INTRODUCTION
Infectious diseases are important drivers of ecological interactions and evolution (Boots et al. Reference Boots, Best, Miller and White2009; Schulenburg et al. Reference Schulenburg, Kurtz, Moret and Siva-Jothy2009), and are of general concern in the context of disease mitigation and conservation biology (Scott, Reference Scott1988; Smith et al. Reference Smith, Dobson, McKenzie, Real, Smith and Wilson2005, Reference Smith, Acevedo-Whitehouse and Pedersen2009). Traditional microparasite models focus on infectious disease from the host point of view by dividing hosts into Susceptible, Infected and Recovered sub-populations (SIR) (Anderson and May, Reference Anderson and May1979; Grenfell and Harwood, Reference Grenfell and Harwood1997; Hagenaars et al. Reference Hagenaars, Donnelly and Ferguson2004; Brooks et al. Reference Brooks, Antonovics and Keitt2008; Ben-Zion et al. Reference Ben-Zion, Cohen and Shnerb2010). Although these models effectively describe epidemics/epizootics of those microparasites for which their numbers per host are irrelevant and/or difficult to quantify, they are less applicable to those microparasites where the size of parasite population within a host is a key to understanding host-parasite population dynamics. Recently, a metapopulation framework has been applied to disease dynamics in order to incorporate spatial structuring of the host population (Arino and Van den Driessche, Reference Arino and Van den Driessche2006; Colizza and Vespignani, Reference Colizza and Vespignani2008; Apolloni et al. Reference Apolloni, Poletto, Ramasco, Jensen and Colizza2014), but in such approaches the unit of the patch is a host population, and the parasite population per host is still overlooked even though dynamics of infection within a host can be affected by individual host characteristics and can have direct impacts on individual host health, on host movement and on the rate of transmission. Macroparasite models, on the other hand, directly consider the parasite population but even these models often do not capture the dynamics in parasite numbers within individual hosts (May and Anderson, Reference May and Anderson1979; Rosà et al. Reference Rosà, Pugliese, Villani and Rizzoli2003; Cornell et al. Reference Cornell, Isham and Grenfell2004). Furthermore, not all parasites fit neatly into the micro- or macroparasite conceptual framework. Together, these limitations have led to the call for a unifying framework which considers both host and parasite populations (Gog et al. Reference Gog, Pellis, Wood, McLean, Arinaminpathy and Lloyd-Smith2015). One possible approach applies traditional metapopulation theory to parasite population dynamics, but views individual hosts (rather than local host populations) as patches that can be colonized by the parasite (Grenfell and Harwood, Reference Grenfell and Harwood1997). To our knowledge, this approach has not yet been developed theoretically nor investigated experimentally, perhaps because very few parasites allow for the possibility of tracking their dynamics over time without destructive sampling. The use of model systems which can experimentally test how characteristics of individual hosts can influence parasite populations at both the individual host and host population levels are thus of fundamental importance.
Gyrodactylus spp. (Monogenea) are ectoparasites which feed on the epithelial cells and mucus of many marine and freshwater teleost fish species (Bakke et al. Reference Bakke, Cable, Harris, Baker, M. and Rollinson2007). They attach to the epidermis of their host via specialized hooks and are directly transmitted primarily by jumping to a new host during contact (Scott and Anderson, Reference Scott and Anderson1984; Kearn, Reference Kearn1994). Gyrodactylus spp. are viviparous, with an unusual method of reproduction: the developing embryo contains within itself a second developing embryo, which allows for rapid population growth of the parasite on an infected host (Kearn, Reference Kearn1994; Bakke et al. Reference Bakke, Cable, Harris, Baker, M. and Rollinson2007). Gyrodactylid infection can result in high rates of mortality (Van Oosterhout et al. Reference Van Oosterhout, Harris and Cable2003), and induce a temporary refractory period in surviving hosts (Scott and Robinson, Reference Scott and Robinson1984; Scott, Reference Scott1985a ). As such, gyrodactylids cause epidemic outbreaks, making their population dynamics typical of microparasites (Anderson and May, Reference Anderson and May1979; May and Anderson, Reference May and Anderson1979) despite being helminth parasites. Furthermore, because they are ectoparasites they can be observed over time without sacrificing the host. Thus this model system has been useful for studying parasite dynamics on individual hosts within a host population (Scott, Reference Scott, Rollinson and Anderson1985b ; Cable and van Oosterhout, Reference Cable and van Oosterhout2007b ; Richards et al. Reference Richards, van Oosterhout and Cable2010; Johnson et al. Reference Johnson, Lafferty, van Oosterhout and Cable2011; Tadiri et al. Reference Tadiri, Dargent and Scott2013), and holds potential for furthering our understanding of host-parasite population dynamics.
