INTRODUCTION
Human leishmaniases are diseases caused by various species of kinetoplastid protozoan parasites of the genus Leishmania. Leishmania (Viannia) braziliensis and L. (V.) peruviana are two of the Leishmania species associated with cutaneous leishmaniasis (CL) in South America. L. braziliensis and more rarely L. (V.) panamensis are responsible for the devastating and life-threatening lesions of the mucocutaneous leishmaniasis form (MCL), whereas L. peruviana causes benign CL and has not been associated with MCL (González et al. Reference González, Pinart, Rengifo-Pardo, Macaya, Alvar and Tweed2009). Both species occur sympatrically in Peru, L. braziliensis/L. peruviana hybrids being identified from patients with either localized CL associated with L. peruviana infection or MCL typical of L. braziliensis infection (Dujardin et al. Reference Dujardin, Bañuls, Llanos-Cuentas, Alvarez, DeDoncker, Jacquet, Le Ray, Arevalo and Tibayrenc1995). The factors behind the distinct clinical pictures are not known. It is consensual that clinical manifestations of Leishmania infection are determined by a combination of factors, including the host's genetic make-up and immune status, in addition to features of the parasite at both specific and intraspecific levels (Bañuls et al. Reference Bañuls, Hide and Prugnolle2007).
Reproduction in natural populations has been considered to be predominantly clonal, with exponential propagation in an ideal environment. Nevertheless, in stressful conditions, genetic exchange might be crucial for the generation of new phenotypes, some with selective advantage and subsequent expansion of Leishmania in a population, by contributing to phenotypic diversity in natural parasite populations (Miles et al. Reference Miles, Yeo and Mauricio2009). The emergence of hybrids associated with MCL in Peru may be an example of such a case (Nolder et al. Reference Nolder, Roncal, Davies, Llanos-Cuentas and Miles2007).
Recombination between different species and strains has been detected in natural populations, such as L. braziliensis/L. panamensis of subgenus Viannia in the New World and, L. major/L. arabica, L. major/L. infantum and within L. infantum strains of the subgenus Leishmania in the Old World (Kelly et al. Reference Kelly, Law, Chapman, Van Eys and Evans1991; Belli et al. Reference Belli, Miles and Kelly1994; Ravel et al. Reference Ravel, Cortes, Pratlong, Morio, Dedet and Campino2006; Chargui et al. Reference Chargui, Amro, Haouas, Schönian, Babba, Schmidt, Ravel, Lefebvre, Bastien, Chaker, Aoun, Zribi and Kuhls2009). However, the occurrence, emergence and behaviour of hybrid strains are relatively unexplained aspects of the epidemiology of leishmaniasis, with important relevance to diagnosis, treatment and control strategies. In addition, genetic exchange in Leishmania has recently been proven to occur in sand flies experimentally infected with L. major, with diverse trait inheritance among the progeny (Akopyants et al. Reference Akopyants, Kimblin, Secundino, Patrick, Peters, Lawyer, Dobson, Beverley and Sacks2009).
The study of hybrids may uncover parasite-specific factors, including their inheritance, the clinical features and their epidemiological importance. In this study we analysed the in vitro growth and adaptive capacity under different stress conditions, as well as the virulence and infectivity, in vivo, of clones of 2 species of the Leishmania subgenus Viannia, L. braziliensis and L. peruviana, and their putative hybrids, in order to investigate a possible hybrid selective advantage.
MATERIALS AND METHODS
Parasites
A panel of 24 clones from 6 strains of L. braziliensis, L. peruviana and L. peruviana/L. braziliensis hybrids, isolated in Huanuco, Peru, was used in this study (Table 1). These clones were initially maintained in Alpha Minimum Essential medium (α-MEM, Sigma, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, BioWhittaker, Switzerland) in the London School of Hygiene and Tropical Medicine (LSHTM) cryobank since their isolation. After thawing, all experimental cultures were carried out in liquid Grace's medium (Sigma) plus 10% FBS, unless specified.
Table 1. Strains and clones of Leishmania (Viannia) species used in phenotypic comparisons

In vitro growth kinetics and parasite densities at different temperatures
To compare and select the optimal conditions for growth of Leishmania, parasites from all the strains were centrifuged and re-suspended in 10 ml of liquid medium to a final density of 1·0E+05 parasites/ml. Parasites were incubated at 20°C, 24°C and 28°C until the end of the experiment. The kinetics of the growth curve and parasite densities were monitored by counting the parasites on the same days until parasite concentrations decreased to a minimum, using a Neubauer haemocytometer. All assays were carried out in duplicate and 4 independent counts were made.
