INTRODUCTION
It is commonly known that the reproductive outcome of a parasite depends on the host it exploits. In fact, reproductive performance of the same parasite species varies among host species (Schmid-Hempel and Schmid-Hempel, Reference Schmid-Hempel and Schmid-Hempel1996; Riquelme et al. Reference Riquelme, George-Nascimento and Balboa2006; Ghimire and Phillips, Reference Ghimire and Phillips2010; Khokhlova et al. Reference Khokhlova, Fielden, Degen and Krasnov2012), between host genders (Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009a ; Luong et al. Reference Luong, Grear and Hudson2009), among host age cohorts (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2013; Veena and Manjunath, Reference Veena and Manjunath2013), among hosts with different reproductive status (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014) and even among hosts of different nutritional status (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005; Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007). This variation in parasite performance may be manifested not only in offspring quantity (Luong et al. Reference Luong, Grear and Hudson2009) but also in their quality (e.g. survival or body size; Campbell and Duffey, Reference Campbell and Duffey1979; Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2013; Veena and Manjunath, Reference Veena and Manjunath2013; Khokhlova et al. Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014). In some studies that simultaneously assessed both quality and quantity of the offspring in parasites exploiting hosts belonging to different species or different cohorts of the same host, a negative relationship between quantity and quality of the offspring was reported (Veena and Manjunath, Reference Veena and Manjunath2013; Khokhlova et al. Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014). This suggests the occurrence of a trade-off between quantity and quality of the offspring with parasites producing more offspring of a lower quality in some hosts and fewer offspring of a higher quality in other hosts.
Originally, the idea about a trade-off between offspring quantity and quality (Lack, Reference Lack1947; Smith and Fretwell, Reference Smith and Fretwell1974) suggested that the balance between offspring number and quality maximizes fitness and, therefore, is expected to be favoured by natural selection (Stearns, Reference Stearns1992). However, observations on the intraspecific variation in the quantity and quality of offspring in different environments (see review in Fox and Czesak, Reference Fox and Czesak2000) have led to the argument that this trade-off should be favoured by natural selection only if females can anticipate the offspring environment and adaptively adjust their quality (Parker and Begon, Reference Parker and Begon1986). If this hypothesis is accepted, then, for parasites, the trade-off between quantity and quality of offspring is more likely (a) in taxa with active rather than passive transmission modes; and (b) across different host species rather than across different cohorts of the same host species (Khokhlova et al. Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014). The latter is because the maternal environment in terms of host species can reliably predict the environment of the offspring (merely because the probability that they will exploit the same host species as their mother did is high). In contrast, maternal environment in terms of the host's age, reproductive status, or nutrition is ephemeral, so that reliable prediction by a mother of the environment that her offspring will face seems to be unlikely. Alternatively, a trade-off between quantity and quality of offspring in parasites, like in other species, can arise merely as a consequence of maternal energetic constraints when a mother has enough energy to invest either in the number of offspring or in their quality (e.g. size) but not both (Stearns, Reference Stearns1992). If this is true, then a trade-off between offspring quantity and quality in parasites exploiting hosts belonging to different cohorts of the same species may also occur. However, such a trade-off has never been studied experimentally.
Fleas represent a convenient parasite taxon for exploration of the condition-dependent adjustment of offspring quality by mothers. They are holometabolous obligatory haematophagous insects that generally parasitize small and medium-sized burrowing mammals. Larvae of most species are not parasitic and feed on organic matter from the burrow substrate. Upon emergence from the pupa, a new adult must locate a suitable host. Given the low mobility of fleas, this new host would likely belong to the same species as the maternal host. Thus, it is not surprising that an across-host species trade-off between offspring quantity and quality has been documented in a host-generalist (although not in a host-specific) flea (Khokhlova et al. Reference Khokhlova, Pilosof, Fielden, Degen and Krasnov2014).
