INTRODUCTION
Land-use change is recognized as one of the most important factors determining modifications to ecosystems, including epidemiological cycles, worldwide (Foley et al. Reference Foley, DeFries, Asner, Barford, Bonan, Carpenter, Chapin, Coe, Daily, Gibbs, Helkowski, Holloway, Howard, Kucharik, Monfreda, Patz, Prentice, Ramankutty and Snyder2002; Patz et al. Reference Patz, Daszak, Tabor, Aguirre, Pearl, Epstein, Wolfe, Kilpatrick, Foufopoulos, Molyneux and Bradley2004). For example, road construction has been linked to increased human contact with wildlife and potentially with novel zoonoses (Wolfe et al. Reference Wolfe, Eifel, Gockowski, Muchaal, Nolte, Proser, Torimiro, Weise and Burke2000), and the formation of culverts has resulted in ponds that are suitable breeding sites for malaria-transmitting anopheline mosquitoes (Marques, Reference Marques1987; Charlwood and Alecrim, Reference Charlwood and Alecrim1989). Parasites are ubiquitous in the lives of animals, and humans and also play a central role in ecosystems, affecting the ecology and evolution of species interactions (Esch and Fernandez, Reference Esch and Fernandez1993), host population growth and regulation (Hudson et al. Reference Hudson, Dobson and Newborn1998; Hochachka and Dhondt, Reference Hochachka and Dhondt2000), and even biodiversity (Hudson et al. Reference Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002). There is, in general, an established ecological and evolutionary balance such that macroparasites are able to survive and reproduce effectively without killing their hosts, thus maintaining their ability to continue reproducing in the host (Dobson and May, Reference Dobson, May, Soule and Sunderland1986; Lyles and Dobson, Reference Lyles and Dobson1993). Such stable systems can, however, be disrupted when new pathogens are introduced, potentially altering the natural dynamics and thus becoming problematic for species survival (Laurenson et al. Reference Laurenson, Sillero-Zubiri, Thompson, Shiferaw, Thirgood and Malcolm1998; Taraschewski, Reference Taraschewski2006).
In Asia, more than 70% of primates are classified on the IUCN Red List as Vulnerable, Endangered, or Critically Endangered, meaning that they could disappear forever in the near future (IUCN, 2011). Since the 1970s, the academic community has recognized that many primate populations are severely threatened by human activities (Chapman and Peres, Reference Chapman and Peres2001). Beside the commonly cited problems for primate conservation – habitat loss to settlement, logging and agriculture, illegal hunting for bushmeat, traditional medicine, and the live primate trade – disease has been recognized as a serious threat to endangered species (May, Reference May1988; Lyles and Dobson, Reference Lyles and Dobson1993; Daszak et al. Reference Daszak, Cunningham and Hyatt2000; Chapman and Peres, Reference Chapman and Peres2001; Lafferty, Reference Lafferty2003; Smith et al. Reference Smith, Sax and Lafferty2006). The dynamics of human–non-human primate interactions are changing radically. As a result of human population increase, combined with illegal hunting, and the fragmentation and degradation of formerly natural habitats, non-human primates are often forced to live in an anthropogenically disturbed landscapes comprising, for example, farmland, human settlements, forest fragments or isolated protected areas (Chapman and Peres, Reference Chapman and Peres2001; Wolfe et al. Reference Wolfe, Prosser, Carr, Tamoufe, Mpoudi-Ngole, Torimiro, LeBreton, McCutchan, Birx and Burke2004). Gillespie and Chapman (Reference Gillespie and Chapman2006) found that the index of forest patch degradation and the presence of humans strongly influenced the prevalence of gastrointestinal nematodes in red colobus monkeys. These, and the genetic similarity between humans and non-human primates may facilitate disease transmission between these species (Wolfe et al. Reference Wolfe, Escalante, Karesh, Kilbourne, Spielman and Lal1998; Chapman et al. Reference Chapman, Gillespie and Goldberg2005). Although there is a growing recognition that the transfer of diseases between humans and non-human primates can be of great significance for conservation biology (Wolfe et al. Reference Wolfe, Escalante, Karesh, Kilbourne, Spielman and Lal1998; Woodford et al. Reference Woodford, Butynski and Karesh2002), only a few studies have focused on helminth parasites (e.g. Legesse and Erko, Reference Legesse and Erko2004; Chapman et al. Reference Chapman, Speirs, Gillespie, Holland and Austad2006; Gillespie et al. Reference Gillespie, Greiner and Chapman2005, Reference Gillespie, Lonsdorf, Canfield, Meyer, Nadler, Raphael, Pusey, Pond, Pauley, Mlengeya and Travis2010; Wenz et al. Reference Wenz, Heymann, Petney and Taraschewski2010; Standley et al. Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, van Tulleken, Kane, van Lieshout, Ajarova, Kabatereine and Stothard2011, Reference Standley, Mugisha, Dobson and Stothard2012).
