INTRODUCTION
Chagas’ disease (CD), caused by the intracellular protozoan parasite Trypanosoma cruzi, is a neglected tropical disease, representing a serious health problem in Latin America. The available chemotherapy based on two nitroderivatives, benznidazol (Bz) and nifurtimox (Nf), is not satisfactory, especially during the later chronic phase of the disease (De Castro et al. Reference De Castro, Batista, Batista, Batista, Daliry, De Souza, Menna-Barreto, Oliveira, Salomão, Silva, Silva and Soeiro2011) and up to now, no therapeutic alternatives are available, which emphasizes the need to identify novel natural and synthetic anti-T. cruzi compounds.
Aromatic diamidines and analogues present broad-spectrum activity against pathogenic microorganisms, and some are in human and veterinary clinical use such as pentamidine (1,5-bis(4-amidino-phenoxy)pentane, Pentacarinat®-Rhodia) and diminazene aceturate (Bouteille et al. Reference Bouteille, Oukem, Bisser and Dumas2003; Wilson et al. Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008; Nyunt et al. Reference Nyunt, Hendrix, Bakshi, Kumar and Shapiro2009). Many of these compounds cause considerable side effects and require parenteral administration, however their excellent antimicrobial activity justifies the synthesis and screening of new analogues and pro-drugs in order to improve their therapeutic window (Ismail et al. Reference Ismail, Arafa, Wenzler, Brun, Tanious, Wilson and Boykin2008; Soeiro and De Castro, Reference Soeiro and De Castro2009). With respect to experimental T. cruzi infection, our group has demonstrated the trypanocidal efficacy of several amidine analogues, and among them, arylimidamides including DB889, DB766 and DB1831 showed remarkable in vitro as well as in vivo effects (Silva et al. Reference Silva, Batista, Mota, De Souza, Stephens, Som, Boykin and Soeiro2007, Reference Silva, Batista, Oliveira, De Souza, Hammer, Silva, Daliry, Araujo, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012; Batista et al. Reference Batista, Batista, Oliveira, Borges, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010; De Souza et al. Reference De Souza, Silva, Nefertiti, Ismail, Arafa, Tao, Nixon-Smith, Boykin and Soeiro2011).
In the present work, our aim was to screen the activity of 10 novel structurally related amidine compounds (DB2238, DB2239, DB2240, DB2242, DB2243, DB2246, DB2247, DB2248, DB2249 and DB2250) against different forms and strains of T. cruzi in vitro, as well as evaluate their potential cytotoxic effects upon uninfected cardiac cells.
MATERIALS AND METHODS