The guppy (Poecilia reticulata) is the host for Gyrodactylus turnbulli (see Harris and Lyles, Reference Harris and Lyles1992). Guppies are a common sexually dimorphic ovoviviparous tropical fish, used as a model species for many ecological studies including exploration of male–female interactions, mate-choice and parasitism (Houde and Torio, Reference Houde and Torio1992; Kolluru et al. Reference Kolluru, Grether, Dunlop and South2009), and shoaling behaviour (Croft et al. Reference Croft, Albanese, Arrowsmith, Botham, Webster and Krause2003; Richards et al. Reference Richards, van Oosterhout and Cable2010). In many guppy populations, females harbour more parasites than males (Gotanda et al. Reference Gotanda, Delaire, Raeymaekers, Pérez-Jvostov, Dargent, Bentzen, Scott, Fussmann and Hendry2013; Stephenson et al. Reference Stephenson, van Oosterhout, Mohammed and Cable2014; Dargent et al. Reference Dargent, Rolshausen, Hendry, Scott and Fussmann2015), and the tendency of females to shoal more tightly together than males may facilitate parasite transmission especially in grouped female fish (Croft et al. Reference Croft, Albanese, Arrowsmith, Botham, Webster and Krause2003; Richards et al. Reference Richards, van Oosterhout and Cable2010). Also, guppy populations vary widely in their ability to resist parasites (Cable and van Oosterhout, Reference Cable and van Oosterhout2007b ; Dargent et al. Reference Dargent, Scott, Hendry and Fussmann2013). Thus, the guppy-gyrodactylid system provides a unique opportunity for experimentally testing how heterogeneity among hosts can influence parasite population dynamics both at the individual host level and at the host and parasite population level. Although the effects of sex and number of guppies on parasite population growth have been studied in separate experiments (Richards et al. Reference Richards, van Oosterhout and Cable2010; Johnson et al. Reference Johnson, Lafferty, van Oosterhout and Cable2011; Stephenson et al. Reference Stephenson, van Oosterhout, Mohammed and Cable2014; Dargent et al. Reference Dargent, Rolshausen, Hendry, Scott and Fussmann2015), the direct comparison between parasite dynamics on isolated hosts and groups has not been made, nor have the combined effects of grouping and sex on parasite epidemic dynamics been investigated.
The goals for this experiment were to determine whether host sex, group size and group composition influenced host-parasite dynamics at the level of individual and grouped hosts. We expected parasite populations to reach higher numbers, and persist for longer in groups of fish when compared with isolated fish due to greater availability of hosts. We also expected higher parasite burdens on females than males, both on isolated fish and in single-sex groups due to greater size and possibly lower resistance of females (Dargent et al. Reference Dargent, Rolshausen, Hendry, Scott and Fussmann2015). For mixed-sex groups, however, our null expectation was that heterogeneity among fish would have an averaging effect on parasite population growth. Although we found that parasites reached higher densities on isolated females than males, this difference did not persist in groups, and heterogeneity in group composition did not influence parasite dynamics.
