INTRODUCTION
Liver flukes, Fasciola gigantica, are one of the most important trematode species and have been recognized as significant plant-borne parasitic zoonosis. These parasites are an important cause of fasciolosis that infects both domestic and wild animals in the tropical regions of Asia and Africa. Fasciolosis causes worldwide economic losses more than US$3.2 billion per annum from its effect on the livestock industry (Torgerson and Claxton, Reference Torgerson, Claxton and Dalton1999; Mas-Coma et al. Reference Mas-Coma, Valero and Bargues2009, Reference Mas-Coma, Bargues and Valero2014a ). In addition, human fasciolosis is recognized by the World Health Organization (WHO) as an emerging serious human health problem, and has been reported on numerous countries. It has been estimated that at least 2·4 million up to 17 million people is infected and more than 90 million people are at the risk of infection (Mas-Coma et al. Reference Mas-Coma, Bargues, Valero and Bruschi2014b ). In Thailand, the prevalence rates of fasciolosis in cattle and water buffaloes are estimated to be 52·94–67·27%, with the highest incidences in the North and North-east, and the lowest in the South. It has been reported that the Thailand annual economic loss due to fasciolosis in cattle and buffaloes is estimated around 350–400 million baht (US$10–11 million) owing to a lower nutrition conversion, a decrease in milk production and a reduction in fertility of the animals (Srihakim and Pholpark, Reference Srihakim and Pholpark1991; Sukhapesna et al. Reference Sukhapesna, Tantasuvan, Sarataphan and Imsup1994; Anuracpreeda et al. Reference Anuracpreeda, Wanichanon, Chawengkirtikul, Chaithirayanon and Sobhon2009a , Reference Anuracpreeda, Songkoomkrong, Sethadavit, Chotwiwatthanakun, Tinikul and Sobhon2011, Reference Anuracpreeda, Tepsupornkul and Chawengkirttikul2017a ; Phalee and Wongsawad, Reference Phalee and Wongsawad2014).
Up to now, there is no vaccine available for the prevention of fasciolosis, and thus a repertoire of drugs remains the most effective mean for treatment and control of the animals in endemic areas (McManus and Dalton, Reference McManus and Dalton2006; Anuracpreeda et al. Reference Anuracpreeda, Chawengkirttikul and Sobhon2016a ). Triclabendazole (TCZ), the most common drug of choice, has been reported to be efficient against Fasciola sp. However, the flukes’ resistance to this drug is increasing since 1995 (Overend and Bowen, Reference Overend and Bowen1995; Fairweather and Boray, Reference Fairweather and Boray1999; Moll et al. Reference Moll, Gaasenbeek, Vellema and Borgsteede2000; Fairweather, Reference Fairweather2005; Keiser et al. Reference Keiser, Engels, Büscher and Utzinger2005). In view of the cost and the resistance of liver flukes to the action of drugs, new anthelmintic drugs that can kill the flukes are urgently needed. Interestingly, a number of different plant extracts have been tested for their anthelmintic activity (Akhtar and Riffat, Reference Akhtar and Riffat1991; Tandon et al. Reference Tandon, Pal, Roy, Rao and Reddy1997; Athanasiadou et al. Reference Athanasiadou, Githiori and Kyriazakis2007; Aswar et al. Reference Aswar, Aswar, Watkar, Vyas, Wagh and Gujar2008; Magalhaes et al. Reference Magalhaes, Kapadia, da Silva Tonuci, Caixeta, Parreira, Rodrigues and Da Silva Filho2010; Mehlhorn et al. Reference Mehlhorn, Quraishy, Rasheid, Jatzlau and Ghaffar2011; Hossain et al. Reference Hossain, Chandra, Nandy, Mandal and Gupta2012).