Compounds
The synthesis of the amidine compounds (DB2238, DB2239, DB2240, DB2242, DB2243, DB2246, DB2247, DB2248, DB2249 and DB2250) (Fig. 1) was performed as previously reported (Liu et al. Reference Liu, Chai, Kumar, Tidwell, Boykin and Wilson2012). Stock solutions were prepared in dimethyl sulfoxide (DMSO) with the final concentration of the solvent never exceeding 0·6%, which did not exert any toxicity towards the parasite or mammalian host cells (data not shown). Benznidazole (Bz) (Rochagan, Roche) was used as reference drug.
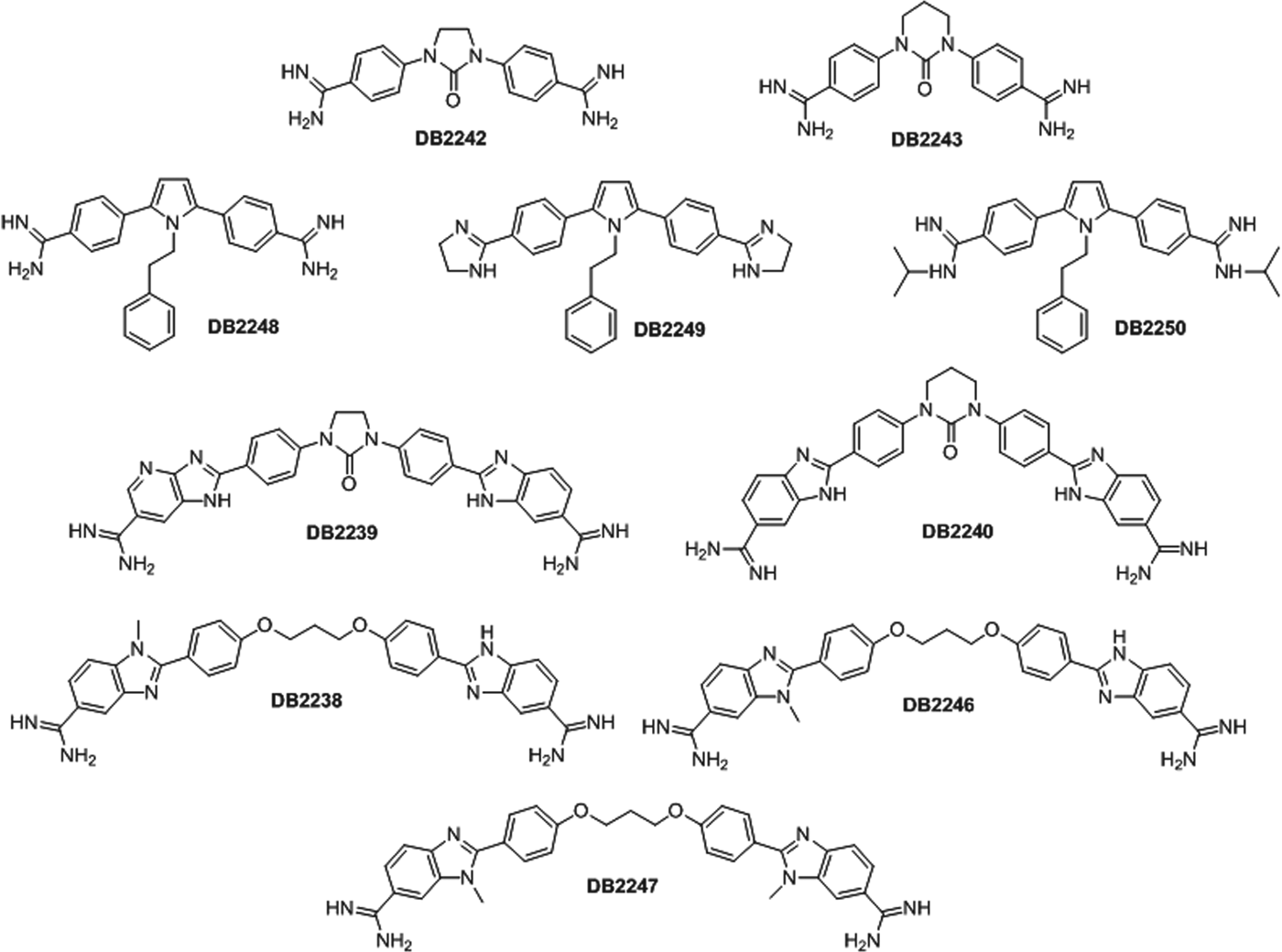
Fig. 1. Structures of the diamidine compounds.
Mammalian cell cultures
For the analysis of compound in vitro toxicity, primary cultures of embryonic cardiomyocytes (CM) were obtained from Swiss mice as previously reported (Meirelles et al. Reference Meirelles, Araujo-Jorge, Miranda, De Souza and Barbosa1986). After purification, the CM were seeded at a density of 0·05×106 cell well−1 into 96-well microplates containing gelatin-coated cover slips. The cultures were then sustained at 37 °C in Dulbecco's modified medium (DMEM) supplemented with 10% horse serum, 5% foetal bovine serum, 2·5 mm CaCl2, 1 mm L-glutamine and 2% chicken embryo extract. For the analysis of the effect on intracellular parasites, monolayers of mouse L929 fibroblasts were cultivated (4×103 cell well−1 into 96-well microplates) at 37 °C in RPMI-1640 medium (pH 7·2–7·4) without phenol red (Gibco BRL) supplemented with 10% foetal bovine serum and 2 mm glutamine (RPMIS), as previously reported (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Parasites
Bloodstream trypomastigote forms (BT) of the Y strain were obtained from the blood samples of infected albino Swiss mice at the peak of parasitaemia. The purified parasites were resuspended in Eagle medium modified by Dulbecco (DME) supplemented with 10% bovine foetal calf serum (FCS) (DMES) as reported (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). Intracellular forms were obtained by the infection of L929 cell lineages with tissue culture derived trypomastigotes (Tulahuen strain expressing the Escherichia coli β-galactosidase gene) (Buckner et al. Reference Buckner, Verlinde, La Flamme and Van Voorhis1996) at 1:10 ratio, respectively, following a previously established protocol (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). Briefly, after 2 h of host cell: parasite interaction, the cultures were washed to remove non-interiorized parasites and 200 μL of fresh RPMIS added and the incubation proceeded for 48 h/37 °C to establish infection.
