Introduction
Gastrointestinal nematodes (GIN) are one of the most pressing health concerns faced by small ruminant producers. Of the small ruminant GIN, Haemonchus contortus (Barber's pole worm) is the most pathogenic, responsible for the majority of economic losses in sheep production worldwide (Veríssimo et al., Reference Veríssimo, Niciura, Alberti, Rodrigues, Barbosa, Chiebao, Cardoso, da Silva, Pereira, Margatho, da Costa, Nardon, Ueno, Curci and Molento2012). The prevalence of GIN resistance to commercial dewormers has created the need for alternative methods of control. Plants rich in secondary compounds, such as condensed tannins, also called proanthocyanidins (PAC), have been explored for their anti-parasitic effects, though the mechanism of action remains unknown. Lespedeza cuneata (Dum. Cours.) G. Don [sericea lespedeza; syn. Lespedeza juncea var. sericea (Thunb.) Lace & Hauech; Chinese bushclover] a tropical perennial, has been shown to be effective in managing GIN infections (Lange et al., Reference Lange, Olcott, Miller, Mosjidis, Terrill, Burke and Kearney2006; Shaik et al., Reference Shaik, Terrill, Miller, Kouakou, Kannan, Kaplan, Burke and Mosjidis2006) but, as with other plants investigated for their anti-parasitic activity (Assis et al., Reference Assis, Bevilaqua, Morais, Vieira, Costa and Souza2003; Marie-Magdeleine et al., Reference Marie-Magdeleine, Hoste, Mahieu, Varo and Archimede2009), it is not adapted to the New England climate. However, birdsfoot trefoil (BFT; Lotus corniculatus L.; Fabaceae), a drought-resistant legume (Hedqvist et al., Reference Hedqvist, Mueller-Harvey, Reed, Krueger and Murphy2000) that tolerates low soil fertility is adapted to the northeastern USA and has exhibited anti-parasitic effects against H. contortus in vivo by reducing fecal egg count (Marley et al., Reference Marley, Cook, Keatinge, Barrett and Lampkin2003) and total worm burden (Marley et al., Reference Marley, Cook, Keatinge, Barrett and Lampkin2003; Heckendorn et al., Reference Heckendorn, Häring, Maurer, Senn and Hertzberg2007), but in some cases showed no effect against GIN (Niezen et al., Reference Niezen, Robertson, Waghorn and Charleston1998; Bernes et al., Reference Bernes, Waller and Christensson2000). Grazing this legume provides additional benefits to the animal by minimizing bloat and improving protein uptake (Min et al., Reference Min, Pinchak, Anderson, Fulford and Puchala2006) and may provide a feasible option for producers to use as hay or forage to manage small ruminant GIN.
Many in vitro anti-parasitic studies using PAC-containing forages have primarily focused on the concentration and effect of PAC (Molan et al., Reference Molan, Waghorn, Min and McNabb2000, Reference Molan, Meagher, Spencer and Sivakumaran2003; Barrau et al., Reference Barrau, Fabre, Fouraste and Hoste2005; Brunet and Hoste, Reference Brunet and Hoste2006; Brunet et al., Reference Brunet, Jackson and Hoste2008). PAC are not absorbed from the lumen of the gastrointestinal tract (Terrill et al., Reference Terrill, Waghorn, Woolley, McNabb and Barry1994); therefore, bioactivity would be expected to occur within the GI tract. Since BFT would be consumed as a hay or forage, H. contortus would theoretically be exposed to PAC as well as countless other secondary compounds from the plant. This study utilizes an aqueous extract for its biological relevance to what the animals would ingest when consuming BFT and uses PAC concentrations of BFT strains to predict which strains will prove the most efficacious. Since PAC concentrations across BFT strains differ substantially (Grabber et al., Reference Grabber, Riday, Cassida, Griggs, Min and MacAdam2014), the BFT strains examined were selected for overall diversity. Fifty-nine strains were originally chosen to include the 48 strains of the Core Collection (Steiner et al., Reference Steiner, Beuselinck, Greene, Kamm, Kirkbride and Roberts2001) as well as experimental populations and commercial varieties. Eight strains from the Core Collection failed to germinate or were unable survive; therefore, 51 strains were screened for anti-parasitic effects.