MATERIALS AND METHODS
Source and maintenance of fish
Animal Care Approval was obtained per McGill University Ethics Guidelines (AUP 2009-5759). Guppies obtained from the Guanapo River and Lower Lalaja tributary in Trinidad (10°38′23″N, 61°14′54″W and 10°39′14″N, 61°15′18″W) were bred to the F3 generation, keeping track of maternal lines, in the McGill University Phytotron. The room was maintained at 27 ± 1 °C with a 12-h light-dark cycle and the fish were raised in common-garden conditions in an Aquaneering Inc. (San Diego, California, USA) flow-through system. Fish were raised on controlled amounts of TetraMin© Tropical Flakes (Tetra Werke, Melle Germany). In order to mimic a history of natural infection, F3 fish were exposed to our isogenic laboratory culture of G. turnbulli (identified by S. King) from birth.
Experimental design
The experiment consisted of two parts, conducted simultaneously. The first part was a 2 × 2 factorial design used to test the effects of host sex (male vs female) and host group size (1 vs 8) on parasite dynamics. As treatments, we established groups of 8 males (4 replicates), groups of 8 females (4 replicates), isolated males (8 replicates), and isolated females (8 replicates). The second part of the experiment tested the effect of host heterogeneity in sex on parasite dynamics. This part consisted of 4 replicates each containing a group of 8 fish (4 males and 4 females), and data were compared with the homogenous sex groups from the first part of the experiment.
Experimental protocol
In order for fish to overcome infection-acquired resistance and regain susceptibility to Gyrodactylus spp. (Scott and Robinson, Reference Scott and Robinson1984; Scott, Reference Scott1985a ; Cable and van Oosterhout, Reference Cable and van Oosterhout2007b ) parasites were eliminated from adult F3 fish by treating them in a 25 g L−1 salt water bath for 15 min (Schelkle et al. Reference Schelkle, Doetjes and Cable2011) 2 months before the start of the experiment. One week later, fish were anaesthetized in 0·02% Tricaine methanesulfonate (MS-222), buffered to a neutral pH with sodium bicarbonate and scanned using a dissection microscope to confirm the absence of parasites. Seven weeks later, adult F3 fish were again scanned for parasites and weighed to the nearest 0·001 g, measured for standard length (SL) to the nearest 0·01 cm with a calliper, and marked for identification with visible implant elastomer dye (Northwest Marine Technologies Inc., Shaw Island Washington, USA) which has been shown to have no impact on fish health or behaviour (Croft et al. Reference Croft, Albanese, Arrowsmith, Botham, Webster and Krause2003, Reference Croft, Krause and James2004). Fish were then assigned to treatments/replicates in a way that would distribute size, population of origin and maternal lines evenly across treatments/replicates and groups of fish were acclimated with one another for 1 week prior to infection.
A total of 112 fish (56 males and 56 females) were used for this experiment, with an SL of 2·34 ± 0·03 cm for females and 1·61 ± 0·01 cm for males and weights of 0·29 ± 0·01 g for females and 0·08 ± 0·002 g for males. Each group of 8 fish was housed in a tank with 6 L of water and each isolated fish was housed in a tank with 1·8 L of water. Each tank was considered an experimental unit for analyses at the population level. Fish were fed daily with TetraMin© Tropical Flakes mixed with conditioned water into a paste and delivered through a glass precision syringe to each tank according to the number and sex of fish in each tank. A low food availability regime was used to prevent compensation of innate resistance through additional food acquisition (Kolluru et al. Reference Kolluru, Grether, South, Dunlop, Cardinali, Liu and Carapiet2006; Tadiri et al. Reference Tadiri, Dargent and Scott2013).
To begin infections on isolated fish, a heavily infected fish was taken from our isogenic lab culture of G. turnbulli and anaesthetized in 0·02% MS-222. Scales with parasites were removed from the donor fish and placed on an anaesthetized recipient until 3 parasites had transferred to the recipient fish (Scott, Reference Scott1982). To introduce infection to a group of fish, a juvenile pet-store guppy (sex undetermined) from a naïve laboratory stock was infected with 3 parasites as above and added to the experimental tank for 4 or 6 days when 3 parasites had naturally transferred to the experimental fish in the group, at which time the juvenile pet-store guppy was removed (defined as ‘Day 0’ for each tank). This procedure eliminated the potential bias that might have occurred by initiating the epidemic on a male or a female in the mixed groups.