Terminalia catappa L. is a medicinal herb species of the family Combretaceae and commonly known in Thailand as ‘Hu-kwang’. This plant is naturally widespread in tropical and subtropical beach areas. The different parts (leaves, bark and fruits) of T. catappa have been used for the treatment of several symptoms and diseases, i.e. fever, diarrhoea, dermatitis, hepatoma, lung cancer, diabetes mellitus and gastric infection (Lin et al. Reference Lin, Chen, Lin and Ujiie1997; Chen et al. Reference Chen, Li, Liu and Lin2000; Tanaka et al. Reference Tanaka, Nonaka, Ishimatsu, Nishioka and Kouno2001; Fyhrquist et al. Reference Fyhrquist, Mwasumbi, Haeggström, Vuorela, Hiltunen and Vuorela2002; Nagappa et al. Reference Nagappa, Thakurdesai, Venkat Rao and Singh2003; Tang et al. Reference Tang, Gao, Wang, Xu, Zhao and Xu2003; Gao et al. Reference Gao, Dou, Tang, Xu, Fan and Zhao2004; Chu et al. Reference Chu, Yang, Liu, Kuo, Chang and Hsieh2007; Nunes et al. Reference Nunes, Viana, Brito Junior, Rabelo, Nunes Filho, Nunes and Martins2012; Yeh et al. Reference Yeh, Hsieh, Hsieh, Chien, Lin, Chiou and Yang2012; Germosén-Robineau, Reference Germosén-Robineau2014; Kumar et al. Reference Kumar, Kumari, RAjeshwar, Umadevi and Kotla2014). In our previous study, the crude extract of T. catappa leaves (TcCE) has shown the anthelmintic activities against the ruminant gut fluke, Fischoederius cobboldi (Anuracpreeda et al. Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ). In the present work, we have investigated the in vitro anthelmintic activity of the ethanol extract from T. catappa leaves on motility, survival and tegumental surface of adult F. gigantica by using relative motility (RM) assay and observation by light microscopy (LM) and scanning electron microscopy (SEM). Examination of tegumental surface alterations is important because the tegument is the interface with the hostile environment of the host, and thus it is the principal route by which the ethanol extract of T. catappa could damage the parasite through its action on the tegument as one of the primary targets.
MATERIALS AND METHODS
Collection of plant materials
Terminalia catappa L. leaves were collected from a local area of Songkhla Province, Thailand. The plant samples were identified and authenticated by Dr Panupong Puttarak, a curator at the herbarium of Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla, Thailand. Voucher specimen numbers of T. catappa were SKP 049 20 03 10, and they were deposited to the herbarium at Department of Pharmacognosy and Pharmaceutical Botany, Prince of Songkhla University, for future reference. The plant name has been checked and corresponded to the latest revision of ‘The Plant List’ (http://www.theplantlist.org).
Preparation of crude extracts
The crude extracts were obtained from the leaves of T. catappa following the method of Anuracpreeda et al. (Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ). Briefly, the dried leaves were powdered (985 g) and macerated with 6000 mL of ethanol at room temperature for 2 days. After filtration, each extract was pooled and evaporated to concentrate under reduced pressure in 22–26 mmHg at 45 °C by rotary evaporator. Finally, the crude extracts (63·8 g) were stored at −20 °C in a refrigerator protected from light until further use.
Assay methods for the anthelmintic activities
Collection of adult parasites
As per the method described by Anuracpreeda et al. (Reference Anuracpreeda, Chawengkirttikul and Sobhon2016b ). Adult F. gigantica were removed from the liver and bile duct of infected cattle and water buffaloes killed for consumption at local abattoirs in Pathumthani Province, Thailand. They were washed and cleaned several times with Hank's balance salt (HBS) solution containing 100 U mL−1 penicillin and 100 µg mL−1 streptomycin. Only intact and actively flukes were selected and immediately used for this study.
Crude extract, drug and media
Crude extract, drug and media were prepared according to the method of Anuracpreeda et al. (Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ). Briefly, the crude extract of T. catappa (TcCE) was dissolved in 0·1% (v/v) dimethyl sulphoxide (DMSO) (Sigma Co., St. Louis, MO), yielding a concentration of 500 mg mL−1. The sterile RPMI-1640 culture medium (Gibco) [pH 7·4 with HEPES 20 mm, supplement with penicillin (50 IU mL−1), streptomycin (50 µg mL−1), gentamycin (50 µg mL−1) and 20% fetal bovine serum (FBS)] was mixed with the crude extract stock solution to obtain the concentrations at 100, 200, 400, 800 and 1000 µg mL−1 of TcCE in the medium. Triclabendazole (TCZ) was purchased from Sigma Chemical (St, Louis, MO, USA). TCZ at a concentration 100 µg mL−1 in RPMI-1640 medium containing antibiotics and FBS was prepared from the stock solution, and used as the positive control in this study. The medium containing antibiotics and 0·1% (v/v) DMSO without TcCE was used as the negative control.