This study utilized in vitro methods to investigate the anti-parasitic effects of 51 BFT strains in the USA and streamlined these efforts by hypothesizing, based on previous studies, that PAC content would be the best predictor of anti-parasitic activity. The specific objectives of this study were to: (1) test the anti-parasitic effects of 51 BFT strains against H. contortus egg hatching and first stage (L1) larval motility; (2) test the effects of the top performing BFT strains (13) on H. contortus third stage (L3) larval motility and exsheathment; and (3) determine if the PAC content of a specific strain of BFT can be correlated to the biological activity of that strain.
Materials and methods
Preparation and analysis of BFT powder and extracts
Harvest
BFT strains (51) were grown from seed in a greenhouse at the University of Rhode Island, under mist, in late January 2012 and hardened off outside in early May before being transplanted to field plots. Accessions were originally chosen based on previously reported PAC content, ranging between 2 and 105 mg g−1 dry matter (Roberts et al., Reference Roberts, Beuselinck, Ellersieck, Davis and McGraw1993; Grabber et al., Reference Grabber, Riday, Cassida, Griggs, Min and MacAdam2014; USDA, 2014) and seed for each strain was acquired (Supplementary Data Table 1). Once transplanted and established in field plots, plant matter was harvested (clipped and bagged) pre-bloom and kept at −20 °C until the frozen tissue samples were freeze-dried (Labconco Bulk Tray Dryer 115 V and FreeZone 6 L Benchtop Freeze Dry System, Labconco Corporation, Kansas City, MO, USA) and ground into fine powder. All freeze-dried material was stored at −20 °C for phytochemical analysis.
BFT crude aqueous extract
Stock solutions of BFT crude aqueous extract (BFT-AqE) powder were prepared for use in egg hatch and first stage (L1) larval motility assay by measuring 360 mg ground BFT powder into a 50 mL polypropylene tube and adding room temperature (RT) tap water up to a volume of 30 mL (12 mg powder mL−1). Ground BFT was soaked in the dark at RT for 24 h. Solid plant matter was centrifuged (200 × g for 5 min) to the bottom of the tube and the supernatant was collected and subsequently used the same day in the in vitro assays. A stock solution of 50 mg powder mL−1 was prepared in the same way for use in the L3 motility and exsheathment inhibition assay.
Phytochemical analysis
Analysis of the BFT powders was performed by the Reed Research Group at the University of Wisconsin-Madison. PAC extract was isolated from the BFT powders following the established DMAC [4-(dimethylamino)cinnamaldehyde] method for PAC isolation (Feliciano et al., Reference Feliciano, Shea, Shanmuganayagam, Krueger, Howell and Reed2012; Krueger et al., Reference Krueger, Chesmore, Chen, Parker, Khoo, Marais, Shanmuganayagam, Crump and Reed2016). Purified (‘Bruce’ strain) PAC, reflective of the natural structural heterogeneity of BFT PAC was used as a reference standard and results are expressed as BFT PAC equivalence (mg g−1).
BFT ‘Bruce’ PAC extract (BFT-PAC)
The ‘Bruce’ variety of BFT was used as the standard for the DMAC assay and was the only variety of BFT that PAC was extracted from. PAC was isolated from the BFT powder by the Reed Research Group at the University of Wisconsin-Madison, using solid-phase chromatography according to the well-established method for PAC isolation (Howell et al., Reference Howell, Reed, Krueger, Winterbottom, Cunningham and Leahy2005). Reverse phase (C18) followed by adsorption chromatography (Sephadex LH-20) was used to extract and isolate the total PAC. Isolated PAC was stored at RT in the dark and re-suspended in water when used for in vitro assays.
In vitro assays
Parasitology parameters
The H. contortus isolate used in this study was maintained by one of the authors (A. Zajac) from a closed flock with minimal drug use. This isolate is effectively treated with the commercial anthelmintics used in this experiment. Third stage larvae (L3 identification >99% H. contortus) were kept at 4 °C until used to infect donor lambs or use in the L3 motility and exsheathment inhibition assay and were no older than 3 months of age when used. Larvae were classified as motile or non-motile based on detection of movement during 5 s of observation (Skantar et al., Reference Skantar, Agama, Meyer, Carta and Vinyard2005; Katiki et al., Reference Katiki, Ferreira, Gonzalez, Zajac, Lindsay, Chagas and Amarante2013).