Recently, we studied a quantitative component of reproductive performance (namely, egg production) in the flea Xenopsylla ramesis in relation to the reproductive status of its rodent host (Meriones crassus) (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014). We found that egg production of fleas fed on pregnant rodents was significantly lower than on fleas fed on non-reproducing and lactating rodents, probably because of changes in hormonal and/or immune status of a female host during pregnancy and lactation (see detailed explanations in Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014). In the present study, we investigated offspring quality of these fleas and asked whether female fleas feeding on hosts belonging to the same species but of different reproductive status trade off quantity of the offspring for quality. We considered two alternative scenarios. If the assumption that offspring quantity/quality trade-off is favoured only if a female can reliably predict the environment of the offspring (Parker and Begon, Reference Parker and Begon1986) holds, then the effect of the maternal host's reproductive status will be translated directly into quality of the new generation of fleas and no trade-off will exist between offspring quality and quantity. Alternatively, if an offspring quantity vs quality trade-off is caused by parental constraints (Stearns, Reference Stearns1992), then the negative relationships between the number of offspring and variables describing their quality will be observed. Most studies of the trade-off between quantity and quality of offspring considered quality in terms of size (e.g. seed size or egg size; see reviews in Stearns, Reference Stearns1992 and Fox and Czesak, Reference Fox and Czesak2000) that reflects the amount of energy invested by mother per individual offspring (Parker and Begon, Reference Parker and Begon1986). However, quality of an offspring can also include other life history traits. In this study, we assessed the quality of newly emerged fleas via their emergence success (proportional survival, that is, what proportion of eggs survived until emergence), developmental time, body size and resistance to starvation. We predicted that if a trade-off between offspring quantity and quality is associated with evolutionary constraints (that is, it applies only when mothers are able to anticipate the conditions offspring will experience; Parker and Begon, Reference Parker and Begon1986), then the proportion of eggs surviving until emergence will be lower in fleas fed on pregnant hosts than on lactating and non-reproductive hosts. In addition, new adult fleas produced by fleas fed on pregnant hosts will (a) develop longer, (b) be smaller and/or (c) die under starvation faster compared with new adults produced by fleas from lactating and non-reproductive hosts. Alternatively, if a trade-off between offspring quantity and quality is associated with physiological constraints (Stearns, Reference Stearns1992), then the proportion of eggs surviving until emergence will be higher in fleas fed on pregnant hosts than on lactating and non-reproductive hosts; new imagoes from mothers fed on pregnant hosts will (a) develop faster, (b) be larger and/or (c) live longer under starvation compared with new imagoes from fleas fed on lactating and non-reproductive hosts. Our interpretation of these variables in terms of quality is as follows. Larvae that develop faster may outcompete slower-developing larvae for food (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005) or may cannibalize them (Lawrence and Foil, Reference Lawrence and Foil2002), and an imago that emerges earlier will have better opportunities to locate a host and start reproduction than an imago that emerges later. An intraspecific positive correlation between body size and fecundity has been shown for insects (Honek, Reference Honek1993). The ability to survive unpredictable and lengthy periods without a blood meal reflects the amount of energy stored in the fat tissue of a new imago accumulated during pre-imaginal development (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2002). In addition, we explored the possible mechanisms underlying the manifestation of quality traits and asked whether the quality traits are correlated with each other. In particular, we tested whether (a) larger body size is associated with longer duration of development; and (b) higher resistance to starvation is associated with either larger body size or longer duration of development or both.
MATERIALS AND METHODS
Rodents and fleas
We used fleas X. ramesis and rodents M. crassus from laboratory colonies started in 1999 and 1997, respectively. Details of rearing procedures and colony maintenance have been described elsewhere (e.g. Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001, Reference Krasnov, Khokhlova, Fielden and Burdelova2002; Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009a , Reference Khokhlova, Serobyan, Krasnov and Degen b ; Khokhlova et al. Reference Khokhlova, Serobyan, Degen and Krasnov2010). All parent fleas were collected 24–48 h after emergence and did not feed prior to experiments. Rodents were 6–8 months old and had not been exposed previously to fleas.