In a previous investigation, we determined the influence of human contact on the presence of gastrointestinal helminths in 2 species of arboreal New World monkey from a tropical rainforest in Peru (Wenz et al. Reference Wenz, Heymann, Petney and Taraschewski2010). Although no direct transmission of helminths from humans to these primates was detected, tamarins foraging in an area of human-altered habitat showed substantial changes in their parasite communities with potential disease consequences.
In the present study, we examined the influence of human contact and habitat modification on the behaviour and on the presence of gastrointestinal helminths in a species of Old World monkey, the long-tailed macaque (Macaca fascicularis) in northeast Thailand, where there is a high rate of interaction with human-modified habitats (Fuentes, Reference Fuentes2006).
MATERIALS AND METHODS
Study site
The study was conducted in 2 forest parks in north eastern Thailand, Kosumpee Forest Park (16°15′N and 103°04′E) in Kosum Phi Sai, and Don Chao Pu Forest Park (15°67′N and 104°86′E) in Pha Na (Fig. 1).

Fig. 1. Broad-view map showing the very general location of the study sites Kosum Phi Sai and Pha Na within Thailand.
Kosumpee Forest Park comprises an area of about 0·2 km2 of mixed deciduous forest and is located next to the Chi River on the northern edge of Kosum Phi Sai. According to the park staff, the Kosumpee macaque population has been isolated from other conspecific populations by extensive agricultural areas and human settlements since at least 1966, when the park was established. Based on information from the park authorities, the park is visited by about 100 000 tourists per year. Designated areas exist in the park for picnicking, sanitation and rubbish collection. Directly in front of the park are a school, a Buddhist temple and villagers’ houses. Park staff estimate Kosumpee supports 400–500 long-tailed macaques (Macaca fascicularis). However, this number may be greatly underestimated.
Don Chao Pu Forest Park in Pha Na, Amnat Charoen Province comprises an area of about 0·4 km2 and is also mixed deciduous forest. It is located about 100 m outside the village along a road. This more than 100 years old park is a designated animal sanctuary, but it has seen encroachment. It is visited mostly by people from surrounding areas and is also used for several municipal and religious occasions. For example, at the end of December the park is used by monks for meditation and Buddhist teaching, while local farmers also graze cattle inside the park. However, there are also some minimally impacted areas of forests inside and next to the park. Macaques living in theses areas have access to clear water and natural food sources throughout the year. The park is home to 300–400 long-tailed macaques (Macaca fascicularis) with some satellite colonies around the park.
Both study areas represent semi-natural settings with a considerable amount of human–wildlife interaction.
Study animals
The subjects of the study were members of 7 wild groups of long-tailed macaques (Macaca fascicularis), 3 groups in the Kosumpee Forest Park and 4 groups in the Don Chao Pu Forest Park. All sampled group members were recognized individually by unique markings such as small injuries in the face, scars, size or pelage colour. The groups investigated differed in their contact with humans and their proximity to human facilities. Additionally, their diet differed with respect to the proportion of human-provided food consumed (see below for details).
In Kosumpee Forest Park, all of the investigated groups had close contact with humans, and consumed daily large amounts of human-provided food. In addition, they had access to the school grounds and the houses in front of the park, where the monkeys would, on occasion, steal food and fight with dogs. In this study, the 3 human contact groups from Kosumpee Forest Park will be referred to as K1, K2 and K3.