Donor lambs
The donor animals used for this study were Dorset/Hampshire crossbred lambs born and maintained inside at the University of Rhode Island Peckham Farm, located in Kingston, RI, USA. Lambs were dewormed orally with levamisole hydrochloride (8.8 mg kg−1 BW, Prohibit®, AgriLabs, St. Joseph, MO, USA) and ivermectin (0.2 mg kg−1 BW, Ivomec®, Merial Inc., Duluth, GA, USA). Seven days post-anthelmintic treatment, lambs were experimentally infected with 10 000 L3 H. contortus. Once eggs were present in the fecal samples (approximately 35 days), feces were used for the collection of H. contortus eggs for use in the egg hatch assays. New donor lambs were infected as needed.
Egg hatch and L1 motility inhibition assay
The anti-parasitic effects of BFT-AqE and BFT-PAC on hatching of H. contortus eggs were determined by modification of previously published procedures (Assis et al., Reference Assis, Bevilaqua, Morais, Vieira, Costa and Souza2003; Marie-Magdeleine et al., Reference Marie-Magdeleine, Hoste, Mahieu, Varo and Archimede2009) as described by Barone et al. (Reference Barone, Zajac, Manzi-Smith, Howell, Reed, Krueger and Petersson2018). Fresh feces were collected directly from the donor animal's rectum and eggs were recovered by running fresh feces in water through a series of sieves (1000, 355, 150, 38 and 25 µm). Eggs retained on the last two sieves were recovered using flotation with a concentrated sodium nitrate solution (Fecasol®, Vetoquinol U.S.A., Inc., Fort Worth, TX, USA) and collected on glass cover slips and rinsed with water. Eggs were placed (100 eggs well−1 in 100 µL H2O) into 24-well flat-bottomed microplates. Stock solutions in water of BFT-AqE (12 mg powder mL−1) and BFT-PAC (12 mg PAC mL−1), prepared fresh prior to each assay, were serially diluted with water and added in equal volume to the wells. Thiabendazole (TBZ, Thermo Fisher Scientific Inc., Waltham, MA, USA), a commercial dewormer, in dimethyl sulfoxide (0.5 µg mL−1) (DMSO, Fisher BioReagents™, Thermo Fisher Scientific Inc.) and water were used as positive and negative controls, respectively. Water (890 µL), DMSO (10 µL) and serial concentrations of BFT-AqE, BFT-PAC or water were added (1 mL) to each well for a total volume of 2 mL. Final concentrations in wells for BFT-AqE were 6, 3, 1.5 and 0.75 mg powder mL−1 and final concentrations in wells for BFT-PAC were 6, 3, 1.5 and 0.75 mg PAC mL−1. Five replicates were run for each set of dilutions and controls. Eggs were incubated at 26 °C for 24 h and determined to be hatched or not hatched, and results were expressed as per cent egg hatch inhibition. The larvae that hatched from the eggs were quantified and determined to be motile or non-motile (based on detection of movement during 5 s of observation). Results were expressed as per cent motility inhibition of eggs hatched. Percentages were adjusted to the negative control (water, 0.0 mg mL−1) values for each individual assay, determined by using the following formula: inhibition (%) = (A − B)/(A) × 100 (Acharya et al., Reference Acharya, Hildreth and Reese2014). Where A = % motility in the water control and B = % motility in the different concentrations of extracts.
L3 motility and exsheathment inhibition assay
Based upon the results in the egg hatch and L1 motility assays (EC50 ⩽ 2.5) as well as agronomic performance (growth habit, winter hardiness, pest resistance, uniformity and vigour; data not published), 13 BFT varieties were selected to investigate the anti-parasitic effects of BFT-AqE and BFT-PAC on L3 motility and exsheathment. Stock solutions of BFT-AqE (50 mg powder mL−1) and BFT-PAC (12 mg mL−1), made fresh for each assay, were serially diluted using tap water. Sheathed larvae were incubated in BFT-AqE (25 mg powder mL−1), BFT-PAC (6, 3, 1.5 and 0.75 mg PAC mL−1) or a water control for 24 h at 37 °C. Following previously described methods (Barone et al., Reference Barone, Zajac, Manzi-Smith, Howell, Reed, Krueger and Petersson2018), the sheathed L3 (2000) were added to Earle's Balanced Salt Solution (EBSS, Sigma-Aldrich®, Inc., Natick, MA, USA) up to a volume of 1 mL in a 15 mL polypropylene tube. Water control (1 mL) or treatment (1 mL) was added for a total volume of 2 mL. After 24 h, per cent motility was determined and exsheathment was induced following established procedures (Conder and Johnson, Reference Conder and Johnson1996) using CO2 and then incubated for 18 h. After incubation, 100 larvae/tube were determined motile or non-motile, and motile larvae were determined sheathed or exsheathed. Results were expressed as per cent motility inhibition of all larvae and per cent exsheathment inhibition of the motile larvae. Percentages were adjusted to the negative control (water, 0.0 mg mL−1) values for each individual assay, determined by using the following formula: inhibition (%) = (A − B)/(A) × 100 (Acharya et al., Reference Acharya, Hildreth and Reese2014). Where A = % motility or exsheathment inhibition in the water control and B = % motility or exsheathment inhibition in the different concentrations of extracts.
