INTRODUCTION
Scabies is a skin condition caused by infestation of the epidermis with the ectoparasitic mite Sarcoptes scabiei. The disease remains prevalent in the developing world, with an estimated 300 million people affected (Taplin et al. 1990). Scabies is endemic in northern and central Australian remote Aboriginal communities, with reported point prevalences of 25% in adults (Carapetis et al. 1997) and up to 50% in children (Currie, Connors and Krause, 1994). Secondary streptococcal infection of skin due to scabies lesions is a significant cause of morbidity, and has been linked to the extreme rates of post-streptococcal disease observed in these communities (Currie and Carapetis, 2000; McDonald, Currie and Carapetis, 2004). Mass treatment programmes using the pyrethroid acaricide 5% permethrin have been successful in reducing overall rates of scabies and streptococcal pyoderma (Carapetis et al. 1997). However, anecdotal reports of clinical failure and in vitro reports of tolerance to permethrin (Walton, Myerscough and Currie, 2000), unpublished observations) suggest the potential emergence of resistance, threatening the success of such programmes.
Ivermectin has been suggested as a suitable alternative for mass treatment programmes (Lawrence, Sheridan and Speare, 1994; Lawrence et al. 2005), and has recently been licensed for the treatment of ordinary scabies in France (Giudice, Chosidow and Caumes, 2003). Ivermectin was introduced for the treatment of crusted (severe) scabies in northern Australia in the early 1990s and has been used successfully in combination with topical acaricides. However, repeat infestations and recrudescence with ivermectin have been reported, with multiple doses required to achieve a cure (Huffam and Currie, 1998; Walton et al. 1999). Importantly, clinical and in vitro resistance to ivermectin has recently been documented in 2 crusted scabies patients who had previously received 30 and 58 doses of ivermectin over 4 and 4·5 years respectively. No in vitro resistance to topical acaricides was observed in these mites, however, and infestations were eventually cleared by increased topical permethrin and benzyl benzoate application (Currie et al. 2004). This raises serious concerns regarding the sustainability of ivermectin treatment for scabies, particularly if it is applied as a public health measure in a community setting. Ivermectin has been successfully used for the annual mass treatment of onchocerciasis in West Africa since 1987, with over 100 million doses distributed (Ardelli and Prichard, 2004). Ivermectin resistance has not yet been documented, although suboptimal responses of Onchocerca volvulus to ivermectin have recently been reported (Awadzi et al. 2004), suggesting resistance may be developing. After more than 20 years of intensive use, ivermectin resistance is widespread in veterinary helminths (Wolstenholme et al. 2004).
As information accumulates, it is becoming apparent that ivermectin resistance mechanisms are complex and probably multifactorial. However, several candidate mechanisms are well established. One proposal involves alterations to the Glutamate (GluCl) or GABA-gated chloride channels, which are members of the ligand-gated ion channel family and the physiological targets of ivermectin in invertebrates (Blackhall et al. 1998a; Feng et al. 2002; Blackhall, Prichard and Beech, 2003; Njue et al. 2004). Interestingly, selection at a beta-tubulin gene has recently been observed in ivermectin exposed O. volvulus, although the functional basis behind this remains uncertain (Eng and Prichard, 2005).
Another potential mechanism for ivermectin resistance is increased cellular export mediated by ATP-binding cassette (ABC) transporters. ABC transporters can confer multidrug resistance by transporting a broad range of substrates across membranes, thus leading to decreased intracellular accumulation. ABC transporters are presently grouped into 8 subfamilies according to sequence similarity and domain organization (Sheps et al. 2004). Several of these proteins have been associated with drug resistance, the most well known of which is the ABC-B transporter P-glycoprotein.
P-glycoprotein was first discovered to be over-expressed in multidrug-resistant cancer cells; hence the common referral of P-glycoprotein as the multidrug resistant gene (mdr). Increased expression of P-glycoprotein in these cells confers resistance to a wide range of hydrophobic drugs (reviewed by (Gottesman et al. 1995). Increased copy number of the P-glycoprotein homologue pfmdr1 in Plasmodium falciparum was initially thought to be associated with chloroquine resistance in malaria parasites (Foote et al. 1990), but more recent evidence suggests a stronger link to mefloquine resistance (Price et al. 1999, 2004).