Parasites on each fish were counted every second day for 36 days or until no parasites were found in a tank on two consecutive counting days. In groups of fish, the first day of infection was noted separately for each group (Day 0 in all cases) and for each individual within the group, based on the day that it was first infected. If a fish in a group died, it was left in the tank for one day in order to allow transmission to other guppies (Scott and Anderson, Reference Scott and Anderson1984; Gheorghiu et al. Reference Gheorghiu, Cable, Marcogliese and Scott2007) and then removed.
Independent variables
Our independent test variables were sex (male vs female), group size (isolated vs grouped), and group composition (homogenous vs heterogeneous).
In addition, to account for variability in the size of fish at the beginning of the experiment (Cable and van Oosterhout, Reference Cable and van Oosterhout2007a ; Tadiri et al. Reference Tadiri, Dargent and Scott2013), we calculated the relative condition index (Kn) of each guppy based on its weight (W) and SL relative to all other fish of the same sex in the experiment. For each sex, a least squares regression of Log(SL) and Log(W) was performed, and the slope (b) and intercept (log(a)) for the line of best fit were obtained. Kn was then calculated for each individual fish as Kn = W/(a × SLb ) (Le Cren, Reference Le Cren1951; Peig and Green, Reference Peig and Green2010) using the sex-specific parameters. Average Kn was also calculated for each group of fish.
Definition and calculation of dependent variables
Peak parasite burden (maximum number of G. turnbulli), time to peak parasite burden, persistence of infection (last day of infection minus first day of infection) and host mortality were recorded for isolated fish, for each individual in a group, and for the population of grouped fish. In addition, asynchrony in when individual fish within groups became infected was recorded as the delay from when infection was introduced into the population. Maximum prevalence (per cent of infected fish in groups) and time to maximum prevalence over the course of the experiment were also recorded for groups of fish.
Statistical analysis
All analyses were done using R Language and Environment for Statistical Computing version 3.1.0 (R Development Core Team, 2014). Generalized Linear Mixed-Effects Models (GLMMs) were constructed to determine the effects of host sex, host group size (isolated vs group of 8), fish size (either W and SL or Kn, and average of the group for group-level response variables) and group composition (homogeneous or heterogeneous) and the interactions thereof on host mortality, peak parasite burden, time to peak and persistence on isolated fish, on individuals in groups and in the group as whole. For each response variable, models were fitted to the distribution of the variable and models for individual fish-level response variables were nested within the random variable tank. Models using SL and W as metrics for size were not significant, so all final models used only Kn. Final models were produced using the stepAIC function to select the combination of factors which produced a model with the lowest Akaike information criteron (AIC). In all cases, the level of significance was set at P < 0·05, and all values reported are means and standard errors.
RESULTS
Basic parasite dynamics
A total of 28 fish (13·2%) died over the course of the experiment, and mortality did not significantly differ between group sizes (P = 0·271) or between sexes (P = 0·433).
In all but two grouped tanks, parasites reached 100% prevalence within 14 days as additional fish became infected asynchronously (Fig. 1b, c, e, and f). In tanks, parasite numbers increased and reached distinct population peaks (Fig. 2). The rate at which fish became infected (delay to infection) did not significantly differ among groups (data not shown). Group composition (females, males, mixed sex) had no impact on peak prevalence, time to infection or time to peak prevalence (data not shown).

Fig. 1. Parasite population dynamics on individual isolated females (a), individual females in a sample all-female tank (b), and individual females in a sample mixed-sex tank (c), as well as individual isolated males (d), individual males in a sample all-male tank (e) and individual males in a sample mixed-sex tank (f). Data are square-root transformed for graphing purposes, but were not transformed for analysis. For all panels, ‘Day 0’ indicates the day on which at least 3 parasites were first found in the tank.