In vitro culture determination of anthelmintic activity
In a laminar flow cabinet, 1050 adult flukes collected as above were randomly allocated to seven groups (150 flukes per group) and incubated in tissue culture dishes (Nunc, Sigma-Aldrich). Group 1 was the negative control with the worms incubated in the RPMI-1640 medium containing antibiotics and 0·1% (v/v) DMSO without TcCE. Group 2 was the positive control with the worms incubated in the medium contained TCZ at 100 µg mL−1. Groups 3–7 were treatment groups incubated in the medium with TcCE at 100, 200, 400, 800 and 1000 µg mL−1. Three replicates were used for each group. The flukes in culture medium were incubated for 1, 3, 6, 12 and 24 h (30 flukes per incubation time) in an incubator with 5% CO2 for 24 h at 37 °C. After incubation, the flukes were categorized and counted as complete inactiveness or paralysis (death) and still moving (alive) after stimulating by vibration, and the tegumental alterations were examined under LM and SEM.
Anthelmintic evaluation criteria
Motility criteria
A stereomicroscope was used to evaluate the motility of worms. The motility of worms at each incubation period was assessed as per the following criteria proposed by Kiuchi et al. (Reference Kiuchi, Miyashita, Tsuda, Kondo and Yoshimura1987). The worms with immobility were stained in 1% (w/v) methylene blue diluted in 0·85% (w/v) NaCl for 3 min. The scoring system was as follows: 3 (complete whole body movement), 2 (partial body movement), 1 (immobile but alive, unstained with 1% methylene blue) and 0 (immobile and dead, stained with 1% methylene blue). The RM value was calculated using the formula listed below (Kiuchi et al. Reference Kiuchi, Miyashita, Tsuda, Kondo and Yoshimura1987). The smaller RM values revealed stronger drug activity, and when all worms died these values were 0.
n, motility score; N, number of worms with the score of ‘n’.
Survival index (SI)
Survival indices were evaluated by examining the parasites under a stereomicroscope, and denoted the percentages of live worms at a given time after treatment. The scoring system was as follows: score 3, 2, 1 = still alive and score 0 = dead (immobile and stained with 1% methylene blue).
Examination of tegumental alterations by LM
For LM examination, parasite specimens were performed according to the method described by Anuracpreeda et al. (Reference Anuracpreeda, Srirakam, Pandonlan, Changklungmoa, Chotwiwatthanakun, Tinikul, Poljaroen, Meemon and Sobhon2014, Reference Anuracpreeda, Watthanadirek, Chawengkirttikul and Sobhon2017b ). Briefly, flukes taken from negative control, TCZ- and TcCE-treated groups were cut and fixed in Bouin's fixative solution at 4 °C for overnight. Then, they were washed several times with 0·01 M phosphate-buffered saline (PBS), pH 7·4 for 15 min each. The tissue blocks were dehydrated with a graded series of ethanol concentrations (from 30 to 100%), cleared with xylene and embedded in paraffin. Subsequently, the sections were cut at 6 µm-thickness using the Leica RM2125 microtome, stained with hematoxylin and eosin, observed and photographed under the light microscope, Olympus BX51.
Examination of tegumental alterations by SEM
According to Anuracpreeda et al. (Reference Anuracpreeda, Phutong, Ngamniyom, Panyarachun and Sobhon2015) flukes were collected from negative control, TCZ- and TcCE-treated groups at each incubation times of the drug concentrations. The parasite specimens were cut and fixed in 2·5% glutaraldehyde in 0·1 M sodium cacodylate buffer (Sigma-Aldrich, USA) containing calcium acetate, pH 7·2, at 4 °C for 2 h. Then, they were washed three times for 15 min each with the same buffer, re-fixed in 1% OsO4 (Sigma-Aldrich) with 0·1 M sodium cacodylate buffer (pH 7·2) at 4 °C for 1 h, and washed again with distilled water three times for 15 min each. Thereafter, they were dehydrated through serial of ethanol concentrations (from 50 to 100%) for 30 min each, and then dried with liquid CO2 in a HCP-2 critical point dryer apparatus (Hitachi, Japan) for 15 min. Subsequently, the dried specimens were mounted on aluminium microscopy stubs and coated with gold particles using an ion-sputtering apparatus (SPI-Model sputter coater; Structure Probe, USA) with a setting at 10–15 mA for 4 min. Finally, they were observed and photographed using a JSM-5400 electron microscope (JEOL, USA), operating at an electron accelerating voltage of 15 kV.