Statistical analysis
Egg hatch, L1 and L3 motility inhibition, and exsheathment inhibition data were analysed statistically using an analysis of variance and means separated with Dunnett's t test using the GLM Procedure in SAS (SAS Institute Inc., Cary, NC, USA). Treatment means were compared using a least significant difference value with significance defined as P ⩽ 0.05. The concentration of extracts required to prevent 50% (EC50) and 90% (EC90) of egg hatching were calculated using PROBIT procedure in SAS (SAS Institute Inc.,), using a confidence interval of 95%. All effective concentrations greater than the actual concentrations tested in the assays were extrapolated values through the PROBIT procedure. The CORR procedure in SAS was used to determine correlations between the PAC content of the BFT powders and the anti-parasitic effects of the corresponding aqueous extracts.
Results
Phytochemical analysis
Freeze-dried powder from 51 strains of BFT had an average PAC concentration of 13.9 ± 2 mg g−1 (mean ± s.e.m.; range: 1.4–63.8 mg PAC g−1; Table 1, last column).
Table 1. Effect of aqueous extracts from 51 strains of BFT and purified BFT-PAC (Bruce strain) extract on Haemonchus contortus egg hatch and larval motility post-hatch

Eggs were incubated in varying concentrations of BFT-AqE and BFT-PAC (‘Bruce’ strain), negative controls (water; 0.0 mg mL−1) and positive controls (TBZ; 0.5 µg mL−1) for 24 h. Egg hatch inhibition was determined and motility inhibition of resulting L1 larvae was determined (non-motile for 5 s). All values are mean ± s.e.m. (5 replicates per mean), adjusted to zero.
a Dash (–) indicates zero L1 larvae counted; 100% egg hatch inhibition.
b Results are expressed as BFT PAC equivalence (mg g−1).
c Insignificant; <5 larvae representing percentage.
*P ⩽ 0.001 vs negative control (0.0 mg mL−1).
Egg hatch and L1 motility inhibition assay
Egg hatch and larval motility inhibition results for the aqueous extracts of 51 strains of BFT (BFT-AqE) are reported in Table 1, showing wide variation in the range of inhibition at the varying concentrations. Of the 51 strains of BFT that were tested, 70% of the strains proved to be >90% efficacious against egg hatching at 6 mg mL−1 and 11 of them exhibited 100% egg hatch inhibition at a concentration of 3 mg powder mL−1 BFT-AqE (Table 1). The purified BFT-PAC (‘Bruce’ strain) that was screened, exhibited 8% egg hatch inhibition (compared with 89% egg hatch inhibition using ‘Bruce’ strain BFT-AqE) and 98% L1 motility inhibition at the highest concentration of 6 mg PAC mL−1 (Table 1). Water control (0.0 mg mL−1) values for each individual assay, averaged 12 ± 0 per cent egg hatch inhibition and 1 ± 0 per cent L1 motility inhibition (mean ± s.e.m.). Average per cent egg hatch inhibition in TBZ was 97 ± 0 (mean ± s.e.m.).