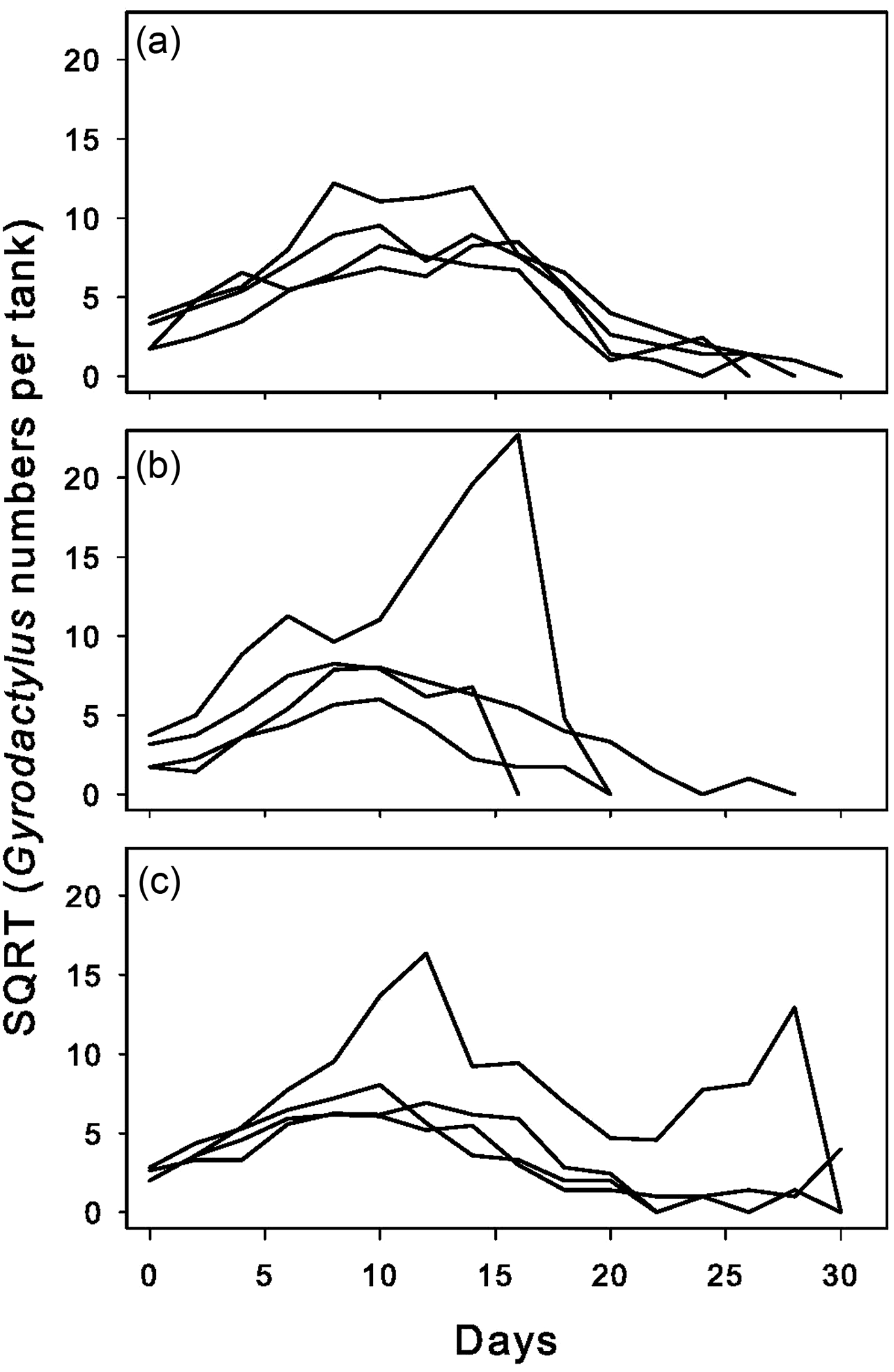
Fig. 2. Total parasite population numbers over the course of the experiment in group tanks for male groups (a), female groups (b) and mixed-sex groups (c). Data are square-root transformed for graphing purposes, but were not transformed for analysis. For all panels, ‘Day 0’ indicates the day on which at least 3 parasites were first found in the tank.