In the Don Chao Pu Forest Park in Pha Na four groups were investigated. Two of these groups had contact with humans and consumed human-provided food almost every day; these were designated groups P1 and P2. The two other groups sampled were sylvatic, referred to as P3 and P4. These groups had nearly no contact at all with humans and fed almost exclusively on natural food sources.
Behavioural observations
Behavioural data were collected while following the monkey groups for whole days using ‘instantaneous scan sampling’ (see Martin and Bateson, Reference Martin and Bateson1993). With this method, a group was scanned every 5 min and the maintenance activity of all visible individuals, their height in the forest and their approximate location in the area were recorded during a 2-min period. This is an appropriate scanning period for rather dispersed groups of primates (Martin and Bateson, Reference Martin and Bateson1993). The 5-min interval between scans allowed for independent data points for group positions. Definitions of maintenance activities are self-evident: locomotion, feeding, drinking, grooming and resting. We differentiated between human-provided food (FH) and natural food sources (FN). The height classes were grouped into 6 categories: ground, 0–5 m, 6–10 m, 11–15 m, 16–20 m and >20 m above the ground. The behaviour of dependent infants was not recorded.
Collection and examination of fecal samples
Fresh fecal droppings from 135 individual animals comprising 7 groups were collected between July and December 2008 by following the groups during their complete activity period (06:00 h–18:00 h). The study period was restricted to the dry season only. With the beginning of the wet season, both study sites flooded; therefore fecal sample collection would not have been possible during that time. Individual fecal samples were gathered directly after defecation. Locality, date, group, sex, individual and time of defecation were recorded. After collection, the fecal samples were mixed well and about 2 g were immediately preserved in separate 15·0 mL vials containing 10% buffered formalin (solution of 10% formaldehyde and sodium phosphate buffer, pH 7·0). Samples were stored at ambient temperature. Three samples were collected from each of the 135 individuals on non-consecutive days. The finding of a recognizable parasite stage in any one of multiple samples for a given individual resulted in a positive report for that individual.
Similarly, 3 fecal samples were collected from people between the ages of 1 year and 87 years in Kosum Phi Sai (105 individuals) and Pha Na (65 individuals) on different days. These participants lived or worked next to the forest parks.
Preserved fecal samples were processed using a modified formalin-ethyl acetate sedimentation technique (see Ash et al. Reference Ash, Orihel and Savioli1994). Samples were filled up with formalin to 1 mL. Samples were examined microscopically for the presence of eggs and larvae of different intestinal helminths. Parasite stages were identified by shape, size or other visible structures. Measurements were made to the nearest 0·1 μm±s.d. Eggs or larvae of each parasite were counted and the number contained in each gram of stool was calculated from the volume of the sample examined (300 μL), the total volume of the sample (1 mL) and the weight of the stool specimen. It was measured as an estimate of intensity of infection, and averaged for the 3 samples.
Hookworms were distinguished from Strongyloides species using coprocultures based on the agar plate method (Koga et al. Reference Koga, Kasuya, Khamboonruang, Sukhavat, Ieda, Takatsuka, Kita and Ohtomo1991). Therefore, fresh fecal samples were stored at 4 °C and processed in the laboratory within 1 day. Agar plate cultures were incubated for 5 days at room temperature. The agar plates were examined under a stereomicroscope for the presence of tracks from moving larvae or free-living adults on the 3rd and 5th days. All microscopically positive dishes were further processed by washing the surface of the agar with a 10% formalin solution to collect worms for species identification. Parasites were identified with the help of keys (MAFF, 1979; Ash and Orihel, Reference Ash and Orihel1987). Our ability to identify most parasite species from host fecal examination, even with cultured larvae, is limited. Consequently, we present the majority of our findings at the level of family or genus.
Statistical analysis
All statistical tests were carried out using IBM®SPSS® 20 for Windows. In all tests, significance levels were set to α = 0·05. To test statistical differences in prevalence of parasites between species, sexes and groups Fisher's exact tests were performed. We then employed binary logistic regression to model the prevalence of each parasite using variables having a P-value <0·20 in the univariate analyses. Significant (P < 0·05) risk factors in multivariate analysis were expressed as odds ratios (OR) with 95% confidence intervals (CI). Mann–Whitney U-tests were used to investigate for variation in parasite egg output between sexes, age classes and groups of macaques. Correlations of behavioural data with parasite prevalences and intensities were sought using Spearman rank correlation tests.