RESULTS
Motility and survival observations
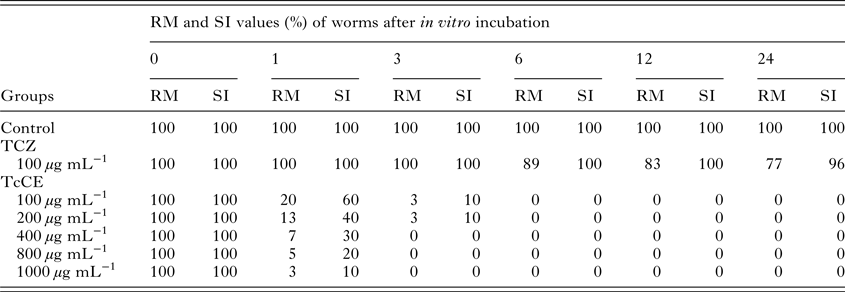
Table 1 showed the motility and survival scores of F. gigantica. During throughout the duration of the experiment (1, 3, 6, 12 and 24 h), all liver flukes in the negative control group displayed active movement throughout the whole body (RM = 100, SI = 100). In the group treated with 100 µg mL−1 of TCZ (Positive control group), flukes showed 23% reduction of RM values (RM = 77), and 4% of them were completely immobile and dead (SI = 96) at 24 h incubation. According to the study, maximum efficacy was observed for TcCE at 400, 800 and 1000 µg mL−1 where all flukes (100%) ceased to be mobile, and most of them took up the vital dye which indicated that they were killed and dead at 3 h incubation (RM = 0, SI = 0).
Table 1. RM and SI values for control and worms treated with triclabendazole (TCZ) and T. catappa crude extract (TcCE) at different hours after incubation
Alterations of tegumental surface as observed by LM
At 24 h post incubation, all negative control parasites appeared normal composition of the tegument which was joined by the tegumental cells’ processes. The spines, muscles and basement membrane also showed normal appearance (Fig. 1A). In the positive control flukes which were treated with 100 µg mL−1 of TCZ, there were some vacuoles, small blebbing and disrupted blebs on the tegument, while the underlying structures still displayed normal appearance at 24 h incubation (Fig. 1B). On the contrary, histopathological changes in the tegumental layer, spines and underlying structures were observed in all worms treated with TcCE after 1 h incubation, the severity increasing with longer incubation times when compared with both negative and positive control groups. After 1 h incubation with 100 and 200 µg mL−1 of TcCE, numerous vacuoles, blebbings and partial disruption on the tegumental surface were observed. These blebs were disrupted and some spines were lost, resulted in the thinning of tegument at 3 h incubation, but the underlying structures still appeared intact (Fig. 1C). At 3 h incubation in 400 µg mL−1 of TcCE, the tegument was partly disrupted and became much thinner and some spines stripped off, while underlying muscles appeared less severe morphological alteration (Fig. 1D). In addition, TcCE at 800 and 1000 µg mL−1 at 3 h incubation caused extensive destruction of the tegument and spines, while the underneath muscle layers and parenchymal cells were less damaged (Fig. 1E and F). These data indicated that the most affected target organ from the treatment with TcCE is the tegument.

Fig. 1. LM micrographs showing the histology of untreated (A), TCZ-treated (B) and TcCE-treated adult F. gigantica (D–F). (A) The negative control of a cross section of fluke incubated in RPMI-1640 medium containing 0·1% DMSO for 24 h, showing tegument (Tg) with normal appearances, i.e. spine (Sp) embedded in the undamaged tegument and muscle (Mu) lying underneath the basement membrane (Ba). (B) The positive control section of fluke treated with TCZ, at concentration 100 µg mL−1, at 24 h incubation, appearing some vacuoles (black arrow), formation of small blebs (Bl) and disrupted blebs (Db) on the tegument, while spine (Sp), muscle (Mu) and other structures underneath the basement membrane show normal. (C) In the fluke treated with TcCE, at concentration 100 µg mL−1, at 3 h incubation, showing numerous vacuoles (black arrow), blebs (Bl) and disrupted blebs (Db), while spine (Sp), muscle (Mu) and other structures underneath the basement membrane appear normal. (D) Fluke treated with 400 µg mL−1 TcCE for 3 h, showings disrupted tegument (Tg, black arrows), while spine (Sp), muscle (Mu) and other structures exhibit normal appearance. (E and F) After 3 h incubation with 800 µg mL−1 (E) and 1000 µg mL−1 (F) of TcCE, showing dislodged spines (DSp) and severe damage of the tegumental surface with extensive degeneration and sloughing (black arrows) from the basement membrane (Ba).