Table 1 gives a full overview of the outcomes of our GLMMs and results are explained below in detail.
Table 1. Outputs of generalized linear mixed-effects models for response variables. For two-way comparisons, the comparison is explained in parentheses next to the variable name

a Kn is the relative condition index based on weight (W) and standard length (SL) of each fish relative to all other fish of the same sex in the experiment. See section Materials and Methods for calculation.
Individual vs grouped fish
Peak total parasite population on groups of fish was higher (123·5 ± 40·0) than on isolated fish (26·4 ± 6·0) (P < 0·001). Parasite populations also persisted longer (P = 0·001) on groups of fish (24·5 ± 1·3 days) than on isolated fish (17·0 ± 1·5 days). Overall, isolated fish had lower peak burdens than individual fish in groups (P = 0·015), but there was an interaction between sex and grouping, with isolated females having higher peak burdens (34·9 ± 10·1) than individual females within groups (17·5 ± 2·6) (Fig. 3). No difference in parasite time to peak or persistence on an individual fish was found between isolated fish or individual fish in single-sex or mixed groups.

Fig. 3. Mean peak burden (±s.e.) vs mean time to peak (±s.e.) for individual fish in each treatment. Abbreviations: SF, single (isolated) females, SM, single (isolated) males, FG, female groups, MG, male groups, MIX, mixed groups.
There was a significant interaction of Kn and grouping (isolated vs in a group of 8) (P = 0·01), with the effect of Kn on parasite burden being stronger on isolated fish than on individual fish within single-sex or mixed groups (Fig. 4).

Fig. 4. Interaction of Kn and sex for isolated (A) and grouped (B) fish. Log(peak burden + 1) used for graphing purposes, but not for statistical analysis.
Male vs female hosts
Parasites reached higher peak burdens on isolated females (34·9 ± 10·1) than on males (15·4 ± 5·0) (P = 0·006). There was an interaction of sex and Kn (P = 0·038) on peak parasite burden both for isolated fish and individual fish in groups, with Kn having a positive impact on parasite load for males but not for females (Fig. 4). Parasite numbers peaked later (P = 0·001) (Fig. 3) on females (9·4 ± 0·7 days) than on males (6·6 ± 0·5 days) and the infection persisted longer (P = 0·033) on females (17·4 ± 0·9 days) than on males (13·6 ± 0·7 days). Infection also persisted longer on fish with a higher Kn (P = 0·0479), regardless of sex.
At the group level, there was no difference in time to peak prevalence, parasite population peak burden, time to peak population burden or parasite persistence in a tank between male and female groups.
Group composition: single-sex vs mixed sex groups
We found no differences between mixed-sex groups and single-sex groups (or individual fish within them) for any of the response variables.
DISCUSSION
Our investigation of parasite dynamics on isolated (single host patch) and grouped (multiple host patches) fish confirms that metapopulation theory is compatible with our model system (Grenfell and Harwood, Reference Grenfell and Harwood1997; Hanski, Reference Hanski1999), as the presence of multiple patches and connectivity among them allowed the parasite total population to grow larger and persist longer than on single isolated fish. There was no difference in time between when fish first became infected and when parasite burden peaked or in duration of infection between isolated fish and individual fish in a group, but time to peak parasite numbers in the tank and duration of infection in the tank were prolonged in groups compared with isolated fish. In this aspect, dynamics on each fish were similar but occurred asynchronously due to consecutive infection, leading to longer persistence of the overall parasite populations. We found that fish characteristics in the form of sex and Kn impacted parasite dynamics in isolation, but that these differences were not observed in grouped fish.