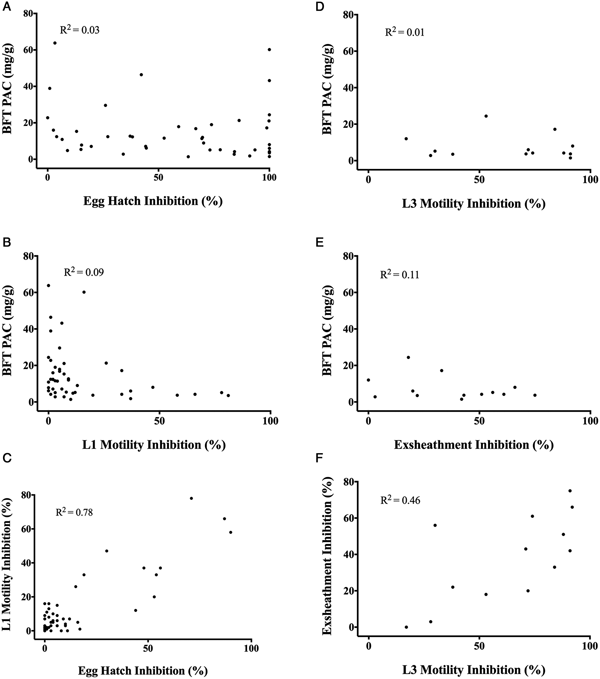
The EC50 and the EC90 of the 51 BFT strains against egg hatching ranged from 0.66 to 9.36 mg mL−1 and 1.48 to 97.15 mg mL−1 respectively (Table 2). BFT-PAC was not effective against egg hatching; the calculated EC50 was 1795 mg mL−1 (Table 2). There was no correlation between the PAC content of each BFT powder and the anti-parasitic effects of the 51 BFT-AqE extracts against egg hatch (R 2 = 0.03, P = 0.23; Fig. 1A) or L1 motility (R 2 = 0.09, P = 0.04; Fig. 1B) at 1.5 mg powder mL−1 BFT-AqE. There was a significant positive correlation between L1 motility inhibition and egg hatch inhibition at 1.5 mg powder mL−1 (R 2 = 0.78, P < 0.0001; Fig. 1C).

Fig. 1. No correlation between the concentration of PAC in the BFT powders and the aqueous extracts (1.5 mg powder mL−1) effect on (A) egg hatching (R 2 = 0.03, P = 0.23), (B) L1 motility (R 2 = 0.09. P = 0.04), (D) L3 motility (R 2 = 0.01, P = 0.7), (E) exsheathment (R 2 = 0.11, P = 0.27). Significant correlation between L1 motility and egg hatch inhibition (C) (R 2 = 0.78, P < 0.0001) as well as L3 motility and exsheathment inhibition (F) (R 2 = 0.46, P = 0.01).
Table 2. Effective concentration that inhibited 50% (EC50) and 90% (EC90) (mg powder mL−1) Haemonchus contortus egg hatching and 95% confidence interval (95% CI) of BFT strains

Eggs were incubated in varying concentrations of BFT-AqE and BFT-PAC (‘Bruce’ strain), negative controls (water; 0.0 mg mL−1) and positive controls (TBZ; 0.5 µg mL−1) for 24 h. Egg hatch inhibition was determined (5 replicates per mean).
a All values higher than actual assay concentrations tested have been extrapolated through the PROBIT procedure in SAS.
b BFT strain 325 379 aqueous extract egg hatch inhibition ranged from 99 to 100% (Table 1, the CI could not be calculated by PROBIT SAS).
L3 motility and exsheathment inhibition assay
Thirteen strains were selected to test their ability to inhibit L3 exsheathment and motility, based on the egg hatch and L1 motility assays as well as agronomic performance. Twelve of the 13 strains inhibited egg hatch by >99% at 6 mg powder mL−1 and 10 of the 13 strains exhibited >84% egg hatch inhibition at 3 mg powder mL−1. The three strains that were <80% effective against egg hatching included 251 143 (78%), ‘Bruce’ (70%) and 306 182 (34%). Five of the top performing strains were commercial varieties; therefore, ‘Bruce’ was also assayed so that all current commercial varieties would be tested. Across the 13 aqueous extracts tested, efficacy ranged from 0 to 75% exsheathment inhibition, and 17 to 92% L3 motility inhibition at a concentration of 25 mg mL−1 BFT-AqE (Table 3). There was a significant correlation between L3 motility and exsheathment inhibition at 1.5 mg powder mL−1 (R 2 = 0.46, P = 0.01; Fig. 1F). There was no correlation between the PAC content of the BFT powder and the anti-parasitic effects of the BFT-AqE extracts against L3 motility (R 2 = 0.01, P = 0.7; Fig. 1D) or exsheathment (R 2 = 0.11, P = 0.27; Fig. 1E) at 25 mg powder mL−1 BFT-AqE. The strains ranked lowest for egg hatch inhibition (‘Bruce’, 306 182 and 251 143) were also ranked lowest for exsheathment inhibition. ‘Bruce’ aqueous extract had the lowest per cent exsheathment inhibition (0%), followed closely by strain 306 182, with 3% exsheathment inhibition. ‘Bruce’ aqueous extract also exhibited 17% L3 motility inhibition, while strains 306 182 and 251 143 exhibited 28 and 30% L3 motility inhibition, respectively.