Experiments
Procedures for obtaining flea eggs from fleas fed on non-reproductive, pregnant or lactating female M. crassus have been described in detail elsewhere (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014). In brief, we used adult, sexually naïve rodents (29 males and 29 females). We placed a male and a female rodent in an individual cage for a week. Three to 4 days after removing a male (that is, in the middle of assumed pregnancy), we applied fleas (see below) to 20 females, 12 of which later gave birth and, thus, were under flea parasitism during pregnancy, whereas eight females were considered to be non-reproductive. Six of the nine remaining females (from the original 29 females) produced pups and were exposed to fleas on the 14th day after parturition (during lactation), whereas three of these nine females did not give birth and thus were assigned to the ‘non-reproductive’ category. In other words, we used data on fleas produced by parents fed on 11 non-reproductive, 12 pregnant, and six lactating females.
To expose hosts to flea parasitism, a female rodent was placed in a plastic cage (60 cm by 50 cm by 40 cm) with a floor of 3–5 mm of clean sand covered by a wire mesh (5 mm by 5 mm). Fifty newly emerged adult fleas (30 females and 20 males) were released into this cage for 3 days. Under these conditions, oviposition starts on the second day or later (Khokhlova et al. Reference Khokhlova, Fielden, Degen and Krasnov2012). Since M. crassus females have an oestrous cycle of 4–4·5 days and a pregnancy of 18–22 days, and pups start eating solid food at 17 days (Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova, Degen and Rogovin1996; Khokhlova et al. Reference Khokhlova, Kam, Gonen and Degen2000; Kam et al. Reference Kam, Cohen-Gross, Khokhlova, Degen and Geffen2003; Degen et al. Reference Degen, Khokhlova and Kam2011), pregnant and lactating females in our experiments were thus parasitized in the middle of pregnancy or at peak lactation, respectively.
Seventy-two hours after releasing fleas in a rodent cage, we collected fleas from the rodent's body (over a white plastic pan using a toothbrush until no flea was recovered) and bedding substrate, placed fleas recovered from the same rodent individual in a Petri dish and transferred them to an incubator (FOC225E, Velp Scientifica srl, Milano, Italy) at 25 °C air temperature and 90% relative humidity (RH) for 24 h. The next day (that is, day 4 of the experiment), we placed each rodent in a wire mesh tube (5 mm by 5 mm mesh; 15 cm length and 5 cm diameter) to prevent self-grooming. Tubes with rodents were placed in individual white plastic cages. Fleas collected earlier from this individual rodent were released onto the animal and allowed to feed for 2 h. Then, fleas were collected and examined for blood in the midgut under a light microscope (40× magnification). Fleas that took a blood meal from the same rodent were placed in a Petri dish (males and females together) and transferred to an incubator for 24 h, after which time we checked for newly laid eggs and counted them. These procedures were repeated daily over 5 consecutive days (days 4–8 of the experiment). We calculated the mean number of eggs produced per female that took a blood meal for each day and for each group of fleas.
Petri dishes with eggs produced by the same group of female fleas on the same day were filled with a 1 mm layer of sand and larval food medium (94% dry bovine blood, 5% millet flour and 1% grinded excrement of M. crassus), and transferred into an incubator (see above) where they were maintained at 25 °C air temperature and 90% RH.
Minimal duration of development for X. ramesis has been previously established at about 25 days (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001). Consequently, starting from the 18th day after oviposition, Petri dishes were checked daily until either eggs developed into adults or for 60 consecutive days. We recorded (a) the day each adult flea emerged and (b) the number of new adults that emerged from each group of eggs. After emergence, each adult was placed in an individual Eppendorf vial containing a thin layer of sand and left in the incubator. Each of these vials was checked daily until the adult died and the date of its death was recorded. After death, we identified the sex of each flea by examining its genitalia under light microscopy and measured the maximal length of its left hind femur. Length of the femur was measured on a screen (using a digital microscope camera Moticam 2000 with the Motic Images Plus 2·0ML program; Motic, Speed Fair Cp., Ltd., Causeway Bay, Hong Kong; ±0·01 mm, 40× magnification and calibration using an object-micrometer). In total, we used data on development and resistance to starvation for 1276 male and 1363 female and body size for 586 male and 1073 female newly emerged X. ramesis.
