents the late-pregnancy group (data from Exp. 2).
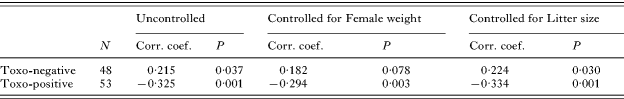
The correlation between the sex ratio and duration of toxoplasmosis was analysed for all 53 Toxoplasma-infected and 48 control mice (from both experiments) (Fig. 4). This time the full model contained also an independent binary variabile experiment (Exp. 1 or Exp. 2) and the binary variable Group (early-pregnant or late-pregnant mice) was substituted with the continuous variable, Time from infection to birth. The effect of Toxo-Time from the infection interaction was highly significant (GLMZ: χ12=7·92, P=0·005, GLM partial Eta squared=0·079). As the data failed in the normality tests, we performed also a nonparametric Kendall correlation and partial Kendall correlation (controlled for Female weight or Litter size). The correlations were very strong and negative for infected mice, appearing weaker and positive for controls (Table 1).

Fig. 4. Correlation between the sex ratio and length of toxoplasmosis infection.
Table 1. Results of the Kendall and partial Kendall tests of correlation between length of infection and sex ratio
(Column 3 shows the correlation coefficients and two-tailed P for the Kendall correlation tests and columns 4 and 5 show the partial correlation coefficients and two-tailed P for the partial Kendall correlation tests.)
DISCUSSION
Our results show that latent toxoplasmosis has a strong effect on the secondary sex ratio. In the early phase of latent toxoplasmosis, i.e. in mice that delivered in the fourth month of infection, the sex ratio was higher than in controls, while the mice in a later phase of infection, i.e. those that delivered in the fifth to eighth month of infection, had a lower sex ratio than controls.
The results for the infected mice are similar to those obtained earlier in infected women (Kaňková et al. Reference Kaňková, Šulc, Nouzová, Fajfrlík, Frynta and Flegr2007). In a large cross-sectional study performed on a sample of 1803 clients of 3 gynaecology and obstetrics clinics, the probability of the birth of a boy increased up to 0·71, which means that 250 boys were born for every 100 girls, in women with the highest concentrations of anti-Toxoplasma antibodies (and therefore probably with recent, but already latent, infection). In the women with a low concentration of anti-Toxoplasma antibodies (and therefore probably with an old infection) the sex ratio was lower than in Toxplasma-free women (Kaňková et al. Reference Kaňková, Šulc, Nouzová, Fajfrlík, Frynta and Flegr2007).
In mice, the mortality and morbidity of acquired toxoplasmosis is relatively high. In our experiments, a statistically significant decrease in the weight of infected animals was observed, even 8 months after infection. The Trivers-Willard hypothesis suggests that females in poor physical condition give birth to more female offspring (Trivers and Willard, Reference Trivers and Willard1973). We assume that in the later phase of infection, the non-specific, long-term negative effects of toxoplasmosis on the health of mice (leading to a reduced proportion of male offspring) outweigh the specific short-term effects of toxoplasmosis on the sex ratio (resulting in an increased proportion of male offspring in earlier phases of the infection). We also propose that the Trivers-Willard effect was probably responsible for the female biased sex ratio in the early-pregnant controls as well as for the gradual increase in the sex ratio of the growing up controls. In the beginning of the experiments, all mice were rather young and small. Under such conditions, the mice are expected to invest more in production of female offspring. This trend (observed in early-pregnant mice in both experiments) was reversed by the specific influence of toxoplasmosis in infected mice.
We can only speculate about the nature of the specific short-term positive effects of toxoplasmosis on the sex ratio of an intermediate host. Classical ‘host oriented’ theories of the biased sex ratio such as the Trivers-Willard hypothesis would predict the female-biased sex ratio in infected mice. The ultimate cause can be a specific adaptive manipulation with the number of the more migratory (congenitally infected) males, which could more efficiently serve as a vector for the spread of the parasite in space. In our experiments, we have never observed vertical transmission of Toxoplasma infection to F1 offspring (unpublished results), although this form of infection is relatively common in wild mice of the genera Mus and Apodemus (Owen and Trees, Reference Owen and Trees1998; Marshall et al. Reference Marshall, Hughes, Williams, Smith, Murphy and Hide2004). However, the congenital infection can only occur in recently infected females and in the acute phase of toxoplasmosis. Therefore, there is only a low chance that congenitally infected females would transmit the infection vertically to the next generation. It means that the congenitally infected migratory males are more important for Toxoplasma than females.