Table 3. Aqueous extracts of the top 13 BFT strains and purified BFT-PAC extract tested against Haemonchus contortus L3 motility and exsheathment inhibition

Third stage larvae (L3) were exposed to BFT-AqE (top ranked strains) and BFT-PAC (‘Bruce’ strain), negative controls (water, 0.0 mg mL−1), and positive controls (TBZ, 0.5 µg mL−1) for 24 h. Motility inhibition of larvae was determined (non-motile for 5 s) and then L3 were exposed to CO2 bubbling and incubated for another 18 h. All L3 were determined motile or non-motile, and motile larvae were determined sheathed or exsheathed. All values are mean ± s.e.m., and have been adjusted to negative control.
a Commercially available in the USA in 2017.
*P ⩽ 0.001 vs negative control (0.0 mg mL−1).
The BFT-PAC (‘Bruce’ strain) that was screened, exhibited 4% L3 motility inhibition and 100% exsheathment inhibition at a concentration of 6 mg PAC mL−1 (Table 3). Compared with the crude ‘Bruce’ BFT-AqE extract, which at 25 mg powder mL−1 exhibited 0% exsheathment inhibition, the purified BFT-PAC extract, at only 6 mg mL−1 exhibited 100% exsheathment inhibition, and 77% exsheathment inhibition at the lowest concentration of 0.75 mg mL−1 BFT-PAC (Table 4). As for L3 motility inhibition, neither extract exhibited high efficacy. The Bruce-AqE (25 mg powder mL−1) exhibited 17% L3 motility inhibition, while the BFT-PAC (6 mg PAC mL−1) exhibited 4% L3 motility inhibition.
Table 4. Purified BFT-PAC ‘Bruce’ variety against Haemonchus contortus egg hatching, L1 and L3 survivability, and exsheathment.

a Eggs were exposed to BFT-PAC, negative control (water, 0.0 mg mL−1) and positive control (TBZ, 0.05 µg mL−1) for 24 h. Egg hatch inhibition was determined and motility inhibition of resulting L1 larvae was determined (non-motile for 5 s).
b Third stage larvae (L3) were exposed to BFT-PAC ‘Bruce’ strain for 24 h and then bubbled with CO2 and incubated for another 18 h. All L3 were determined motile or non-motile and of the L3 that were motile, they were determined to be exsheathed or ensheathed. All values are mean ± s.e.m..
*P ⩽ 0.05 vs negative control (0.0 mg mL−1) within row
Discussion
This study evaluated the potential anti-parasitic effect of 51 strains of BFT by in vitro screening against H. contortus egg hatch, L1 and L3 motility, and L3 exsheathment. It is evident from our findings that plant secondary compounds play a key role in the observed anti-parasitic effects, but that PAC may not be the only deciding factor. The widespread variability in anti-parasitic effects observed across the 51 BFT strains has also been observed in studies of other plant aqueous extracts (Acharya et al., Reference Acharya, Hildreth and Reese2014). Twenty-one of the 51 aqueous extracts had an EC50 of 1–2 mg powder mL−1, comparable to previous reports of BFT egg hatch inhibition of PAC (<76% eggs hatched) at concentrations <1 mg PAC mL−1 (Molan et al., Reference Molan, Waghorn and McNabb2002; Molan and Faraj, Reference Molan and Faraj2010). Although previously reported PAC concentrations cannot be directly compared with the aqueous extract concentrations, these numbers potentially indicate that similar levels of bioactivity are observed in much lower concentrations of water-soluble PAC in the aqueous extracts.
Efficacy was compared of the 13 varieties in both the egg hatch and L1 motility inhibition assay and the L3 motility and exsheathment inhibition assay. Although the bottom ranked strains remained poor performers in both assays, the 10 strains that were >84% effective at 3 mg powder mL−1 against egg hatching, exhibited additional variation when screened for L3 motility and exsheathment inhibition. The activity observed against egg hatching and L1 motility was not reflective of how those same extracts performed against L3 larvae and exsheathment, supporting the hypothesis that anti-parasitic activity can differ depending on the developmental stage of the parasite (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006). The mechanism of action behind how secondary compounds negatively affect H. contortus at different life stages remains unknown and might provide explanation for the differences in efficacy observed in this study, against egg hatching, larval stages and exsheathment.