Although peak parasite total populations were higher on groups than on isolated fish, they were not 8 times higher, and the existence of additional hosts lowered the average parasite burden per fish for female hosts. The addition of multiple hosts presumably provided the parasite with more options if their host mounted an immune response, died, or became overcrowded with parasites (Bagge et al. Reference Bagge, Poulin and Valtonen2004), and thus allowed it to reach a population growth rate closer to the parasite's innate reproductive potential. However, parasite population growth and dispersal were likely constrained due to trade-offs between carrying capacity, reproductive potential and the cost of migrating. Our study would indicate that the costs of transmission and the parasite's own reproductive potential may have had a greater impact on parasite dynamics than overall quality of the host (carrying capacity). Of course, these inferences are limited by the fact that our epidemics were run in a highly controlled, experimental setting and began with only 3 parasites. It is possible that host abundance and sex could have a greater impact if more parasites had been introduced.
Consistent with our hypothesis, parasites reached higher burdens and persisted longer on isolated female guppies compared with isolated male guppies. One reason could be that females from the populations we used have been shown to be less resistant to parasites than males (Dargent et al. Reference Dargent, Rolshausen, Hendry, Scott and Fussmann2015). Another reason could be that the larger size of females compared with males provided more resources for the parasite in terms of food, space and ability to move to another region of the host to avoid local defence reactions (Poulin and Rohde, Reference Poulin and Rohde1997). Previous work has shown that larger guppies harbour more gyrodactylids than smaller ones (Cable and van Oosterhout, Reference Cable and van Oosterhout2007a ) and that the parasites disperse more rapidly through a group of fish when introduced on a fish with a higher Kn (Tadiri et al. Reference Tadiri, Dargent and Scott2013). In this study, we found a positive relationship between Kn and peak parasite burden on isolated males, but not on isolated females (which were overall larger than males). However, despite differences in parasite dynamics between the sexes observed at the individual level, we did not find a difference in parasite burden between individual grouped males and grouped females, nor did we find any effect of Kn on parasite burden for grouped fish, indicating that there was also an effect of group size on individual burden.
In contrast to previous reports of higher transmission in female than male groups (Richards et al. Reference Richards, van Oosterhout and Cable2010) and higher transmission in male than female groups (Richards et al. Reference Richards, van Oosterhout and Cable2012), we did not observe any differences in peak prevalence, time to first infection or time to peak prevalence between our single-sex groups. In both previous studies, the measure of transmission was the number of non-focal fish that became infected within 3 days of introduction of a focal fish infected with either 30 (Richards et al. Reference Richards, van Oosterhout and Cable2012) or 100 gyrodactylids (Richards et al. Reference Richards, van Oosterhout and Cable2010). This contrasts with our protocol in that we explored transmission from an initial population of 3 parasites to the time of peak prevalence in populations of smaller feeder guppies at higher density compared with the larger ornamental guppies kept at lower density. Richards et al. (Reference Richards, van Oosterhout and Cable2012) suggested that transmission may be a function of initial parasite load and the impact it has on shoaling behaviour or courtship displays but given the number of differences between our experiment and the two previous studies, it is difficult to attribute the different findings to a single factor.
We found that parasites peaked earlier on males than females, despite having similar burdens in groups. One possibility for the lower parasite growth rate in grouped females could be that females increase investment in parasite resistance (rather than growth) when grouped at a high density, where infection is more likely to occur, an effect observed in many invertebrate systems (Wilson and Cotter, Reference Wilson, Cotter, Ananthakrishnan and Whitman2008), and potentially also in ours (Pérez-Jvostov et al. Reference Pérez-Jvostov, Hendry, Fussmann and Scott2015). While we did not find a significant difference in somatic growth between isolated and grouped females as Pérez-Jvostov et al. (Reference Pérez-Jvostov, Hendry, Fussmann and Scott2015) did, this could have been an issue of power, since there were only 8 isolated females and changes in weight were much less drastic than differences in parasite loads.