Ivermectin is known to be an excellent substrate for ABC transporters such as P-glycoprotein (Nobmann, Bauer and Fricker, 2001), with mammals deficient in P-glycoprotein displaying hypersensitivity to ivermectin (Schinkel et al. 1994; Roulet et al. 2002). Several molecular studies suggest the involvement of P-glycoprotein in ivermectin resistance, although a functional association has not yet been demonstrated. Early research found alterations to P-glycoprotein and increased mRNA levels in ivermectin resistant Haemonchus contortus (Xu et al. 1998). Selection at a P-glycoprotein allele following ivermectin treatment has been observed in H. contortus (Blackhall et al. 1998b), and more recently Onchocerca volvulus (Eng et al. 2005).
Thus it is possible that P-glycoproteins or other ABC transporters might play an important role in the development of ivermectin resistance in S. scabiei. Better monitoring tools will enable more accurate assessment of treatment failures and provide a more sensitive evaluation method, particularly in the management of emerging resistance in a remote community setting. To investigate mechanisms of ivermectin resistance, an important preliminary step is to identify and characterize candidate resistance genes. In this study, we utilized an EST library and database to identify potentially relevant ABC transporter genes from S. scabiei.
MATERIALS AND METHODS
Searching the Sarcoptes scabiei var. hominis EST dataset
A Sarcoptes scabiei var. hominis cDNA library was constructed using the shed skin of crusted scabies patients as previously described (Fischer et al. 2003a,b). An EST dataset of 43776 sequences was established from the library (Holt et al. 2003). Putative ABC transporters were initially identified by comparing the EST dataset to GenBank using BLASTx (Altschul et al. 1990). Additional putative ABC transporters were identified by searching the EST dataset for homologues of the P-glycoprotein ABC transporters, Pgp-49 (Q00449) from Drosophila melanogaster, and Pgp-A (AAC38987) from H. contortus. Contigs identified as potential ABC transporters were further analysed using BLASTp.
Sequence extension of EST contigs
Sequences of the individual clones within contigs were completed by sequencing with vector and specific primers as required. In addition, a semi-nested PCR approach on the cDNA library using a combination of vector and nested specific primers was employed to try and extend the contig sequences. Primers used for clone sequence completion and contig extension are listed in Table 1. All primers were synthesized by Proligo (Lismore, NSW, Australia).

PCR reactions contained 1× PCR buffer, 0·2 mM dNTPs, 1·25 μM each of vector and sequence-specific primer, 1 U Taq polymerase (Qiagen, Brisbane, QLD, Australia) and 1 μl cDNA library template DNA. Reactions were cycled at 94 °C for 30 sec, 56 °C for 30 sec and 72 °C for 1 min and 30 sec for 35 cycles, followed by a final extension step of 72 °C for 10 min (annealing temperatures varied slightly according to primer Tm). First round PCR products were diluted 10-fold and 100-fold, and subjected to a second round of PCR using the sequence-specific nested primer.
PCR products were either excised from agarose gels prior to purification or directly purified using the MinElute PCR purification kit (Qiagen). PCR products were cloned using the pGEM-T Easy cloning kit according to the manufacturer's protocol (Promega, Annandale, NSW, Australia). Ligations were electroporated into E. coli XL1-Blue cells (Stratagene, LaJolla, CA, USA) and plasmid DNA was extracted using a miniprep kit according to the manufacturer's protocol (Bio-Rad, Regents Park, NSW, Australia). Plasmids were sent to the Biomolecular Research Facility (Newcastle, NSW, Australia) for sequencing with M13 vector primers (Table 1).
Identification of a P-glycoprotein using degenerate PCR
To remove E. coli contamination, the cDNA libraries were treated with DNaseI to digest non-phage DNA. A fresh aliquot of the normalised cDNA library was mixed with 10 U DNaseI (Roche, Castle Hill, NSW, Australia) and 2·5 mM of MgCl2 and incubated at 37 °C for 2 h. The reaction was heat inactivated for 10 min, cooled and purified using the QiaQuick reaction clean-up kit (Qiagen).