In vitro survival at shock temperatures
To evaluate whether temperature-induced stress could differentially influence parasite growth, 1 clone of each parental strain and putative hybrids were chosen randomly. Parasites in the exponential growth phase, were re-suspended at a concentration of 1·0E+05 parasites/ml in liquid medium and incubated at high shock temperatures (36°C, 38°C, 40 °C, 42°C, 44°C and 46°C) for 10, 30 and 60 min. After incubation under each stress condition, the parasite cultures were re-incubated for 1 week at 24 °C, the temperature considered optimal for promastigote survival in the sand fly vector. In addition, control cultures of each strain/clone were incubated at 24°C during the entire period. After the incubation period, parasites were counted using a Neubauer haemocytometer. For each clone, parasite density ratios were calculated in relation to control cultures. All assays were carried out in duplicate, and 4 independent counts were made.
In vitro hydrogen peroxide sensitivity assay
To analyse the growth inhibitory effect of reactive oxygen species on Leishmania, parasite viability, in the presence of hydrogen peroxide, was assessed. Three clones of L. peruviana, 4 clones of L. braziliensis and 2 clones of the hybrid strains from cultures in the exponential growth phase, were adjusted to 1·0E+07 parasites/ml in Schneider's medium (Sigma) with 10% FBS. Parasites were exposed to varying concentrations of H2O2 (Sigma) (100 μ m, 200 μ m, 300 μ m, 400 μ m, 500 μ m, 600 μ m, 700 μ m, 800 μ m, 900 μ m, 1000 μ m) in 96-well plates and incubated at 24°C for 2 h. Parasite viability was analysed by adding XTT solution 0·3 mg/ml (sodium 3′-[1-(phenylaminocarbonyl)- 3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulphonic acid hydrate) (Roche Diagnostics, Germany) to each well. After incubation for 1–2 h at 24°C, orange formazan solution was formed and was quantified spectrophotometrically on an ELISA plate reader (Awarness Technology INC, USA) at 630 nm. Relative viability was calculated from the ratio of the OD readings in parasites exposed to H2O2 versus those not exposed to H2O2. All assays were carried out in triplicate.
In vivo studies
Several clones of the 6 L. braziliensis, L. peruviana and L. peruviana/L. braziliensis hybrid strains were used for the in vivo infection experiments (Table 1). Promastigotes were maintained in liquid medium at 24°C and metacyclic promastigotes were obtained according to morphological characteristics described by Almeida et al. (Reference Almeida, Cuba Cuba, Sá, Pharoah, Howard and Miles1993). A total of 50 male golden hamsters (Mesocricetus auratus) aged 6–8 weeks were purchased from Harlam Interfauna Ibérica SL (Barcelona, Spain) and housed at the Instituto de Higiene e Medicina Tropical (IHMT), Lisbon, under stable climatic and dietary conditions.
A group of 3 hamsters was inoculated intradermally in the snout with 1·0E+05 promastigotes/50μl/animal from each Leishmania clone/strain (L. peruviana, L. braziliensis and L. peruviana/L. braziliensis hybrids), plus a control group of 3 animals inoculated with saline solution. Prior to infection, hamsters were anaesthetized with 150 mg of ketamin (Imalgene® 1000, Rhône Mérieux, France) and 15 mg of xylazin (Rompun®, Bayer, Germany). The animals were followed for 52 weeks (1 year). One hamster from each group was sacrificed at 10, 26 and 52 weeks post-infection (p.i.). Some animals were euthanised out of the pre-established time points, to meet the Humane End Points’ policy.
The diameter of the lesions was measured with a calliper, weekly until the 10th week and monthly after that. Statistical analysis of lesion diameter measurements (dependent variable) from the first 10 weeks was performed with software SPSS 19.0 through a Linear Mixed Model with repeated measurements, considering time and strain as independent variables. Results were considered statistically significant for values of P<0·05. After 10 weeks statistical analysis was not possible due to the reduced number of animals.