Ethical considerations
The research protocol was approved by the Ethical Committee of the Medical Department of Khon Kaen University. Human sample collection was conducted in collaboration with the local healthcare centres. Participation was voluntary; all participants were informed about objectives and procedures of this study and sufficient time was given to ask questions. Participants provided informed consent to the study (guardians consented in the cases of minors), associated procedures and use of results and all participants could withdraw from the study at any time without any further obligates. Free, ethically prescribed anthelminthic treatment according to World Health Organization recommendations (World Health Organization, 2006) and health education were provided by the local healthcare centres for those who harboured intestinal parasitic infections.
RESULTS
Human fecal samples
Fecal samples from the inhabitants of the 2 villages studied, Kosum Phi Sai (105 individuals) and Pha Na (65 individuals), contained 7 different types of helminth: Opisthorchis viverrini, Taenia sp., Strongyloides stercoralis, Trichostrongylus sp., hookworm eggs (Necator americanus/Ancylostoma duodenale) and small trematode eggs. These belonged to the family Heterophyidae and were possibly Haplorchis sp. (referred to here as minute intestinal flukes (MIF)). Prevalences in both villages were similar. For Opisthorchis, Strongyloides and hookworms infection rates were moderately high, whereas Taenia, Trichostrongylus and MIF infections were less common (Table 1). Mean intensities of infection were generally low and are presented in Table 1.
Table 1. Prevalence of parasite infection with 95% confidence intervals in parentheses and mean intensities (eggs g−1, larvae g−1) of parasite infection with standard deviation (±) for humans sampled in Kosum Phi Sai and Pha Na

Macaque fecal samples
One trematode and at least 5 species of nematodes were recovered from macaques. The MIF belonged to the family Heterophyidae and was morphologically identical to that found in human samples. One of the nematodes was identified as Strongyloides fuelleborni, another as Oesophagostomum sp., while a third nematode was identified as a Trichuris sp. The eggs of a nematode taxon from the Order Rhabditida, probably Globocephalus sp. were also detected. Furthermore, nematode larvae belonging to the Superfamily Metastrongyloidea were found.
There were no significant differences between the prevalence of any of the parasites relating to host sex (Fisher's exact test, P > 0·05) or host age class (infant, juvenile, adult; Fisher's exact test, P > 0·05). Further, binary logistic regression revealed no significant association between prevalence of any parasite with either of the variables. Thus the data were pooled for further analyses.
Differences in prevalences among macaque groups
Parasite prevalences between the investigated macaque groups differed markedly from each other (Table 2). Strongyloides fuelleborni was absent in the sylvatic group P4 from Pha Na, revealing significant differences with all human-contact groups. In the second sylvatic group, P3, only 1 of the macaques was infected. All groups from Kosumpee Forest Park had significantly higher prevalences of S. fuelleborni infection than the sylvatic groups P3 and P4 from Pha Na (Fisher's exact test, P < 0·001). In addition, the prevalence of infection was significantly higher in group K2 than in the human contact P2 group in Pha Na (Fisher's exact test, P = 0·026). There were also significant differences in Strongyloides infection between the groups from Pha Na. The human contact group P1 showed significantly higher infection rates than either of the non-contact groups P3 and P4 (Fisher's exact test, P1 vs P3 P = 0·05, P1 vs P4 P = 0·004) and the second human contact group P2 was significantly more often infected than sylvatic P4 (Fisher's exact test, P = 0·021). Multivariate analyses showed 2 significant variables. Macaques originating from Kosumpee Forest Park are at greater risk of being infected with S. stercoralis (OR 4·02; CI 1·71–9·42) than macaques from Pha Na. Additionally, human contact raises the risk of being infected with this parasite (OR 17·44; CI 2·15–141·53).
Table 2. Prevalence of parasite infection for the different macaque groups

The MIF was totally absent from the sylvatic groups P3 and P4, whereas the parasite was present in all the human contact groups with prevalences ranging from 14·3% (n = 21) to 30% (n = 20).