Estimates of quantity and quality of flea offspring
Quantity of offspring (parent reproductive effort) was evaluated as the mean number of eggs produced by a female flea per day. Quality of new imagoes produced by fleas exploiting non-reproductive, pregnant, and lactating hosts was estimated using the following variables. Emergence success was calculated as mean number of newly emerged imago per egg for eggs produced by the same group of fleas fed on the same host individual on the same date. Duration of development was estimated for each egg/new imago as the number of days from the day of oviposition until the day of emergence. We used length of the left hind femur of each new imago as a proxy for body size because these traits have been shown to be positively correlated (Krasnov et al. Reference Krasnov, Burdelov, Khokhlova and Burdelova2003). Resistance to starvation was calculated for each offspring as the number of days from emergence to death.
Data analyses
In previous studies, we found that duration of development, body size and resistance to starvation differed between male and female fleas with males being smaller (e.g. Krasnov et al. Reference Krasnov, Burdelov, Khokhlova and Burdelova2003), developing more slowly (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001) and usually dying under starvation faster (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2002) than females, all else being equal. Consequently, we analysed the effect of host reproductive status on these variables separately for male and female new imagoes. Gender differences in emergence success could not be evaluated in our experimental design because gender could not be attributed to an egg. Therefore, the effect of host reproductive status on the emergence success of new imagoes was analysed for both genders together. Prior to analyses, the data were subjected to angular (emergence success) or log+1 (the remaining variables) transformations. Figures present untransformed data.
We tested for the effect of host reproductive state on the variables describing offspring quality using linear mixed-effects models (LME; Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009) with host reproductive status as a fixed explanatory variable. The day of oviposition nested within individual hosts (using their individual numbers) were included in the models as random factors to control for variation among individual hosts and dates when an egg was laid. To fit the models, we used the lme function as implemented in ‘nlme’ package (version 3.1–106; Pinheiro et al. Reference Pinheiro, Bates, DebRoy and Sarkar2013) in R (version 3.02; R Development Core Team, 2013). Then, we used Tukey's HSD tests for multiple comparisons adjusted for mixed-effects models to test for differences among non-reproductive, pregnant, and lactating hosts in their effect on dependent variables using the glht function in ‘multcomp’ R package (version 1.2–15; Hothorn et al. Reference Hothorn, Bretz and Westfall2008).
To test for trade-offs between quantity and quality of offspring as well as among quality variables, we ran two series of linear models. In the first series, we modelled the effect of the offspring quantity measured as egg production effort by parent fleas (mean number of eggs produced per female) on each of the quality variables. In the second series, we related each quality variable to one, two or three other quality variables. In this series, a dependent variable represented an earlier event, while independent variable(s) represented later event(s), namely we modelled (a) the effect of emergence success on duration of development; (b) the effect of emergence success and duration of development on body size (that is, femur length); and (c) the effect of emergence success, duration of development and body size on time to death under starvation. Prior to these analyses, we controlled for the confounding effect of flea sex and reproductive status of a host (see Results) by substitution of the original values with their residual deviations from the linear mixed-effects models of the effect of flea sex and/or rodent reproductive status on each of the variables.