Alterations of tegumental surface as examined by SEM
For negative control group, all flukes exhibited normal appearance of the tegument with no damage of the oral and ventral suckers at 24 h of incubation in the RPMI-1640 medium containing 0·1% DMSO. Fluke had a flattened leaf-like shaped with tapered anterior and posterior regions. The ventral surface consisted of two suckers: the oral sucker is located at the anterior region, while the ventral sucker is positioned at one third of the body from the anterior edge. The genital pore is located ventrally near the ventral sucker (Fig. 2A). The tegument exhibited transverse folds alternating with grooves and covered with numerous spines. These spines had serrated edges and are more congregated on the cephalic cone and middle region, but reduced in number and size at the posterior and tail regions. There were groups of bulbous papillae, which are supposed to be sensory receptors on the surface (Fig. 2B). The dorsal side of the body appeared similar surface features as the ventral surface, but the spines and papillae had less numerous and were smaller than ventral surface (Fig. 2C).

Fig. 2. SEM images of the tegument of adult F. gigantica. Flukes were incubated in maintenance RPMI-1640 medium containing 0·1% DMSO (A–C) and exposed to 100 µg mL−1 of triclabendazole (TCZ) for 24 h (D–F). (A and B) Anterior, middle and posterior regions of the ventral surface of adult fluke. (A) The anterior region showing oral sucker (Os), ventral sucker (Vs), and opening of genital canal (Ge). (B) The surface of anterior region shows numerous clusters of papillae (pa), and rows of serrated spines (Sp) with highly corrugated surface. The surface between the spines exhibits a series of alternating folds (fo) and grooves (gr). (C) The dorsal surface area appears the surface of fold with small ridges (ri) separated by furrows (fu). (D) The posterior region appears swollen tegument (Sw) covered with blebs (Bl) and some disrupted blebs (Db). The spines (Sp) are also partially submerged in the swollen tegument. (E) A medium magnification micrograph of the anterior region exhibits mild swelling of the tegument with wide and deep grooves (Dg), and the spines (Sp) are partially submerged in the swollen tegument. (F) At medium magnification micrograph of the middle region, the spines (Sp) are partially submerged in the swollen tegument, with some detached, exposing empty sockets (white arrows). Disrupted blebs (Db) are also observed on this region.
For positive control group, the general features of the tegument of flukes treated with TCZ were similar to the negative control group at 24 h incubation. However, the spines appeared to be submerged by the swollen tegument between them (Fig. 2D), and some spines were dislodged from their sockets (Fig. 2F). Blebbing of the surface was observed and also some blebs were disrupted (Fig. 2D and F). Likewise, deep grooves were exhibited on the folds on the ventral and dorsal surfaces (Fig. 2E). These tegumental surface alterations affected the anterior and ventral regions more severely than the posterior and dorsal regions.
For TcCE group, the sequences of pathological alterations and damages in the tegument of adult F. gigantica after treatment with TcCE consisted of seven levels as followings: (1) swelling, (2) blebbing formation, (3) disruption of blebs, (4) spine submerging, (5) spine dislodging, (6) erosion and lesion and (7) total demolition of the tegument. Grading of the tegumental alterations and damages in the flukes during the course of incubation with TcCE is shown in Table 2. The TcCE at concentrations 100 and 200 µg mL−1 exhibited similar pattern of changes. After 1 h incubation, the ventral surface of the tegument showed swelling and deep grooves at the anterior region, particularly along the oral and ventral suckers (Fig. 3A and B), and middle region of the ventral surface (Fig. 3C). The spines in this region were submerged in the swollen tegument (Fig. 3B and C). Following 3 h incubation, there were numerous blebs on the posterior region and some blebs were ruptured (Fig. 3D). In addition, the spines were detached exposing empty sockets (Fig. 3D). On the dorsal surface of anterior and middle regions showed moderated swelling of tegumental ridges and folds, which were covered with small blebs, while posterior region appeared slightly swollen. At 6 h incubation, the tegument of treated flukes showed a great damage on the body. The damaged anterior and posterior regions of both ventral and dorsal surfaces were sloughed away and lesions were more marked throughout the ventral surface. Almost all of the dorsal surface also appeared complete lesion.