We also found no effect of group composition (homogenous vs heterogeneous) on parasite dynamics, as our mixed-sex groups did not differ from all-male or all-female groups, nor did individuals within these groups. This finding is inconsistent with theoretical work that suggests heterogeneity would promote asynchrony in local population dynamics and therefore parasite persistence (Hagenaars et al. Reference Hagenaars, Donnelly and Ferguson2004; Singh et al. Reference Singh, Rao, Ramaswamy and Sinha2004; Colizza and Vespignani, Reference Colizza and Vespignani2008). However, since parasite dynamics were similar between single-sex groups of males and females in our study, mixing the sexes in our system may not have generated the heterogeneity in individual hosts that we had expected and can thus explain why we found no influence of heterogeneity on parasite dynamics. Similar results have also been reported in mice (Scott, Reference Scott1991), where grouping susceptible and resistant strains together resulted in similar nematode burdens among mice of both strains, but that increasing transmission rates effected a distinction between the two strains (Scott, Reference Scott2006). However, those studies did not investigate parasite dynamics in single-strain groups, and our results indicate that grouping, rather than group composition, has the greatest impact in homogenizing parasite dynamics.
Although this study set out with the intention of determining how host heterogeneity may influence parasite population dynamics, we found that group composition and factors which influenced parasite dynamics on fish in isolation (Kn and sex) had almost no effect on parasite dynamics on fish in groups or at the group level. These findings indicate that factors associated with grouping fish become more relevant than the effects of the individual host characteristics sex and Kn of individual hosts for both individual and group-level outcomes, but we are cautious about over-generalizing these interpretations, given that our study comes with the limitations of using a specific experimental system.
Our ability to detect some biologically important differences may have been limited by having only 4 replicates per treatment. The relatively small size of the fish tanks probably limited our ability to detect differences in parasite dynamics that would have been driven by host behaviours including shoaling of females but not males. We did not know the infection history of individual fish, other than the fact that they had been previously exposed to parasites, and as such could not explore any possible impact of differences in acquired resistance to parasites (Scott and Robinson, Reference Scott and Robinson1984; Scott, Reference Scott1985a ; Richards and Chubb, Reference Richards and Chubb1998) or of an interaction between sex and acquired resistance. Finally, this study only looked at two host traits (sex and size) and it is possible that other host characteristics, such as Major Histocompatibility Complex (MHC) profiles (Fraser et al. Reference Fraser, Ramnarine and Neff2009), colour (Houde and Torio, Reference Houde and Torio1992) or population of origin (Van Oosterhout et al. Reference Van Oosterhout, Harris and Cable2003; Dargent et al. Reference Dargent, Scott, Hendry and Fussmann2013), could have a stronger impact on parasite dynamics.
Metapopulation theory, while compatible in our system in the sense that additional hosts allowed for asynchronous dynamics to promote parasite persistence, predicts that heterogeneity in patch quality prolongs persistence due to greater asynchrony in local patch dynamics (Dennis and Eales, Reference Dennis and Eales1997; Thomas et al. Reference Thomas, Bourn, Clarke, Stewart, Simcox, Pearman, Curtis and Goodger2001; Fleishman et al. Reference Fleishman, Ray, Sjögren-Gulve, Boggs and Murphy2002; Bonte et al. Reference Bonte, Lens, Maelfait, Hoffmann and Kuijken2003; Schooley and Branch, Reference Schooley and Branch2007; Franzén and Nilsson, Reference Franzén and Nilsson2010). Our study has shown that the ability of a parasite to move from host to host (connectivity) may override individual host differences in the absence of connectivity, thus rendering the expectation of persistence over heterogeneous patches weaker for our system. This study served as the first step towards conceptualizing a theory that incorporates dynamics within individual hosts rather than focusing solely on infection status of individuals (like microparasite models) or the total parasite populations (like macroparasite models), and further investigation into these dynamics is necessary to develop a more unifying framework for parasite population growth and dissemination.
ACKNOWLEDGEMENTS
We would like to thank Stanley King for identification of the parasite. We would also like to thank Mark Romer and Claire Cooney for the management of the McGill Phytotron.
FINANCIAL SUPPORT
Funding for this research was provided by a Fonds de recherche du Québec – Nature et technologies (FQRNT) grant (57516) and National Sciences and Engineering Research Council (NSERC) Discovery grants were awarded to M. E. S. and G. F. F. Research at the Institute of Parasitology is supported by a FQRNT regroupement grant to the Centre for Host–Parasite Interactions.