Degenerate primers for amplifying P-glycoprotein fragments were employed based on the highly conserved ATP-binding domains of P-glycoprotein (Table 2). This approach has been successfully used to isolate P-glycoprotein in several other studies (Kwa et al. 1998; Xu et al. 1998; Huang and Prichard, 1999; Sangster et al. 1999). S. scabiei var. hominis cDNA libraries (Fischer et al. 2003a,b) and multi-mite genomic DNA preparations (Mounsey et al. 2005) were used as templates for nested PCR. First round primers were used in all 16 possible combinations. Cycling conditions were 94 °C for 30 sec, 55 °C for 30 sec, and 72 °C for 1 min and 30 sec, for 35 cycles followed by a final extension step of 72 °C for 10 min. PCR reactions contained 20–200 ng DNA, 0·2 mM dNTPs, 2 μM of each primer and 1·25 U of Taq polymerase. One μl of the first round PCR products was used as a template for the second round reaction with the above parameters employed.

PCR-based library screening for P-glycoprotein
The P-glycoprotein cDNA fragment obtained through degenerate PCR was used to probe the normalised cDNA library. A high-stringency PCR-based screening approach was employed, based on the method developed by Israel (1993). Primers corresponding to the sequence were designed to generate a 280 bp PCR product (236F1/R1, Table 1). All PCR reactions used for screening contained 1 μl of template DNA, 1× PCR buffer, 0·2 mM dNTPs, 1·25 μM primers and 1·25 U Taq polymerase. Reactions were cycled at 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 sec, 50 °C for 30 sec and 72 °C for 45 sec.
Library titration and a dilution series PCR on the normalised cDNA library determined the minimal starting amount of library phage DNA required to generate a PCR product. For the first round of screening, 106 pfu (in 1 ml of SM buffer) were used to infect 1 ml of E. coli XL1-Blue MRF' cells at OD590 1.0 (Stratagene). Then 18 ml of LB broth were added and the mixture plated in an 8×8 matrix (100 μl/well) in a 96-well plate. The plate was sealed and incubated at 37 °C with shaking for 5 h. Rows and columns were pooled by taking a 20 μl aliquot from each well and diluting 1[ratio ]1 with distilled water. Ten μl of chloroform were added to the pooled phage suspensions and reactions were centrifuged briefly prior to PCR. Single PCR-positive wells were selected and the above process repeated several times to enrich the number of positive clones for each round of screening.
After the 4th round of screening, approximately 2000 pfu from the PCR-positive well were plated onto NZY agar plates. Plaque lifts were performed using Hybond-XL membranes according to the manufacturers' protocol (Amersham, Castle Hill, NSW, Australia). A 280 bp probe was generated using the 236F1/R1 primers, purified and labelled with [α-32P] dCTP using the Redivue random priming labelling kit and hybridization was performed at 55 °C overnight according to the manufacturer's protocol (Amersham). Following hybridization, filters were washed 3 times at 55 °C in 2× SSC, 0·1% SDS. Membranes were sealed, exposed to X-ray film at −80 °C overnight and developed.
Hybridization-positive plaques were cored from the agar and placed into 500 μl of SM buffer and 20 μl of chloroform, vortexed, and stored at 4 °C. PCR on the eluted phage stocks was performed as described above. Single-clone phagemid excision was performed on the hybridization and PCR-positive plaques according to the Stratagene Zap Express protocol. Plasmid DNA was extracted using the QiaPrep Spin Miniprep Kit (Qiagen), and sequenced as described above, using the bacteriophage T3 and T7 vector primers (Table 1).
Sequence analysis
Sequence chromatograms were viewed, edited and aligned using the Lasergene software package (DNAstar Inc, Madison, WI, USA). Edited DNA sequences were translated into amino acid sequences using Flip6 frames (Brossard, 1997), accessed via Biomanager (www.angis.org.au). Amino acid sequences were then compared to the conserved domain databases accessed via the NCBI server (http://www.ncbi.nlm.nih.gov/blast/) using BLASTp. Additionally, BLASTn searches were performed between the nucleotide sequences and E.coli genomic databases to assess potential contamination. Sequences reported in this paper are available in the GenBank™ database under the Accession numbers DQ146410-DQ146418.