Fig. 3. SEM images of the adult F. gigantica exposed to 100 µg mL−1 (A–D) and 400 µg mL−1 (E and F) of TcCE. (A–E) Anterior, middle and posterior regions of the ventral surface of adult fluke. (A and B) One hour post incubation with 100 µg mL−1 of TcCE, the tegument on the anterior region and the rim of the oral sucker (Os) (A), and the ventral sucker (Vs) (B) are swollen (Sw). Spines submersion (Sp) are found in the swollen tegument (Sw) divided by deep grooves (Dg). (C) At 1 h incubation with 100 µg mL−1 of TcCE, the middle region shows severe swelling (Sw), with the spines (Sp) submerged in the swollen tegument. (D) Following 3 h examination time point, blebs (Bl), disrupted blebs (Db) and loss of spines with empty spine sockets (white arrows) appear at the posterior region. (E) One hour incubation in TcCE at 400 µg mL−1, the anterior region exhibits deep grooves on the swollen tegument. All spines become dislodged, leaving only spine sockets (white arrows). (F) Following 3 h post incubation, the dorsal surface of the anterior region of the tegument shows severe erosion (Er), which eventually becomes lesion (Le). Some spines are dislodged from their sockets (white arrows).
Table 2. Conclusions of the sequence of tegumental pathological alterations in adult F. gigantica after in vitro exposure to triclabendazole (TCZ) and T. catappa crude extract (TcCE) as investigated by scanning electron microscopy (SEM)
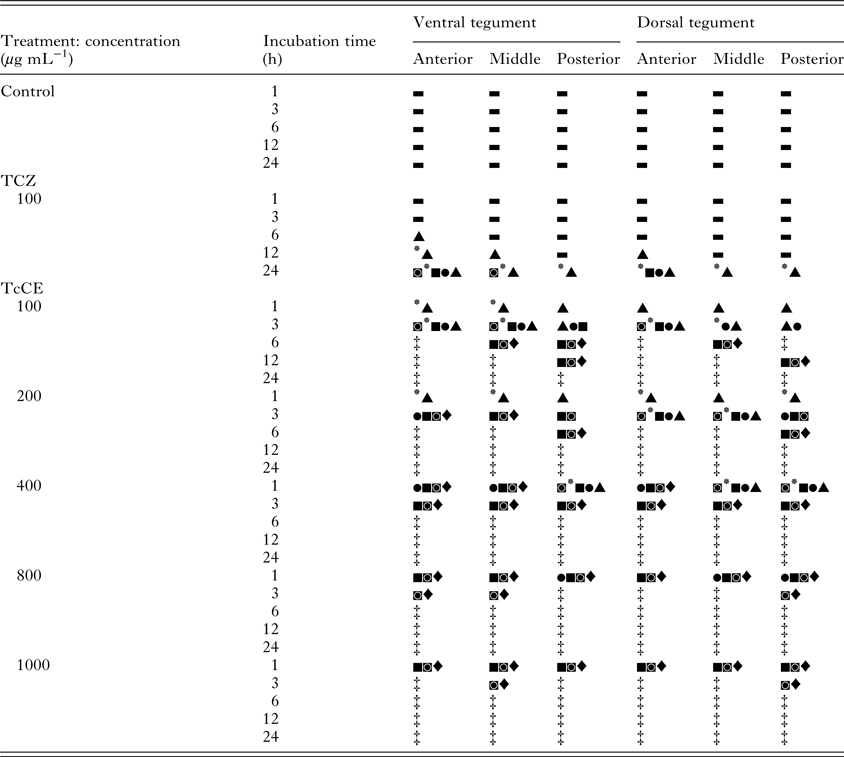
▃, Normal appearance; ▲, Swelling of the tegumental ridges and folds; ●, Blebbing or eruption formation on the tegument; ■, Disruption of the blebs on the tegument; ٭, Submerging of the spines in the swollen tegument; ◙, Shedding of the spines from their sockets; ♦, Erosion and lesion of the tegument; ‡, Total destruction of the tegument.
At the 1 h examination time point with 400 µg mL−1 TcCE, swelling and deep grooves of the tegument were shown on the anterior, middle and posterior regions of the ventral surface. The blebs were disrupted, and erosion and lesions were also observed. Tegument on all regions of the dorsal surface also appeared swollen, disrupted blebs and detached spines (Fig. 3E). After 3 h incubation time point, the treated flukes showed body deformity and extensive destruction of the tegument on both surface. Erosion and lesions were found, and spines were also dislodged from their sockets. As well, all parts of the dorsal surface appeared to be highly disrupted and exhibited complete lesion (Fig. 3F).