Trichuris sp. showed significantly lower prevalences in the sylvatic groups P3 and P4 than in the human contact groups from Kosumpee Forest Park (Fisher's exact tests, P < 0·05). In addition, the human contact group P1 from Pha Na had a significantly lower prevalence than K3 (Fisher's exact test, P = 0·028). Binary logistic regression revealed that macaques originating from Kosumpee Forest Park are at greater risk of being infected with Trichuris sp. (OR 3·94; CI 1·67–9·30) than macaques from Pha Na. Contact with humans, however, showed no significant association.
Oesophagostomum sp. was detected only in the groups from Pha Na. For Globocephalus sp. and the nematode larvae, no significant differences in the prevalences of these parasites were detected between any of the macaque groups.
Differences in egg output among macaque groups
Eggs and larvae excreted per gram of macaque stool samples were also examined. In general, the number of parasite stages voided in feces was low for all macaques sampled during the study period (Table 3). The highest mean intensities of infection were observed for S. fuelleborni and Trichuris sp., with maximum counts of 15 760 eggs per gram (epg) for S. fuelleborni and 770 epg Trichuris sp.
Table 3. Mean intensities (eggs g−1, larvae g−1) of parasite infection with standard deviation (±) for the different macaque groups

Inter-group differences in mean intensity were also recorded for S. fuelleborni and Trichuris sp. For S. fuelleborni the mean intensity from Kosum group K3 was significantly higher than from groups P1 and P2 (MWU K3 + P1 = 0·05, K3 + P2 = 0·018). Statistics for S. fuelleborni infection intensity was not useful with either forest group, since only 1 individual was infected in group P3, and no infection occurred in P4.
Mean infection intensity of Trichuris sp. was significantly higher in all Kosum groups compared with group P1 (MWU K1 + P1 P = 0·008, K2 + P1 P = 0·043, K3 + P1 P = 0·001). In addition, groups K1 and K3 showed significantly higher intensities than P4 (MWU K1 + P4 P = 0·018, K3 + P4 P = 0·004) and group K3 showed higher outputs than P3 (MWU P = 0·013). K3 showed higher egg outputs than group K2 (MWU P = 0·006). All other parasite species had similar mean egg outputs (Table 3).
Correlations of parasitological with behavioural data
The behaviour of human contact groups (K1–3 and P1 + 2) differed significantly from non-contact groups (P3 + 4) by spending more time being fed by humans (MWU P = 0·001) and on the ground (MWU P = 0·001), while the non-contact groups spent significantly more time 16 m to 20 m in trees above the ground (MWU P = 0·001). Time spent moving, resting, feeding, drinking and grooming were significantly different between these groups.
There were positive correlations between the prevalence of Strongyloides, Trichuris and the nematode larvae and the habit of accepting human-provided food, while the correlation with Oesophagostomum was significantly negative. The corresponding correlations of parasite prevalences with the time spent eating natural food sources were also highly significant but in the opposite direction. Similarly, the prevalences of Strongyloides, Trichuris and the nematode larvae were positively correlated with the time spent on the ground, while for Oesophagostomum the correlation was negative and highly significant (Table 4).
Table 4. Spearman rank correlation of parasite prevalence with behavioural data of macaques
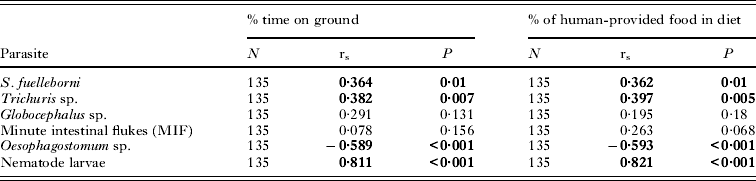
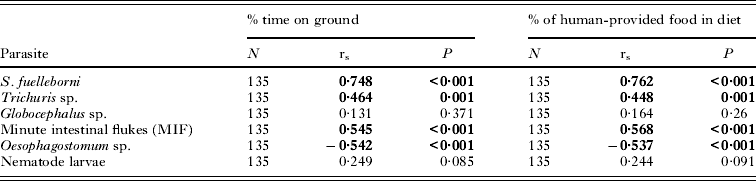
There were strong positive correlations between mean intensity of infection of Strongyloides, Trichuris, MIF and the nematode larvae with the time spent eating human-provided food while the correlation for Oesophagostomum was significantly negative (Table 5). The converse was true for time spent eating natural food sources. There were also significant positive correlations between the intensity of infection and the time spent on the ground for Strongyloides, Trichuris and the MIF while the correlation was again significantly negative for Oesophagostomum.