Cluster analysis
Further analysis of the S. scabiei sequences was conducted to confirm the assignment of ABC-subgroups, and to examine the relationship of the S. scabiei ABC transporters to sequences from Caenorhabditis elegans and Drosophila melanogaster (Table 3). Peptide sequences corresponding to the conserved ATP-binding regions were aligned using ClustalW and bootstrapping for confidence determination was performed using Seqboot. Consensus dendrograms were constructed using the Parsimony (Protpars) algorithm. Because the contig 7008C03 did not appear to contain an ATP-binding motif it was excluded from this analysis.

RESULTS
Identification and extension of EST contigs with homology to ABC transporters
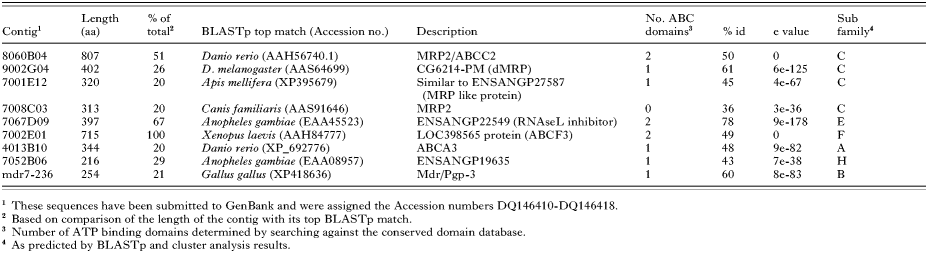
Preliminary analysis of a S. scabiei EST dataset of 43776 sequences, identified 9 contigs with homology to ABC transporters. Contig extension using a semi-nested PCR approach on the cDNA libraries and on further sequencing of individual clones yielded additional sequence information. During this process, 2 of the previously separate contigs were found to overlap and were combined.
Sequence analysis of contigs
Four of the resulting 8 S. scabiei contigs displayed significant homology to the multidrug resistance protein (MRP/ABC-C) family of ABC transporters (Table 4). 8060B04, 9002G04 and 7001E12 all aligned over the C-terminal region of their MRP homologues, while 7008C03 aligned over the central region of the protein, possibly between ATP-binding domains. This was supported by the absence of ATPase consensus regions in the 7008C03 sequence. Although the contigs presented here were homologous to similar proteins, alignments between the contigs indicated they were sufficiently different from each other not to be considered duplicate or different isoforms of a single S. scabiei protein.

Contig 7067D09 shared high levels of homology (77%) with the conserved RNAse L inhibitor proteins from many organisms. These proteins belong to the ABC-E subfamily of ABC transporters. Two ATP-binding domains were identified, and a conserved domain associated with the RNAase L inhibitor ATPase was also detected (Table 4).
The deduced amino acid sequence of the contig 7002E01 was determined to have significant similarity to the ABC-F transporter GCN20 from several organisms (Table 4). From alignment with the other proteins in this family, the sequence of 7002E01 was determined to be complete. Conserved domains of ATPase components were detected in 2 regions of the sequence (Table 4). No transmembrane domains were identified in the protein, which is consistent with other ABC-F transporters.
Contig 4013B10 displayed significant similarity to the C-terminus of ABC-A proteins from various organisms (Table 4). The 7052B06 clone contig appeared to be chimeric, with BlastP detecting no homology over the first 150 bp. The remainder of the protein aligned with the N-terminal ATP binding domain of ABC-H proteins from Anopheles gambiae and D. melanogaster, and ABC-G proteins from other organisms (Table 4).
As none of these contigs appeared to be members of the ABC transporter subfamily B, which includes the P-glycoproteins implicated in drug resistance in some organisms, a further degenerate PCR approach was utilized in order to identify a member of this group.