Spleen, liver, lymph nodes, bone marrow, skin from the ear (not the inoculation site) and from the snout (inoculation site) were aseptically harvested for direct parasite detection by NNN (Novy, MacNeal and Nicolle) medium culture and PCR. DNA was extracted using a commercial kit (PCR-template Preparation kit, Roche Diagnostics), quantified (GeneQuant, Amersham Biosciences, Germany) and PCR was performed as previously described by Zhang et al. (Reference Zhang, Miranda-Verastegui, Arevalo, Ndao, Ward, Llanos-Cuentas and Matlashewski2006). Parasite load was estimated by realtime TaqMan® PCR, as described by Rolão et al. (Reference Rolão, Cortes, Rodrigues and Campino2004). Mass cultures of L. braziliensis promastigotes were used to construct the standard curve ranging from 10·0E+05 to 1·0 parasites. The diluted parasite cultures were processed for DNA extraction, as above and mixed with DNA from a healthy hamster.
Animal manipulation was approved by the Ethics Committee of the IHMT (approval ID 28/2006) and Veterinary Authorities (‘Direcção Geral de Veterinária’, approval ID 520/000/000/2006) and followed the guidelines of the Portuguese legislation (Lei n°92/95, 12.9).
RESULTS
In vitro growth behaviuor
Growth kinetics and parasite densities of clones and uncloned strains of L. peruviana, L. braziliensis and L. peruviana/L. braziliensis putative hybrid were compared at 20°C, 24°C and 28°C. At 20°C, all L. braziliensis parasites presented relatively consistent and distinctive growth patterns with higher parasite densities than L. peruviana (reaching 2·0E+ 07 parasites/ml) (Fig. 1). Hybrids were broadly divided into those with kinetics and high parasite densities (approx. 1·0–2·0E+07 parasites/ml) similar to L. braziliensis, even exhibiting a late peak, and clones with lower densities (max. 1·0E+07 parasites/ml) more similar to L. peruviana strains/clones used in this study.

Fig. 1. Growth patterns of Leishmania (Viannia) strains. Growth kinetics and parasite densities obtained for cloned and uncloned strains of Leishmania peruviana, L. braziliensis and L. peruviana/L. braziliensis hybrid clones at 20°C, 24°C and 28°C. The results are mean values of 4 independent counts.
At 24°C, all L. braziliensis clones presented consistent kinetics, reaching the highest parasite densities (2·5E+07 parasites/ml). At this temperature, L. peruviana parasites presented lower densities (until 1·5E+07 parasites/ml) than L. braziliensis parasites. The hybrid cultures again showed variable groups of behaviours, with high densities more similar to L. braziliensis (approx. 2·5E+07 parasites/ml) and low densities more similar to L. peruviana. This was also observed to a certain extent at 28°C (Fig. 1).
In vitro survival after temperature shock and growth inhibitory effect of H2O2
The influence of high temperature shock on parasite growth recovery was analysed 7 days post-exposure. At 36°C, 38°C and 40°C, parasite densities of L. peruviana, L. braziliensis and hybrids were not substantially affected for short incubation periods (10 and 30 min), although L. braziliensis generally achieved the highest densities, hybrid parasites presented an intermediate parasite density ratio between the parental species (Fig. 2). Following a 60 min incubation at temperatures equal to or above 40°C, parasites from all strains survived but did not multiply. At 42°C there was an abrupt decrease in growth for all strains, regardless of the incubation time. Following shocks at 44°C and 46°C, there was no parasite growth.

Fig. 2. Behaviour of the parasites in the presence of different conditions of high temperature shock. Parasite density ratios (percentages) of Leishmania peruviana (LC2434cl3), L. braziliensis (LC2873cl3) and L. peruviana/L. braziliensis hybrid (HR434cl2) clones in comparison with controls, at different high shock temperatures for different time periods: (A) 10 min incubation; (B) 30 min incubation; (C) 60 min incubation. Parasite densities were measured after 1 week in optimal culture conditions after shock. The results are mean values of 4 independent counts in different experiments. Standard deviation is represented by error bars.
Growth inhibition of Leishmania strains by exogenous H2O2 was analysed through relative parasite viability. All strains were sensitive in a concentration-dependent manner and behaved similarly in response to increasing concentrations of H2O2.