The increased sex ratio in Toxoplasma-infected mice and humans might be just a non-adaptive side-effect of Toxoplasma-induced immunosuppression. Toxoplasmosis induces the production of immunosuppressive lymphokines IL-10 and TGF-beta (Filisetti and Candolfi, Reference Filisetti and Candolfi2004). Also, non-specific stress axis activation is known to result in systemic immunosuppression (Elenkov and Chrousos, Reference Elenkov and Chrousos2002). The implantation success rate is higher for male zygotes (Kirby et al. Reference Kirby, McWhirter, Teitelbaum and Darlington1967; Kirby, Reference Kirby1970) and, consequently, the sex ratio at the early embryonic stage is heavily male biased (Evdokimova et al. Reference Evdokimova, Nikita, Lebedev, Sukhanova and Nazarenko2000; Milki et al. Reference Milki, Jun, Hinckley, Westphal, Giudice and Behr2003; Vatten and Skjaerven, Reference Vatten and Skjaerven2004). Maternal immunological reaction against male specific HY-antigens is an important cause of selective mortality of the Y-chromosome-bearing embryos and, consequently, of the secondary sex ratio adjustment (Shalev et al. Reference Shalev, Nelson and Hamerton1980; Christiansen et al. Reference Christiansen, Pedersen, Nielsen and Andersen2004). Therefore, the toxoplasmosis-associated immunosuppression, or immunomodulation, could be responsible for the enhanced survival of male embryos.
Indirect evidence for this hypothesis was obtained from the study of Hostomská et al. (Reference Hostomská, Jírovec, Horáčková and Hrubcová1957). The authors found the prevalence of toxoplasmosis in 94 mothers of children with Down's syndrome to be 84%. The toxoplasmosis prevalence rate in the control population of the same age, as well as in the fathers of the mongoloid children, was 32%. The high prevalence of toxoplasmosis among mothers of children with Down's syndrome can be most easily explained by their released quality control, i.e. less stringent selection against genetically disadvantaged embryos, which may have increased the risk of bringing children with developmental defects to full term. Similar effects of maternal age and inter-birth interval on stringency of quality control have been recently reported by Neuhauser and Krackow (Reference Neuhauser and Krackow2007).
The association of latent toxoplasmosis and an increased sex ratio was originally found in an observational cohort study on human subjects. Nevertheless, such a study cannot resolve whether Toxoplasma infection is the cause of the increased sex ratio, or whether a third factor, such as a higher level of testosterone in the infected subject (Flegr et al. Reference Flegr, Hrušková, Hodný, Novotná and Hanušová2005), is responsible for both the higher risk of Toxoplasma infection and increased sex ratio (James, Reference James1986). In contrast, the present (experimental and not observational) study clearly shows that the increased sex ratio is the effect of latent toxoplasmosis.
The secondary sex ratio may be influenced by genetic (Krackow and Gruber, Reference Krackow and Gruber1990) and environmental factors such as stress (Kinsley and Svare, Reference Kinsley and Svare1985; Clutton-Brock and Iason, Reference Clutton-Brock and Iason1986), hormone levels (James, Reference James1996), timing of mating (Krackow and Burgoyne, Reference Krackow and Burgoyne1998), age (Jacobsen et al. Reference Jacobsen, Moller and Mouritsen1999), number of preceding pregnancies (Krackow and Hoeck, Reference Krackow and Hoeck1989), sex of preceding siblings (Renkonen et al. Reference Renkonen, Makela and Lehtovaara1962), and socioeconomic status of the parents (Chacon-Pugnau and Jaffe, Reference Chacon-Pugnau and Jaffe1996). In comparison with these effects, the observed effect of toxoplasmosis on the sex ratio estimated from partial Eta squared is rather strong. Nevertheless, as the sample size in both experiments was relatively small, the results need to be viewed with caution.
The scientific and demographic significance of the observed effects of latent ‘asymptomatic’ toxoplasmosis on the secondary sex ratio of infected intermediate hosts could be considerable. Even more considerable, however, could be the possible clinical impact of the phenomenon, if the shift of the sex ratio were just a side-effect of other (immunological or hormonal) changes in the physiology of the host. The study of possible immunosuppressive or endocrinological effects of latent toxoplasmosis seems to be highly desirable.
This research was supported by the Grant Agency of Charles University (Grant no. 151/2006/B-Bio/PrF), Czech Ministry of Education (grant No. 0021620828) and Grant Agency of the Czech Republic (Grant no. 206/05/H012). The experiments comply with the current laws of the Czech Republic.