Degenerate PCR
PCR of the DnaseI-treated cDNA library using degenerate primers designed to the highly conserved ATP-binding domains of P-glycoproteins, successfully yielded products of the expected size for 2 primer combinations--mdrF2/R3/R5 and mdrF2/R3/R6. The fragment from the latter (designated mdr7-236) was cloned and sequenced. The sequence was found to share significant homology to P-glycoprotein from several organisms. No significant similarity was detected from BlastN against the E. coli database indicating the sequence was not derived from E. coli contamination. Degenerate PCRs on genomic mite DNA preparations resulted in the cloning of 2 fragments. The first clone was amplified using the primer combination mdrF1/R4/R6. It was found to be identical to the mdr7-236 sequence but containing a 70 bp intron. The second genomic clone was amplified with the primer combination mdrF3/R3/R5. Although no obvious introns were present, there were multiple stop codons and thus the sequence could not be translated to a single open reading frame. tBLASTx was performed on the nucleotide sequence, with the highest match being the N-terminal ATP-binding domain of CG3879/mdr 49 from Drosophila melanogaster (63% ID, 2e-12). The presence of multiple stop codons suggested the sequence might represent a pseudogene so it was not included for further analysis.
PCR-based library screening
The cDNA was enriched for mdr7-236 phage, through several rounds of PCR-based screening and amplification. Hybridization of the plated enriched stock with a mdr7-236 probe, yielded 2 positive plaques. These phages were confirmed to be positive via PCR with mdr7-236 specific primers then excised to phagemids and sequenced. The clones were found to contain a 1094 bp insert which included the original mdr7-236 sequence. This extended mdr7-236 sequence showed homology to the C-terminal region of many P-glycoproteins, with the top BlastP match being P-glycoprotein 3 from Gallus gallus (Table 4).
Cluster analysis of ABC transporters from S. scabiei
The EST contigs from S. scabiei all grouped closely with their respective homologues from D. melanogaster and C. elegans (Fig. 1). The proteins clustered according to predicted ABC-subfamily. Although the designated ABC-H proteins from S. scabiei and D. melanogaster grouped together, the ABC-H from C. elegans instead grouped with ABC-G. The MRP-like proteins from S. scabiei all fell within the ABC-C group. 7001E12 sat apart from other members in this subgroup, supporting the BLASTp result which indicated that 7001E12 was more divergent, whereas 8060B04 and 9002G04 were more similar to MRP 1 and 2 from other organisms. ABC subfamilies B and C were closely related, with group B apparently derived from group C.

Fig. 1. Dendrogram of Sarcoptes scabiei (Ss) and selected Drosophila melanogaster (Dm) and Caenorhabditis elegans (Ce) ABC transporter ATP-binding domains. Accession numbers for the sequences used are listed in Tables 3 and 4.
DISCUSSION
In this study we identified 9 ABC transporter genes from Sarcoptes scabiei. ABC-subfamilies A, C, E, F and H were represented in the EST database, with an ABC-B protein subsequently identified by further library screening. Cluster analysis found that most S. scabiei contigs clustered closely with their D. melanogaster homologue in each respective subgroup.
Contig 4013B10 was found to belong to the ABC-A subfamily. These are among the largest ABC transporters, averaging 1700aa or more. Anopheles gambiae has 6 ABC-A proteins, whereas Drosophila has 19 (Roth et al. 2003). The physiological roles of these proteins in invertebrates remain unexplored. In humans, ABC-A proteins may be involved in cholesterol transport and have also been implicated in drug resistance (Dean, Rzhetsky and Allikmets, 2001). ABC-A proteins are not present in the yeast chromosome, indicating they evolved following multicellularity (Sheps et al. 2004). Contig 7067D09 was found to encode the RNAse-L inhibitor protein, belonging to the ABC-E family. This gene is highly conserved and represented as a single copy across most genomes, which suggests an essential housekeeping function. In humans it is involved in the antiviral immune response and is implicated in mRNA turnover. Unlike other ABC transporters, this subfamily contains no transmembrane domains, and apparently duplicated ATP-binding domains. It is consequently often classified as a ‘non-transport’ ABC protein. The S. scabiei 7002E01 sequence displayed homology to the ABC-F protein GCN-20. ABC-F proteins share a similar domain organization to ABC-E, with no transmembrane domains. GCN-20 is involved in translational regulation via the activation of eIF2α kinase (Marton et al. 1997). This group of proteins is well conserved between most genomes studied, with 3 members each.