In vivo studies
All infected animals with L. braziliensis and hybrid clones revealed the first cutaneous lesions of infection 2 weeks p.i. and at 3 weeks p.i. in hamsters infected with L. peruviana strains, with swelling of the snout, local alopecia and hyperkeratosis. The first ulcers in the snout were observed at 3 weeks p.i. in one hamster infected with L. braziliensis (LC2873cl1) and in a hamster infected with the hybrid clone HR434cl3. Ulcerative lesions appeared progressively in all animals infected with the other L. braziliensis and hybrid strains between the 3rd week p.i. and the 8th week p.i. (Fig. 3). Lesions, which were measured weekly from the time of lesion appearance until 10 weeks p.i., were larger in hamsters infected with hybrid clones (ranging from 1·4 to 8·5 mm diameter) than those produced by L. braziliensis clones (1·6 to 7·9 mm) (P=0·026) (Fig. 4). There was no significant variation between strains regarding lesion behaviour through time (P=0·435). In addition, at 10 weeks p.i. parasites were detected, in culture or their DNA by conventional PCR, in lymph node and snout of the animals infected with L. braziliensis and hybrid clones (Table 2) and at 26 weeks p.i. also in the skin (not the inoculation site). At this time point (26 weeks p.i.), the spleens of hamsters infected with hybrids also revealed parasites. Throughout the study no Leishmania infection was detected by culture or conventional PCR in hamsters inoculated with L. peruviana strains.
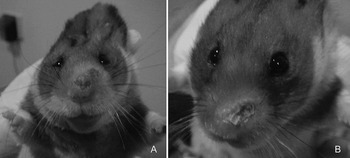
Fig. 3. Photographs of hamsters infected with Leishmania braziliensis and hybrid parasites at an early phase. Lesions observed in golden hamsters inoculated intradermally in the snout with 1·0E+05 Leishmania promastigotes. Nasal swelling with hyperkeratosis of the snout and a crust caused by L. braziliensis parasites (LC2452cl1), at 5 weeks p.i. (A) and by L. peruviana/L. braziliensis hybrid parasites (HR434cl2), at 8 weeks p.i. (B).

Fig. 4. Evolution of lesion diameter measurement during the first 10 weeks post-infection. Mean of lesion diameter of hamsters infected with Leishmania braziliensis and L. peruviana/L. braziliensis hybrid clones from 5 weeks p.i. until 10 weeks p.i. Dotted lines represent 95% confidence intervals.
Table 2. Detection of parasites through NNN (Novy, MacNeal and Nicolle) cultures and PCR from hamsters inoculated with the different Leishmania (Viannia) species and necropsied at 10, 26 and 52 weeks p.i.

−, Negative cultures or no DNA amplification; +, positive cultures or DNA amplification.
From the 10th to the 26th week p.i., animals presented a regression of clinical signs, particularly snout lesions, although with delayed healing in animals infected with hybrid clones. From 26 weeks p.i. to 52 weeks p.i., only the hamsters infected with 2 of the L. braziliensis clones (LC2452cl3 and LC2873cl1) presented slight lip and snout swelling. In contrast, all animals infected with hybrid clones started to present new lesions, some of them quite aggressive (hamsters infected with HR434 cl2, HR434cl3, LC2902cl1 LC2902cl3), such as lip retraction, and prominent swelling (Fig. 5) and even from 20 weeks p.i. (in hamsters infected with HR434cl1). Most of those more aggressive clones also presented higher parasite densities in the in vitro growth in almost all studied temperatures than the other hybrids. During the entire infection period, cutaneous lesions were never observed in other parts of the hamsters’ bodies.

Fig. 5. Photograph of infected hamster with hybrid parasites at a late phase. Lesions observed in golden hamsters inoculated intradermally in the snout with 1·0E+05 Leishmania peruviana/L. braziliensis hybrid parasites (HR434cl3). Deep nasal and superior lip swelling with lip retraction and a crust at 50 weeks p.i.
Quantification by real-time PCR revealed parasite loads in hamsters infected with L. peruviana, L. braziliensis and L. peruviana/L. braziliensis hybrid strains in all organs, although L. peruviana parasitism was much more reduced (Fig. 6). By the end of the infection period, parasite densities were much higher in animals infected with the hybrid clones. During the entire study no parasite DNA was detected in the control group.