The flukes treated with the TcCE at concentrations 800 and 1000 µg mL−1, exhibited similar sequence of damages on the tegument, with more severe destruction occurring at the earlier treatment time. At 1 h incubation, tegumental damages were similar but occurred more rapidly and severely than those seen at concentration 100, 200 and 400 µg mL−1 of TcCE. Blebbing formation, disruption of blebs, spine dislodging, erosion and lesion were prominently on anterior region (i.e. oral sucker) and middle region of the ventral and dorsal surfaces (Fig. 4A, B, D and E). The posterior region of the ventral and dorsal surfaces also showed severe disruption of the tegument and dislodging of spines. After 3 h examination time point, the flukes exhibited severe erosion, lesion and disruption of the spines and basement membrane. Severe deformity and total destruction were appeared on both ventral and dorsal surfaces of the tegument (Fig. 4C and F).

Fig. 4. SEM images of the adult F. gigantica exposed to 800 µg mL−1 (A–C) and 1000 µg mL−1 (D–F) of TcCE. (A–F) Anterior, middle and posterior regions of the ventral surface of adult fluke. (A) One hour of incubation with 800 µg mL−1 of TcCE, damageable oral sucker (Os) with severe lesion (Le) of the muscular rim causing loss of spines (white arrow) are observed on the anterior region of the tegument. (B) By 1 h after incubation, there are severe erosion (Er), large lesion (Le), disappearance of spines (white arrow), and depression in some areas (De) on the middle region of the tegument. (C) Following 3 h post incubation, tegument on the anterior region shows severe erosion (Er), which eventually come to be lesion (Le) exposing a large area of the basement membrane (Ba) and the spines are completely lost (white arrow). (D) At 1 h of incubation with 1000 µg mL−1 of TcCE, loss of spines (white arrow) with empty spine sockets (SS, inset) and depression of some area (De) are observed in the tegument in the anterior region of the fluke. (E) By 1 h post incubation, severe erosion (Er), large lesion (Le), and loss of spines (white arrow) are visible on the middle region of the tegument. (F) Following 3 h observation time point, severe alterations in the anterior region of the tegument characterized by erosion (Er), lesions (Le), and loss of spines (white arrow) and complete demolition of the basement membrane (Ba) is also observed.
DISCUSSION
Nowadays, the application of natural products of plant extracts has been reported to be important alternative sources of the botanical control of the parasites (Geary et al. Reference Geary, Chibale, Abegaz, Andrae-Marobela and Ubalijoro2012; Hossain et al. Reference Hossain, Chandra, Nandy, Mandal and Gupta2012). In our work, this is the first report on the fasciolicidal properties of the ethanol extract from T. catappa L. (TcCE) leaves on adult F. gigantica as judged on significantly lower RM and SI values as well as pathological changes of the parasites’ tegument using LM and SEM.
The RM and SI values of parasites exposure with TcCE began to decrease clearly from 1 to 3 h incubation for all dosages, and complete immobilization (RM = 0) were detected in the treated-flukes for 6 h. These findings indicate that the crude extract could kill the parasites and showing 100% mortality at suitable dosages. These results are consistent with previous report on F. gigantica (Saowakon et al. Reference Saowakon, Tansatit, Wanichanon, Chanakul, Reutrakul and Sobhon2009), which used the doses ranging from 750 to 1000 µg mL−1 of Artocarpus lakoocha crude extract, and showed adult treated-flukes died within 12 h after incubation. Likewise, total immobilization and death of adult rumen flukes, Paramphistomum sp., was examined after 12 h incubation with 500 µg mL−1 of root-tuber extract from Flemingia vestita (Tandon et al. Reference Tandon, Pal, Roy, Rao and Reddy1997). Furthermore, Anuracpreeda et al. (Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ) reported that adult gut parasite, Fischoederius cobboldi, which was treated with 500–2000 µg mL−1 of T. catappa leaves crude extract showed a complete immobilization and death within 12 h post incubation. On the other hand, in this work, we have employed TCZ (as the positive control) to treat the flukes and shown high RM value when compared the observations with TcCE. It was likely that TCZ-treated parasites required more time (24 h after incubation) for paralysis or death as previously reported by Tansatit et al. (Reference Tansatit, Sahaphong, Riengrojpitak, Viyanant and Sobhon2012).