Table 5. Spearman rank correlation of parasite intensity with behavioural data of macaques
DISCUSSION
In this study, the macaques that foraged near human-inhabited areas and ingested human-provided food differed markedly from the groups that had less overlap with humans and human-modified habitats. Human contact groups displayed significantly higher prevalences of Strongyloides fuelleborni and of minute intestinal flukes (MIF). We conclude that the presence of humans and the associated changes of primate behaviour can lead to substantial changes in the community structure of intestinal helminths in wild non-human primate populations.
Land unaffected by humans is increasingly rare (Lilly et al. Reference Lilly, Mehlman and Doran2002) and human activities can alter the ecology of wildlife and environmental parameters in ways that increase the probability of an infection. In a previous study, we showed significantly higher infection rates of Prosthenorchis elegans (Acanthocephala) in 2 tamarin species living in a human-modified habitat compared with their conspecifics in more natural settings (Wenz et al. Reference Wenz, Heymann, Petney and Taraschewski2010). Human modification of the habitat may favour the intermediate host of this potentially pathogenic parasite thereby increasing the probability of exposure. With the modification of wildlife habitat humans may also bring about a change in the behaviour and diet of non-human primates (Weyher et al. Reference Weyher, Ross and Semple2006); indeed many such changes are attributed to the availability of anthropogenic nutrition (Else and Lee, Reference Else, Lee, Else and Lee1986; Box, Reference Box1991; Weyher et al. Reference Weyher, Ross and Semple2006). In addition, a change in foraging strategy may also alter the relationship that primates have with the parasites already present in their environment as shown in wild-foraging and crop-raiding baboons in Nigeria (Weyher et al. Reference Weyher, Ross and Semple2006).
Our behavioural observations show a positive correlation of S. fuelleborni infection with the time macaques spent on the ground. Further, as a consequence of foraging on human-provided food, the daily activity and ranging patterns were different from those of the completely wild foraging macaques in the same area. Human-contact macaques spent more time on the ground than the wild foraging groups resulting possibly in greater exposure to, for example, the infective L3 larvae of S. fuelleborni. Similar observations have been made for baboons. As a consequence of rubbish foraging, daily activity and ranging patterns differed from those of wild foraging baboons (Altman and Muruthi, Reference Altman and Muruthi1988). Semi-provisioned baboons rested significantly more and their home ranges were significantly smaller than non-provisioned individuals (Altman and Muruthi, Reference Altman and Muruthi1988).
Strongyloides fuelleborni belongs to a genus of widely distributed nematodes parasitic in the intestine of humans and other mammals. While it is transmitted mainly by non-human primates (Crompton and Savioli, Reference Crompton and Savioli2006), it is also considered a helminth of human health importance, especially in Central Africa and Papua New Guinea (Viney et al. Reference Viney, Ashford and Barnish1991; Grove, Reference Grove1996). Although infections of humans in Thailand have not been reported, this highlights the potential for zoonotic transmission. Infection is assumed to occur when the infective L3 larvae penetrate the skin (Crompton and Savioli, Reference Crompton and Savioli2006). Animals often defecate, cough, vomit, bleed or urinate on or near the ground or low-lying substrates dispersing infective stages of parasites (Nunn et al. Reference Nunn, Gittleman and Antonovics2000). Thus, it is reasonable to expect that terrestrial primates experience greater infection risk than arboreal primates (Nunn et al. Reference Nunn, Gittleman and Antonovics2000).