The anti-parasitic diversity observed across the 51 strains of BFT, combined with the finding that the anti-parasitic activity of the aqueous extracts did not correlate to the PAC content of the original plant material, suggests a potential role of other secondary compounds. The aqueous extraction may not have extracted all the available PAC in the plant matter, but the amount of PAC should have been proportionally extracted, as cell membrane disruption during the grinding process was the same for all strains. Other secondary compounds might have also been extracted, contributing to the anti-parasitic effects observed. This has been suggested of similar crude aqueous extracts showing anti-parasitic effects against H. contortus, attributing this activity to alkaloids, saponins (Adamu et al., Reference Adamu, Oshadu and Ogbaje2010) and phenolic compounds (Ferreira et al., Reference Ferreira, Castro, Chagas, Franca and Beleboni2013). Other investigators have concluded that PAC concentration is not necessarily related to anti-parasitic activity, as other secondary compounds were found to exert anti-parasitic effects (Mengistu et al., Reference Mengistu, Hoste, Karonen, Salminen, Hendriks and Pellikaan2017).
Additionally, aside from PAC content and other secondary compounds, the structure of the PAC between BFT strains could potentially influence the level of bioactivity that a specific strain exhibits. The structural features of PAC have proven to play a key role in the anti-parasitic activity against GIN through studies comparing PAC from different plants species (Quijada et al., Reference Quijada, Fryganas, Ropiak, Ramsay, Mueller-Harvey and Hoste2015). Differences in PAC structure of BFT strains (Hedqvist et al., Reference Hedqvist, Mueller-Harvey, Reed, Krueger and Murphy2000) has been reported and may have the potential to correlate to anti-parasitic effects and contribute to our understanding of the mechanism of action of PAC against GIN parasites. Unfortunately, an extensive phytochemical analysis of the aqueous extracts was beyond the scope and resources of the current study. Determining the composition of the aqueous extracts could provide additional support for the anti-parasitic effects observed in this study. Our laboratory has previously investigated a cranberry vine aqueous extract (Barone et al., Reference Barone, Zajac, Manzi-Smith, Howell, Reed, Krueger and Petersson2018), prepared in the same way, and showed that based on the amount of PAC present in the freeze-dried plant material (108 mg PAC g−1 powder), and the amount of PAC present in 30 mL of the aqueous extract (4.8 mg PAC mL−1), the efficiency of extraction was 12%. If a similar assumption on extraction efficiency was made for the BFT there might have been at least a weak correlation with anti-parasitic activity, which was not the case.
In addition to the crude aqueous extracts, one purified PAC extract was prepared for this study as a standard for PAC analysis and to compare the efficacy of the BFT-AqE to the efficacy of BFT-PAC. The commercial variety chosen, ‘Bruce’, was selected at the onset of the study because it contained the greatest concentration of PAC compared to the other five commercial varieties. This extraction of PAC differs from both the crude aqueous and the crude organic extraction methods because it isolates only PAC, excluding other impurities. Although this method does include an organic extraction process, it continues to purify the extract by elution through a Sephadex LH-20 column to separate the PAC from the other compounds. This purification of PAC has been used in some studies (Molan and Faraj, Reference Molan and Faraj2010), but many previous investigations have used crude organic extracts (Hernández-Villegas et al., Reference Hernández-Villegas, Borges-Argáez, Rodriguez-Vivas, Torres-Acosta, Méndez-Gonzalez and Cáceres-Farfan2011; Monteiro et al., Reference Monteiro, Bevilaqua, Morais, Machado, Camurça-Vasconcelos, Campello, Ribeiro and Mesquita2011).
The efficacy differences noted between the crude aqueous extract and the purified PAC extract once again suggests that there are other secondary compounds contributing to the bioactivity observed in the BFT-AqE extracts that could be inhibiting egg hatching, but having much less of an effect on exsheathment. Both extracts appeared to affect the motility of L1 larvae similarly, and both had slight deleterious effects on the L3 larvae, but BFT-PAC (‘Bruce’ strain) did not inhibit egg hatching while BFT-AqE (‘Bruce’ strain) did. The BFT-AqE (‘Bruce’ strain) did not inhibit exsheathment, while the efficacy of BFT-PAC (‘Bruce’ strain) against exsheathment was comparable to previous studies of exsheathment showing inhibition at concentrations of 1.2 mg mL−1 PAC (Brunet et al., Reference Brunet, Aufrere, El Babili, Fouraste and Hoste2007; Alonso-Díaz et al., Reference Alonso-Diaz, Torres-Acosta, Sandoval-Castro, Aguilar-Caballero and Hoste2008) and a dose-dependent response. In a study using purified PAC extracts (utilizing the Sephadex LH-20 column to isolate PAC), one of which was from Lotus corniculatus, against egg hatching of Teladorsagia circumcincta, egg hatch inhibition was observed in concentrations <1 mg mL−1 (Molan and Faraj, Reference Molan and Faraj2010). The current study used notably higher concentrations (6 mg PAC mL−1) and did not reach egg hatch inhibition values near what was observed by Molan and Faraj (Reference Molan and Faraj2010). Differences could be due to the difference in species of GIN tested and the strain of BFT used. However, because the extract should solely contain isolated PAC, this would suggest that the structure of the PAC is different between the two strains used at the same concentration.