Fig. 6. Parasite loads of Leishmania sp. DNA by quantitative real-time PCR in different tissue samples. Parasite loads in hamsters infected with Leishmania peruviana, L. braziliensis and L. peruviana/L. braziliensis hybrids at 10, 26 and 52 weeks p.i. Bone marrow DNA from hamsters infected with L. peruviana clones necropsied at 52 weeks p.i. was lost. Standard deviation is represented by error bars.
DISCUSSION
Leishmania reproduction seems to be mainly clonal, although a number of hybrids have been described and there are suggestions that some of them may have enhanced or at least similar fitness to their putative parents (Volf et al. Reference Volf, Benkova, Myskova, Sadlova, Campino and Ravel2007). Recently, it has been shown experimentally that genetic exchange can occur in the digestive tract of the sand fly, with apparent Mendelian inheritance of genomic markers and with segregation of phenotypic traits including parasite virulence (Akopyants et al. Reference Akopyants, Kimblin, Secundino, Patrick, Peters, Lawyer, Dobson, Beverley and Sacks2009). Leishmania interspecies hybrids have been reported from both the Old and New Worlds (Belli et al. Reference Belli, Miles and Kelly1994; Ravel et al. Reference Ravel, Cortes, Pratlong, Morio, Dedet and Campino2006). Genetic exchange has also been previously demonstrated in the trypanosomatids Trypanosoma brucei and Trypanosoma cruzi (Gibson and Whittington, Reference Gibson and Whittington1993; Machado and Ayala, Reference Machado and Ayala2001; Gaunt et al. Reference Gaunt, Yeo, Frame, Stothard, Carrasco, Taylor, Mena, Veazey, Miles, Acosta, de Arias and Miles2003).
Reproducible methods for phenotypic characterization are a pre-requisite for associating differences in parasite growth, virulence and tropisms. Several studies have compared phenotypic properties and fitness between different Leishmania species (Garin et al. Reference Garin, Sulahian, Pratlong, Meneceur, Gangneux, Prina, Dedet and Derouin2001; Gamboa et al. Reference Gamboa, Torres, Doncker, Zimic, Arevalo and Dujardin2008; Vanaerschot et al. Reference Vanaerschot, Maes, Ouakad, Adaui, Maes, De Doncker, Rijal, Chappuis, Dujardin and Decuypere2010), but only one has been made with hybrid strains and their putative parents in order to understand better the epidemiological implications of hybridization events among natural populations (Torrico et al. Reference Torrico, De Doncker, Arevalo, Le Ray and Dujardin1999).
Here, we have analysed the in vitro and in vivo behaviour of several strains and their clones of sympatric L. braziliensis, L. peruviana and L. braziliensis/L. peruviana putative hybrid isolates obtained from natural infections. We compared their ability to sustain growth at 3 different temperatures 20°C, 24°C and 28°C, resilience to high temperature shock and vulnerability to oxidative stress. Under the experimental conditions, 24°C was the optimal incubation temperature for in vitro growth for all the studied New World strains to achieve the highest parasite densities.
All L. braziliensis clones presented consistent and indistinguishable growth patterns at each temperature, suggesting that the subpopulations (clones) within each strain were homogenous. Furthermore, at 24°C, L. braziliensis produced higher parasite densities than L. peruviana strains, indicating greater vigor during in vitro growth. These observations are in accordance with those of Torrico et al. (Reference Torrico, De Doncker, Arevalo, Le Ray and Dujardin1999), who found that, when compared with L. peruviana and L. peruviana/L.braziliensis hybrids, L. braziliensis displayed higher growth capacity and plasticity under different culture conditions. Notably, our data revealed that hybrid clones had heterogeneous phenotypes and divergent behaviours, with some clones having lower growth rates, similar to the putative L. peruviana strains, whilst others had higher rates, more similar to the putative L. braziliensis strains. Interestingly, and importantly, this is reminiscent of the segregation of phenotypic traits seen among the progeny of the experimental L. major strain cross (Akopyants et al. Reference Akopyants, Kimblin, Secundino, Patrick, Peters, Lawyer, Dobson, Beverley and Sacks2009). This suggests, not that hybrids may be uniformly vigorous but that they display a diversity of phenotypes, with different propensities for adaptation to mammalian host, sand fly vectors and environmental conditions.