S. fuelleborni infections can have fatal consequences. The parasite was implicated in the death of several rhesus monkeys (Remfry, Reference Remfry1978). The pathological effects of S. fuelleborni range from a cough, bronchopneumonia and diarrhoea to peritonitis (Flynn, 1973). In a young rhesus male, for example, a small abscess on the caecum, blood-staining of the omentum and peritoneal fluid and ulceration of the mucosal surface of the small and large intestines were detected post-mortem. In that case it was suggested that the peritonitis was caused by perforation of a Strongyloides-induced ulcer (Remfry, Reference Remfry1978).
Although S. fuelleborni infections occur naturally in non-human primates, high prevalence and intensity of infection may have negative consequences for a population of wild animals. Prevalences of more than 70% in the macaques from Kosumpee Forest Park are therefore a potentially disturbing observation, particularly since the mean intensity of infection was also significantly higher than in sylvatic groups. On the other hand, the number of parasite eggs measured in fecal material is affected by many factors (e.g. bias due to variation in egg production, clumping of eggs and differences in stool consistency; Stuart and Strier, Reference Stuart and Strier1995, Engels et al. Reference Engels, Sinzinkayo and Gryseels1996, Reference Engels, Sinzinkayo, DeVlas and Gryseels1997; Ross et al. Reference Ross, Li, Sleigh, Williams and McManus1998). This was considered when choosing the sampling regime. Since a roughly linear, positive relationship between numbers of eggs g−1 of feces and parasite burden has been observed for a number of parasites (Keymer and Hiorns, Reference Keymer and Hiorns1986; Pritchard et al. Reference Pritchard, Quinnell, Slater, McKean and Dale1990; Sithithaworn et al. Reference Sithithaworn, Tesana, Pipitgool, Kaewkes, Pairojkul, Sripa, Pauparoj and Thaiklar1991), we suggest that intensity of infection by the adults of S. fuelleborni was also higher in the groups with human contact compared to the sylvatic ones.
Although Globocephalus has a similar life cycle and transmission mode to S. fuelleborni, we did not detect any significant differences in prevalence of infection. However, only a few individuals were infected with this parasite and the time macaques spent on the ground seems not to have had an influence on parasite transmission. Mean intensity of infection was also very low, and it is possible that only a few infective stages were present in the environment.
The heterophyid trematode (MIF) was present only in human contact groups. Exact identification of the MIF was not possible based on fecal examination alone. Adult worms would have assisted, but a treatment of wild monkeys with antihelminthics to expel the parasites was not possible. Heterophyid flukes live as adults in the intestine of mammals and birds, and they infect snails and freshwater fish as intermediate hosts. Human infection occurs through consumption of raw or undercooked fish which harbour the metacercariae (Fried et al. Reference Fried, Graczyk and Tamang2004). Haplorchis taichui is the most prevalent trematode in the gastrointestinal tract of humans in northern Thailand (Pungpak et al. Reference Pungpak, Radomyos, Radomyos, Schelp, Jongsuksuntigul and Bunnag1998; Radomyos et al. Reference Radomyos, Wongsaroj, Wilairatana, Radomyos, Praevanich, Meesomboon and Jongsuksuntikul1998), and in cyprinid fish (Srisawangwong et al. Reference Srisawangwong, Sithithaworn and Tesana1997; Sukontason et al. Reference Sukontason, Piangjai, Muangyimpong, Sukontason, Methanitikorn and Chaithong1999). Sukontason et al. (Reference Sukontason, Upunyo, Sukontason and Piangjai2005) presented the pathology associated with H. taichui in the small intestine for 3 human subjects. Microscopic examination revealed mucosal ulceration, mucosal and submucosal haemorrhages, fusion and shortening of the villi, chronic inflammation and fibrosis of the submucosa. Significant pathology in the heart, brain and spinal cord of humans may also occur, and is thought to be caused by the atypical carriage of fluke eggs through the circulatory system (Fried et al. Reference Fried, Graczyk and Tamang2004).
Haplorchis taichui is also known to occur in macaques (Yamashita, Reference Yamashita1963), but information on pathogenicity of this parasite in non-human primates is scarce. Given the knowledge of pathological effects of intestinal flukes in humans, it is likely that there is also a health risk for the monkeys.