Cytotoxicity tests
For these experiments, CM were incubated for 24 h at 37 °C with different concentrations of each compound (up to 96 μ m) diluted in RPMIS; their morphology and spontaneous contractibility were then evaluated by light microscopy and their cellular viability (LC50 = concentration that reduces by 50% the cellular viability) determined by the AlamarBlue test following the manufacturer's instructions (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Trypanocidal analysis
Bloodstream trypomastigotes of the Y strain (5×106 mL−1) were incubated for 2 and 24 h at 37 °C in RPMI in the presence or absence of serial dilutions of the compounds (0 to 32 μ m). After compound incubation, the parasite death rates were determined by light microscopy through the direct quantification of the number of live parasites using a Neubauer chamber, and the EC50 (compound concentration that reduces by 50% the number of parasites) was then calculated.
For the assay on intracellular forms, T. cruzi infected-cell cultures (Tulahuen strain expressing β-galactosidase) were incubated for 96 h at 37 °C in RPMIS in the presence or absence of serial dilutions of the compounds (0 to 32 μ m). After this period, 500 μ m chlorophenol red glycoside in 0·5% Nonidet P40 was added to each well; the plate was incubated for 18 h at 37 °C, then the absorbance was measured at 570 nm. Controls with uninfected cells and infected cells both treated only with vehicle and/or with BZ were run in parallel. The EC50 was calculated based on the percentage of T. cruzi growth inhibition by comparing the optical density (O.D.) data of the tested compounds with those obtained from those infected cell cultures exposed only to vehicle. (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Triplicate assays were run in the same plate and at least two assays performed in each analysis and all procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA 0028/09).
RESULTS
Our first analysis was to verify the effect of all novel amidines against bloodstream forms (Y strain). The findings demonstrated that after short exposure (2 h of incubation), 8 out of the 10 amidines gave a greater effect compared with the reference drug (Bz), exhibiting EC50 values lower than 66 μ m (Table 1). Among them, DB2247, DB2246, DB2240 and DB2249 were the most active, exhibiting EC50 values of 3·0, 14·5, 14·3 and 18·9 μ m (Table 1). After 24 h of incubation at 37 °C, seven compounds (DB2239, DB2240, DB2246, DB2247, DB2248, DB2249 and DB2250) displayed superior effects to that of Bz; the diamidino bis-benzimidazole DB2247 exhibited the greatest parasiticidal effect (EC50 = 2·4±0·04 μ m) (Table 1). To further explore the activity of these novel amidines against intracellular forms of the parasite using non-toxic concentrations towards mammalian host cells, in vitro analysis of cytotoxicity on cardiac cell cultures were performed by light microscopy and through colorimetric cellular viability assays. The results showed that up to 96 μ m, none of the compounds studied cause significant loss of cardiac cell viability as evaluated by an Alamar blue assay. The amidine that displayed the highest selectivity index was DB 2247, giving a SI >40 (Table 1). However, when these novel compounds were tested on T. cruzi-infected host cells (Tulahuen strain transfected with β-galactosidase gene), none presented superior trypanocidal activity against the intracellular forms as compared with Bz: the activity of these amidines ranged from 11·4 to >32 μ m while the reference drug showed an EC50 value of 2·6±0·9 μ m (Table 1).
Table 1. Activity of the diamidines on bloodstream trypomastigotes and intracellular forms of T. cruzi (Y strain), cytotoxicity to cardiac cell cultures and selectivity index (SI)
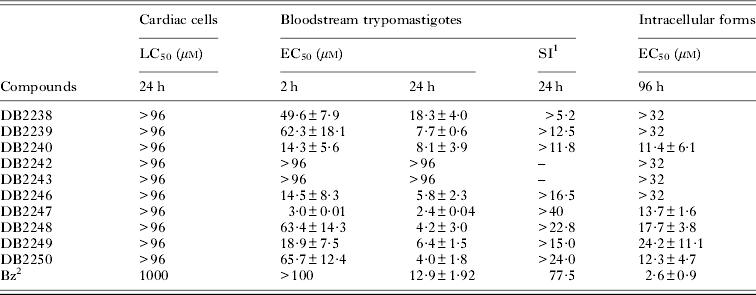
1 Selectivity index (SI) = LC50/EC50 for 24 h.
DISCUSSION
Despite the large number of new classes of compounds tested, many of them based on genomics and target-derived strategies for screening purposes, up to now, no therapeutic alternatives to benznidazole are available to treat Chagas’ disease (Soeiro et al. Reference Soeiro, Werbovetz, Boykin, Wilson, Wang and Hemphill2013). This fact may be due to the low investment for research and technical development in this area, but also, in part, due to the lack of translation from the target-based effects to whole-cell assays or to in vivo activities. Also, it is important to consider that even though medium- to high-throughput screenings of large compound libraries help identify hit compounds, in vitro assays directly performed on whole cells associated with suitable in vivo models are fundamental to better characterization of the biological activity of promising new molecules (Don and Ioset, Reference Don and Ioset2013). Herein, 10 novel amidino compounds were assayed directly against the two relevant parasite forms for the infection of mammalian hosts by T. cruzi: bloodstream trypomastigotes and intracellular parasites (Romanha et al. Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). In addition, we further evaluated in vitro toxicity aspects using primary cultures of cardiac cells, which represent an excellent experimental model since heart damage is one of the main manifestations of chronic disease, representing a target tissue for both parasite infection and triggered inflammation (Henriques-Pons and Gomes, Reference Henriques-Pons, Gomes, Milei and Ambrosio2013).