Following high extreme temperature shock (36–46°C), L. braziliensis and hybrids, showed an apparently greater resilience than L. peruviana to recover to high parasite densities, although this may have been, in part, simply a reflection of the growth rates rather than differential survival. In a previous study (Callahan et al. Reference Callahan, Portal, Bensinger and Grogl1996), it was found that Leishmania visceralizing species (L. donovani and L. infantum) are more resistant to high temperatures than the cutaneous species (L. major, L. tropica, L. mexicana, L. braziliensis, L. panamensis, and L. amazonensis) and, among the New World cutaneous species that L. braziliensis promastigotes replicate more slowly than L. mexicana and L. amazonensis. We have here shown that among the studied strains of the subgenus L. (Viannia) species, L. braziliensis presents higher in vitro temperature shock tolerance than L. peruviana. Concerning the leishmanicidal effect of reactive oxygen species, we did not find detectable differences in sensitivity to hydrogen peroxide between parental and hybrid strains.
As far as we are aware, this was the first in vivo assessment of hybrid strains. Hamsters infected with hybrid clones had, in an early phase, a clinical aggressive pattern similar to hamsters infected with the L. braziliensis clones. However, and surprisingly, in a later phase of the experiment, the tissues from hamsters infected with hybrid clones revealed high parasite densities, associated with the animals’ clinical manifestations (relapses) which were found to be quite aggressive, with greater lesions than hamsters infected with strains of the putative parental species used in this study. These observations suggest that hybrids could be more virulent than parental strains, which would need to be confirmed by further research. Furthermore, we also detected some correlation between in vitro and in vivo behaviour, as, among hybrid clones, we observed a generic higher in vitro growth in those that showed more aggressive behaviour in hamsters. Such a correlation should be further investigated with other phenotypic studies. In addition, in the present study, even though no lesions were observed away from the inoculation site, Leishmania DNA was detected in the skin away from the inoculation site, revealing the capacity of metastasis. Parasites were also detected in internal organs (liver and spleen) although animals were infected intradermally. Unusual visceralization in infections with Leishmania dermotropic species had already been reported in experimental murine and natural canine infections (Almeida et al. Reference Almeida, Cuba Cuba, Sá, Pharoah, Howard and Miles1993; Reithinger et al. Reference Reithinger, Lambson, Barker, Counihan, Espinoza, González and Davies2002). Visceralization could be the result of the ability to metastasize, which appears to be a biological characteristic of some L. braziliensis spp. (Rey et al. 1991).
The overall results suggest that L. peruviana/L. braziliensis hybrid strains are more resilient than the parental species, have higher virulence than L. peruviana, and even more than L. braziliensis in vivo. As mentioned, these differences in hybrid behaviour could have important eco-epidemiological implications. Volf et al. (Reference Volf, Benkova, Myskova, Sadlova, Campino and Ravel2007) observed that L. infantum/L. major hybrids acquired the capacity to be transmitted by the widespread sand fly vector Phlebotomus papatasi, which is normally only competent to transmit L. major. Thus, there may be significant emergence and epidemic spread of Leishmania hybrids, in both the Old and New Worlds. The existence of hybrid strains having diverse or enhanced fitness and selective advantages is also likely to have an impact on virulence, pathogenesis, diagnostic sensitivities, response to treatment, and consequently on the success of disease control.
Our data support the concept that genetic exchange in Leishmania may yield progeny that have a strong selective advantage and can expand clonally. Further studies would help us to elucidate the genetic factors behind the phenotypes observed.
ACKNOWLEDGMENTS
The authors thank Bruno Sousa (IHMT) for assistance with statistical analysis. We also thank Michael Lewis (LSHTM) for advice and Debbie Nolder (LSHTM), Alejandro Llanos Cuentas (Instituto de Medicina Tropical Alexander von Humboldt, Peru) and the late Clive Davies (LSHTM), for provision of the Huanuco isolates.
FINANCIAL SUPPORT
This study was funded by the EC project LeishEpiNetSA (INCO-CT2005-015407) and Project PTDC/CVT/112371/2009 from Fundação para a Ciência e a Tecnologia (FCT), Ministério da Ciência, Tecnologia e Ensino Superior, Portugal. S. Cortes (SFRH/BPD/44450/2008) and C. Maia (SFRH/BPD/44082/2008) hold a fellowship from FCT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.