Infection of macaques with H. taichui implies that they come into contact with raw or undercooked fish. Our observations point to the conclusion that human-provided food is an important factor influencing non-human primate infection. Behavioural observations show a positive correlation between this MIF infection and the amount of human-provided food in the diet of the macaques. Since the wild foraging macaques were not infected with this parasite and their preferred foraging height strongly reduced the likelihood of a diet containing fish, it seems likely that infected intermediate hosts of the MIF formed part of the human-provided food.
Consuming human foods can have significant epidemiological costs due to an increased risk of disease transmission for both humans and primates. Some studies have documented the costs to baboons from foraging in human rubbish dumps, including infections with antibiotic-resistant bacteria, probably acquired from humans (Rolland et al. Reference Rolland, Hausfater, Marshall and Levy1985; Routman et al. Reference Routman, Miller, Phillips-Conroy and Hartl1985) and infections with tuberculosis from eating contaminated meat (Sapolsky and Else, Reference Sapolsky and Else1987; Keet et al. Reference Keet, Krick, Bengis, Grobler and Michel2000). In contrast, Eley et al. (Reference Eley, Strum, Muchemi and Reid1989) found no increased risk of baboons in Kenya acquiring gut parasites from rubbish dump foraging.
The divergences observed in the parasite community structure of the macaques appear not to be related to a transfer of parasites directly from humans. Sampled humans harboured important parasites with pathological effects (O. viverrini, MIF, N. americanus/A. duodenale, Trichostrongylus, Taenia and S. stercoralis). Their presence can mainly be traced back to diet and hygienic standards. The only concurrence that was found was the minute intestinal fluke that was present in both humans and monkeys. This parasite may be indirectly transmitted to the monkeys by humans through contaminated food. To determine the reason why macaques were not infected by O. viverrini detailed studies will be needed of the items which the macaques are picking out of the food offered to them by humans. On the other hand, a low suitability of macaques as hosts of Opisthorchis has been reported (Wykoff, Reference Wykoff1964).
In addition, the macaques in the present study harboured several parasite species which exhibit potential for zoonotic transmission. The eggs identified as Trichuris sp. in this study – although resembling the human whipworm T. trichiura – were larger. On the other hand, morphological studies on adult parasites identified as T. trichiura showed differences between specimens collected from non-human primates and humans using light and scanning electron microscopy (Ooi et al. Reference Ooi, Tenora, Itoh and Kamiya1993). Additionally, in our study none of the humans had eggs of T. trichiura in the feces and both sylvatic and human contact macaques displayed similar prevalences of infection. Therefore, infection with Trichuris sp. seems to occur naturally in macaques and not as a result of anthropozoonotic transmission. The same seems to be the case for Oesophagostomum. Additionally, we did not detect any evidence of zoonotic transmission or double infection of either species of Strongyloides. The villagers were infected only with S. stercoralis, whereas the macaques harboured only S. fuelleborni.
Results from the present study in Thailand, as with the results of a previous study by us in Peru (Wenz et al. Reference Wenz, Heymann, Petney and Taraschewski2010), lead to the conclusion that the presence of humans and/or an environment modified by humans can lead to substantial changes in the community structure of intestinal helminths in wild primates. These changes may even have pathogenic consequences. In terms of conservation, it follows that geographical areas designated for the protection of non-human primate populations should be placed far enough from human settlements to prevent foraging on disturbed land and that the food provisioning by humans requires more effective control in areas like forest parks.
ACKNOWLEDGEMENTS
We thank the two anonymous referees for their valuable comments to the manuscript and their constructive suggestions. The presentation of these data would not have been possible without the invaluable assistance of the staff and students from the Parasitology Department, Khon Kaen University. Dr Kunnyarat Duenngai, Dr Weerachai Saijuntha and his family kindly helped with accommodation, authorities and the organization of human stool sample collection. We thank the authorities of the Khon Kaen forestry office for permission to conduct the study in Kosumpee Forest Park and the officials of Pha Na Administration for permission to conduct the study in Don Chao Pu Forest Park. We thank the authorities of Kosum Phi Sai and Ph Na hospital for their support in collecting human stool samples. Financial support to A.W-M. was given by the German Academic Exchange Service (DAAD) and the Landesgraduiertenförderung Baden-Württemberg. This study was part of the PhD project of A.W-M.