The amidines may be divided into two groups, the smaller benzamidines (DB2242, DB2243, DB2248, DB2249 and DB2250) and the larger benzimidazole amidines (DB2238, DB2239, DB2240, DB2246 and DB2247). The two benzamidines with cyclic urea linkers (DB2242, DB2243) are inactive against both parasite forms and the three with pyrrole ring linkers (DB2248, DB2249 and DB2250) show moderate activity with EC50 values ranging from 4·0–6·4 μ m and 12·4 and 24·2 μ m for bloodstream and intracellular forms, respectively (see Table 1). Upon bloodstream trypomastigotes, the larger benzimidazole amidines, with the exception of DB2238, show EC50 values between 2·4 and 8·1 μ m. It is interesting that the two isomeric benzimidazole amidines (DB2238 and DB2246) show a 3-fold difference in activity towards trypomastigotes; the one with the N-methyl group meta to the amidine group (DB2246) being the most active. The most active compound (DB2247), with both terminal rings with N-methyl groups meta to the amidine groups with an EC50 value of 2·4 and 13·7 μ m for bloodstream and intracellular forms, respectively, is at least 2·3-fold more active than its mono-N-methyl analogue DB2246. This result suggests that the meta amidine-N-methyl group relationship is important for activity in the benzimidazole series and that this array should be explored in other systems.
Although DB2247 is at least 6-fold more effective than benznidazole against bloodstream trypomastigotes, it is less active on intracellular forms as compared with this reference drug (Table 1). Against bloodstream forms, DB 2247 is also at least 30 times faster acting than Bz achieving an EC50 value of 3 μ m after a short incubation time (2 h). Faster action is a desirable characteristic for acute situations such as T. cruzi reactivation due to immunosuppressed conditions. The reduced cell toxicity of DB2247 represents another favourable characteristic for a hit molecule for Chagas’ disease. In vitro analysis by both light microscopy and AlamarBlue assay showed that up to 96 μ m none of the tested compounds induced loss of cellular viability towards cardiac cells, giving a high selectivity index for DB2247 when bloodstream parasites were assayed (SI>40). When tested against intracellular forms located in mammalian host cells, none of the amidines presented an efficacy approaching that of benznidazole (Table 1). The differences in susceptibility profiles between trypomastigotes and amastigotes may reflect (a) distinct uptake/extrusion mechanisms and/or (b) different cellular targets of the non-dividing flagellated form and the multiplicative intracellular stage hence multiple modes of action may be involved and (c) the use of different strains (Y for bloodstream and Tulahuen for intracellular forms) (Nyunt et al. Reference Nyunt, Hendrix, Bakshi, Kumar and Shapiro2009; Soeiro et al. Reference Soeiro, Werbovetz, Boykin, Wilson, Wang and Hemphill2013). The last hypothesis is less probable since previous studies using other aromatic amidines tested against the same strain of T. cruzi (Y strain) also demonstrated differences in susceptibility of some diamidines between intracellular and bloodstream forms, the latter parasites being more sensitive than the amastigotes (Batista et al. Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeiro2009; Pacheco et al. Reference Pacheco, Silva, De Souza, Batista, Silva, Kumar, Stephens, Boykin and Soeiro2009; De Souza et al. Reference De Souza, Silva, Nefertiti, Ismail, Arafa, Tao, Nixon-Smith, Boykin and Soeiro2011) as presently observed.
It will be important to learn more about the molecular characteristics of diamidines required to achieve efficacy against both trypomastigotes and amastigotes. Our data provide useful knowledge for the future design of novel amidine compounds with improved activity against amastigotes while retaining their low mammalian cell toxicity profile. Such information should aid in the design of novel anti-T. cruzi agents which are more active and selective than the current clinical drugs.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Dr Solange Lisboa de Castro (LBC/IOC/Fiocruz) for careful review.
FINANCIAL SUPPORT
The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro, Conselho Nacional Desenvolvimento científico e Tecnológico and Fundação Oswaldo Cruz, PROEP/CNPq/Fiocruz, PDTIS and CAPES. Support was received from The Bill and Melinda Gates Foundation through a subcontract with the Consortium for Parasitic Drug Development (D.W.B.).