It is commonly accepted that the structure of PAC, in terms of monomer units, the degree of polymerization, and interflavanoid linkages, affects the bioactivity of different PAC-containing forages (Foo et al., Reference Foo, Newman, Waghorn and McNabb1996; Molan and Faraj, Reference Molan and Faraj2010). PAC are highly complex and can be characterized by degree of polymerization, as either procyanidin (PC)-type tannins or prodelphinidin (PD)-type tannins. Studies have demonstrated that PD subunits are more potent than PC subunits (Desrues et al., Reference Desrues, Fryganas, Ropiak, Mueller-Harvey, Enemark and Thamsborg2016) regarding anti-parasitic activity against GIN egg hatching (Molan et al., Reference Molan, Meagher, Spencer and Sivakumaran2003) and L3 motility and exsheathment (Brunet and Hoste, Reference Brunet and Hoste2006). Chinese bushclover is well known for its high anti-parasitic activity against H. contortus and has an abnormally high level of PD-type tannins (Kommuru et al., Reference Kommuru, Barker, Desai, Burke, Ramsay, Mueller-Harvey, Miller, Mosjidis, Kamisetti and Terrill2014, Reference Kommuru, Whitley, Miller, Mosjidis, Burke, Gujja, Mechineni and Terrill2015). In contrast, although PD/PC ratios varied between BFT varieties, there is a higher proportion of PC-type tannins, which may explain the lack of anti-parasitic activity seen using PAC isolated from BFT (Hedqvist et al., Reference Hedqvist, Mueller-Harvey, Reed, Krueger and Murphy2000). Research led by the ‘LegumePlus’ project, on PAC-containing legumes such as Sainfoin (Onobrychis viciifolia), found extensive variability between the PAC content and composition of 27 varieties of Sainfoin (Malisch et al., Reference Malisch, Luscher, Baert, Engstrom, Studer, Fryganas, Suter, Mueller-Harvey and Salminen2015). Correlation of a structural characteristic found within PAC to high anti-parasitic effects could provide a technique that would determine the potential anti-parasitic effect of a plant species or variety based on PAC structure (Waterman and Mole, Reference Waterman and Mole1994; Kraus et al., Reference Kraus, Yu, Preston, Dahlgren and Zasoski2003; Mueller-Harvey, Reference Mueller-Harvey2006; Häring et al., Reference Häring, Suter, Amrhein and Luscher2007) and make it possible to breed strains specifically for anti-parasitic use.
Overall, our hypothesis that PAC concentration would be a good predictor of anti-parasitic activity was not supported by this study. Regardless of the PAC concentration of the original plant material, some aqueous extracts of BFT showed high anti-parasitic activity and others did not prove efficacious. Using isolated PAC extracts from other BFT strains could potentially differentiate between the role of other secondary compounds and structural differences. The in vitro anti-parasitic activity of the 51 BFT strains makes BFT a promising bioactive crop and warrants further investigation of BFT using bioactivity-driven fractionation and screening of BFT populations for the identified anti-parasitic compounds.
Supplementary materials
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018002214.
Author ORCIDs
Carly D. Barone 0000-0003-1008-6170
Acknowledgements
The authors of this study would like to thank Dr Rodrigo Feliciano for his training and expertise in DMAC analysis. The authors would also like to thank the many individuals who contributed to the harvesting, collection and freeze-drying of the BFT plant material used for this study.
Financial support
This work was supported by the USDA National Institute of Food and Agriculture, Animal Health project, accession number 1007290 and Hatch/Multi State project, accession number 1011343.
Conflict of interest
None.
Ethical standards
This study was conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Rhode Island.