An interesting discovery was the assignment of contig 7052B06 to the ABC-H subfamily. This recently discovered group of proteins has been identified in the D. melanogaster and A. gambiae genomes (Misra et al. 2002; Roth et al. 2003), but is apparently absent from higher eukaryotes. Although there are suggested ABC-H representatives in C. elegans, our results indicate they are phylogenetically distinct from the arthropod protein (Sheps et al. 2004). Nothing is known about the physiological function of this subfamily, but because of its apparent uniqueness to arthropods it has been earmarked as a potential insecticide target (Roth et al. 2003).
Four of the 9 S. scabiei ABC transporters identified in this study belonged to the ABC-C subfamily. ABC-Cs are well represented across other genomes, with Homo sapiens and Drosophila containing 12 members, and Anopheles having 14 members. ABC-C proteins have been extensively studied due to their implication in multidrug resistance, hence their alternate title of multidrug resistance proteins (MRP). They are closely related to P-glycoproteins with a broad, sometimes overlapping substrate profile. One of the main differences is that MRPs transport substances complexed with glutathione. Additionally, several MRPs are distinct from P-glycoproteins, in that they contain an additional N-terminal transmembrane domain of unknown function. Three S. scabiei contigs (8060B04, 9002G04 and 7008C03) were found to have homology to MRPs 1 and 2 from mammals and Drosophila. This gene has received recent attention in both Anopheles and Drosophila genome annotation. Splice variants from a single MRP gene from Anopheles can encode 10 isoforms (Roth et al. 2003), whereas the Drosophila ortholog (CG6214/dMRP) can encode 14 isoforms (Grailles, Brey and Roth, 2003). The implication of the presence of multiple isoforms is not known, but since exon variation occurs in regions thought to be involved in substrate recognition, these proteins may have an even broader range of substrates, which has implications for drug resistance. Multiple sequence alignments of the S. scabiei ABC-C contigs suggest that they are independent proteins rather than isoforms of a single gene. However, further investigations are needed to fully characterize this important group of proteins in S. scabiei, and particularly to determine whether ivermectin is a substrate.
The identification of a P-glycoprotein (ABC-B) homologue from S. scabiei was highly significant for our search into ivermectin resistance candidates. Our initial survey of the S. scabiei EST database failed to identify P-glycoprotein sequences. However, the EST dataset represents only a small proportion of the library, and P-glycoproteins were subsequently identified from the cDNA library and genomic DNA via degenerate PCR and library screening. The resulting clone had over 50% identity to the C-terminal region of many P-glycoproteins across a range of organisms.
Homologues of several of the ABC transporters identified in this study have been implicated in drug resistance in other organisms and thus are of interest to future studies detailing mechanisms of ivermectin resistance in scabies mites. In humans, the ABC-A3 is found in association with MRP and is also thought to play a role in resistance to anticancer drugs (Klugbauer and Hofmann, 1996). Of the greatest significance is the identification of 2 S. scabiei ABC transporter groups strongly associated with drug resistance: P-glycoprotein (ABC-B) and multidrug resistance proteins (ABC-C).
An important aspect of future research will be to explore, compare and contrast the copy number and expression levels between MRP and P-glycoprotein, and importantly to determine any significant differences between ivermectin resistant and susceptible mites. Recent studies have shown evidence of ivermectin exerting selection pressure at a P-glycoprotein gene from Onchocerca volvulus (Ardelli, Guerriero and Prichard, 2005; Eng et al. 2005). It will be important to determine whether similar selection is observed in our mite populations subjected to multiple doses of ivermectin and presumably under extremely high selection pressure.
The ABC transporters identified in this study will facilitate more detailed investigations into potential mechanisms of ivermectin resistance in S. scabiei. In light of in vitro and clinical evidence of emerging ivermectin resistance, careful monitoring and surveillance for further cases is critical to help avert the scenario of widespread ivermectin resistance observed in other parasites such as veterinary helminths. The development of a new class of acaricides is unlikely in the near future, and therefore prolonging the life of the limited available drugs is essential. Future applications of this work may be the development of more sensitive molecular-based methods for resistance monitoring, enabling strategies for early management of resistance.
This work was supported by the Australian National Health and Medical Research council Project Grant 288301. K.E.M. is supported by a Cooperative Research Centre for Aboriginal Health post-graduate research scholarship. We thank Professor David Kemp for helpful comments.