The tegument of F. gigantica is a primary target for the action of any rational drugs because it can direct contact with the compounds. The tegument is the outermost layer that isolates it from the hostile environment and maintains the parasite's homeostasis. Furthermore, it is responsible for synthesis and secretion of antigens, protection against host immune responses, absorbing nutrients, tissue proliferation and repair, osmoregulation and perception of sensory stimuli and the selective absorption of drugs (Dangprasert et al. Reference Dangprasert, Khawsuk, Meepol, Wanichanon, Viyanant, Upatham, Wongratanacheevin and Sobhon2001; Meaney et al. Reference Meaney, Fairweather, Bernnan, Ramasamy and Subramanian2002; Anuracpreeda et al. Reference Anuracpreeda, Wanichanon, Chaithirayanon, Preyavichyapugdee and Sobhon2006, Reference Anuracpreeda, Wanichanon and Sobhon2009b ). The use of LM and SEM in this study empowered to determine the vital target of the treatments as the surface changes of the tegument have been investigated. LM observation revealed that TcCE caused vacuolization, blebbing formation, disruption of blebs and disintegration of the tegument disrupting the fluke's surface which included detachment of spines. Similar result has been reported on F. cobboldi treated with TcCE in vitro (Anuracpreeda et al. Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ). However, LM was used to investigate a limited area of the earliest surface change, which the pathological changes in the tegument cytoplasm of the parasites could be more observed by SEM.
When examined by SEM, it was found that TcCE has a swift and severe effect on F. gigantica. The sequences of tegumental alterations were composed of the tegumental swelling. The swelling of the tegument is the earliest sign of change and probably possesses the osmotic disturbance due to the damage caused to the tegumental membrane and associated ion pumps present on the apical and basal plasma membranes (Threadgold and Brennan, Reference Threadgold and Brennan1978; Skuce et al. Reference Skuce, Anderson and Fairweather1987). Subsequently, there were blebs formed on the surface, which were then ruptured. It has been suggested that the blebbing is believed to be a device for shedding surface membrane (Bennett et al. Reference Bennett, Hughes and Harness1980). Following the disruption of blebs, spines were dislodged since the apical plasma membrane of the tegument covering the upper surfaces of spines was ruptured and sloughing away. Thereafter, the erosion and lesion was occurred on the tegument, and finally the exposure and destruction of the basement membrane, which lead to the total demolition of the tegument over huge regions. In TCZ treatment, the surface alterations of the parasites exhibited similar pathological sequence as that of TcCE but with less severity. The severity of tegumental change increased at higher treatment concentration and longer exposure times. Following these tegumental alterations, the flukes became immobile and death. The damage of the tegument evidently visible to the naked eye, were examined in all samples at the higher concentrations. The surface of the parasites showed dark and then appeared the shedding of the tegument.
The effect of TcCE varied in different regions of the parasite surface, with the ventral surface were harshly more affected than the dorsal surface, and also the anterior, middle and posterior regions of the parasites were mainly more demolished than the lateral region. The surface alterations observed in the present study resemble that investigated in adult F. gigantica after treated with the sulphoxide metabolite of triclabendazole (TCZ-SX) (Meaney et al. Reference Meaney, Fairweather, Bernnan, Ramasamy and Subramanian2002), and with artesunate (Tansatit et al. Reference Tansatit, Sahaphong, Riengrojpitak, Viyanant and Sobhon2012). As well, similar surface changes were observed on adult F. hepatica after treatment with clorsulon (Meaney et al. Reference Meaney, Fairweather, Bernnan, McDowell and Forbes2003), nitroxynil (McKinstry et al. Reference McKinstry, Fairweather, Brennan and Forbes2003) and with artemether (Keiser and Morson, Reference Keiser and Morson2008). Moreover, similar severe damages were also found in adult F. cobboldi treated with TcCE (Anuracpreeda et al. Reference Anuracpreeda, Chankaew, Puttarak, Koedrith, Chawengkirttikul, Panyarachun, Ngamniyom, Chanchai and Sobhon2016c ). The regional differences in response to various anthelmintics probably depend on the thickness, variation in the anatomy and physiology, routes of crude extract or drug uptake and metabolism of crude extract or drug in different areas of the tegument.
In conclusion, we have clearly shown that the ethanol extract of T. catappa L. leaves (TcCE) exert an anthelmintic effect on F. gigantica. Hence, i n vivo studies should be performed to examine whether the TcCE may serve as a powerful fasciolicide for treatment of fasciolosis.
FINANCIAL SUPPORT
This work was financially supported by Research Grants from Agricultural Research Development Agency (ARDA), National Research Council of Thailand (NRCT), The Thailand Research Fund (TRF) and Mahidol University to Panat Anuracpreeda.
ACKNOWLEDGEMENTS
We are grateful to research grants for supporting this study.