RESULTS
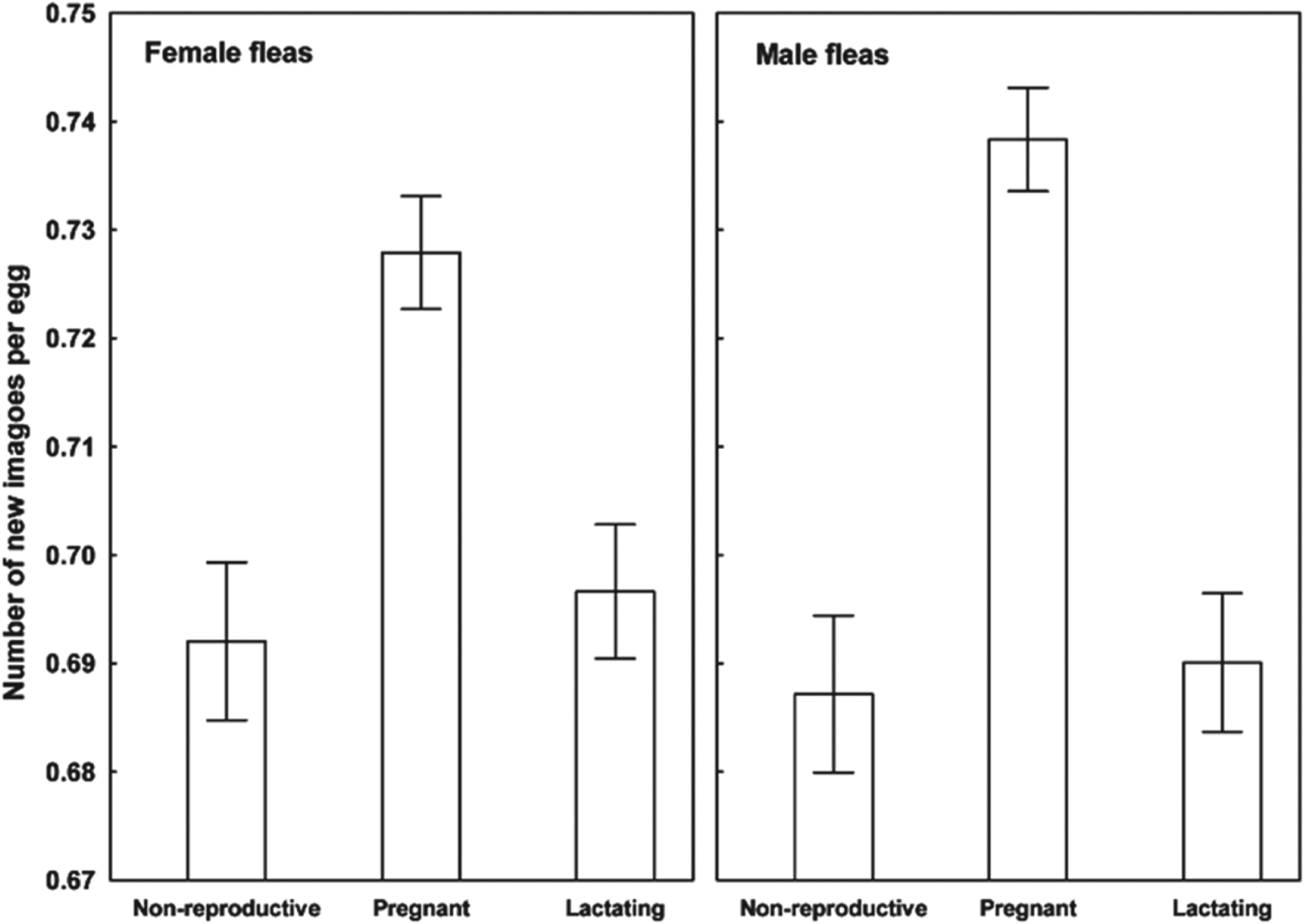
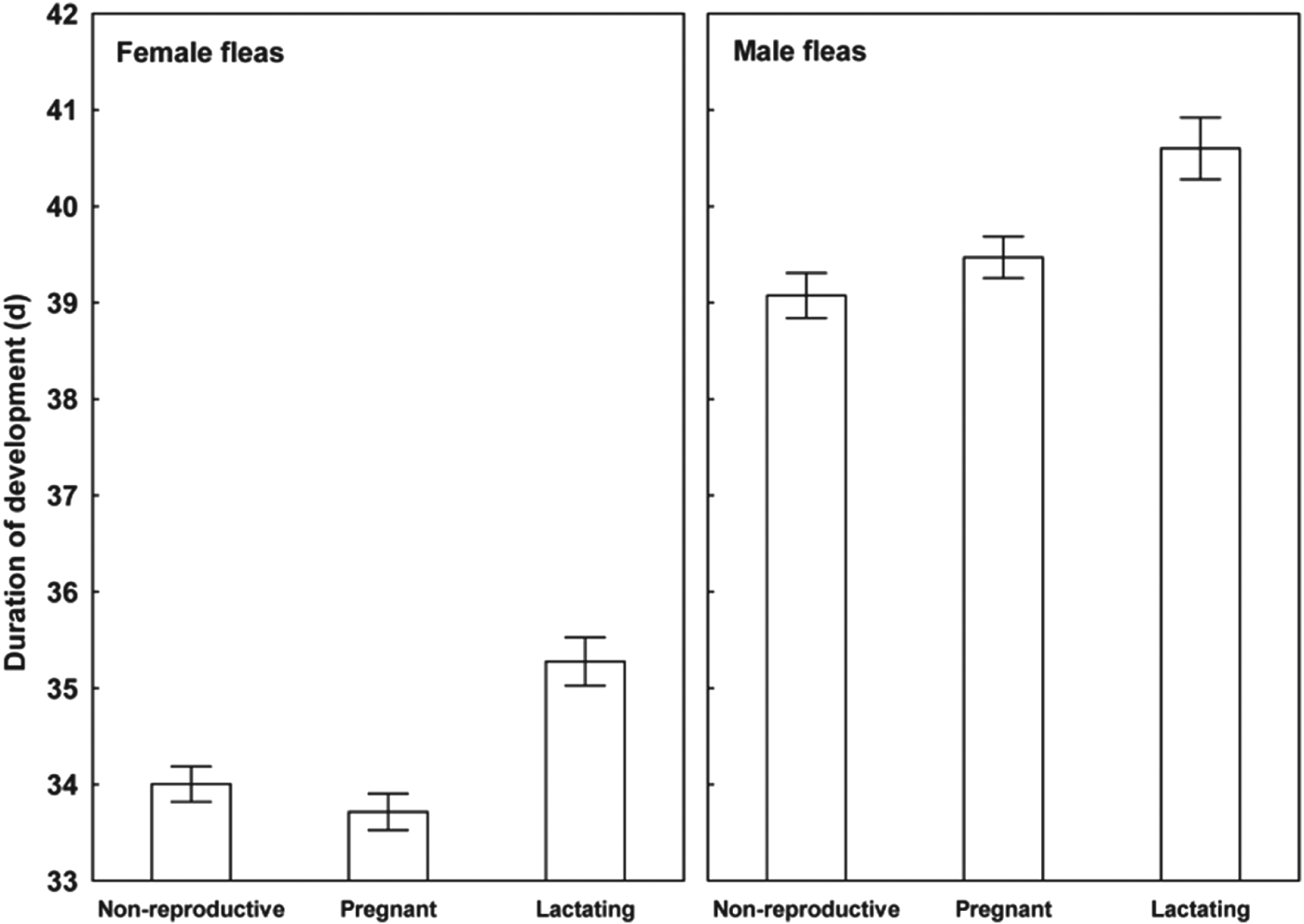
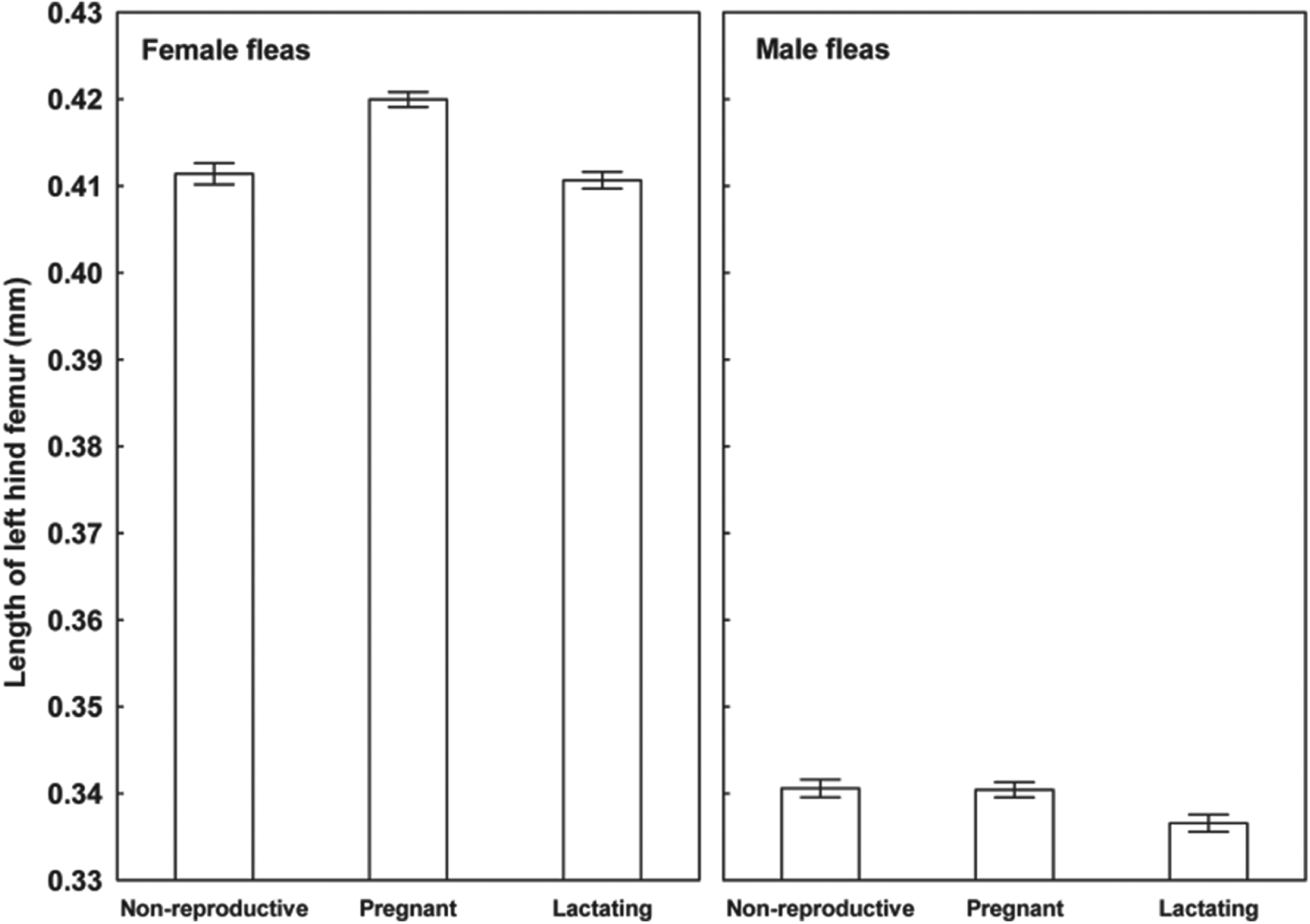
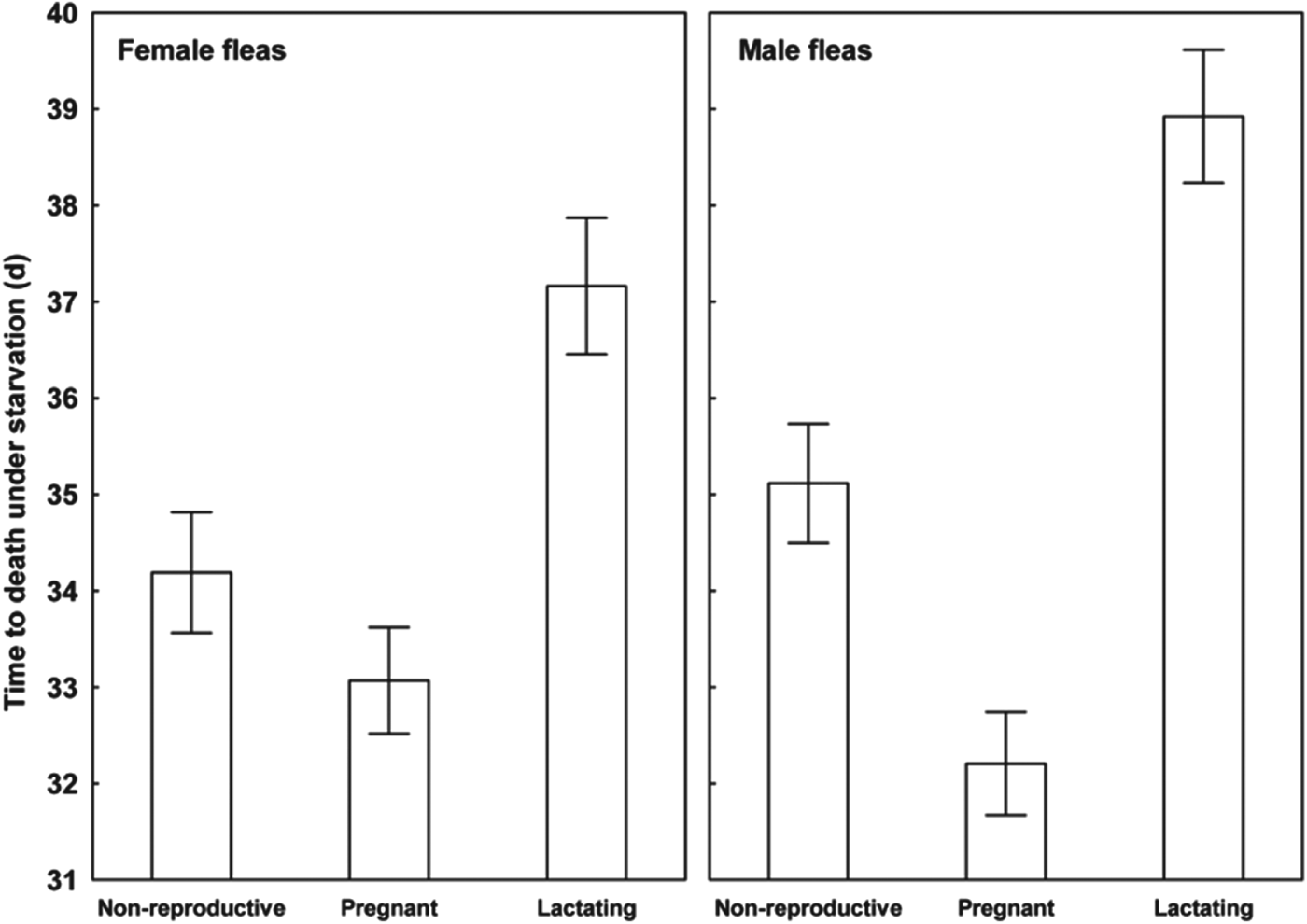
Results of the linear mixed-effects analyses demonstrated that each of the variables describing quality of flea offspring was affected by the reproductive status of a host on which their parents fed (Table 1). Emergence success of new imagoes was significantly higher when their parents exploited pregnant hosts than non-reproductive or lactating hosts (Tukey's tests; z = 6·8 and z = 6·3, respectively, P<0·001 for both; Fig. 1). Offspring from parents fed on non-reproductive or lactating hosts did not differ significantly in emergence success (Tukey's test; z = 0·02, P = 0·98; Fig. 1). Male fleas from parents fed on lactating hosts had significantly longer development times than those from parents fed on non-reproductive hosts (Tukey's test, z = 2·9, P<0·01; Fig. 2), although no significant difference in duration of development between males from parents fed on pregnant hosts and those fed on non-reproductive hosts was found (Tukey's tests; z = 0·9 and z = 2·1, respectively; P>0·10 for both; Fig. 2). Female fleas from parents fed on lactating hosts had significantly longer development times than females produced from parents feeding on either of the two remaining host categories (Tukey's tests; z = 3·7 and z = 4·5, P<0·001 for both; Fig. 2), whereas fleas from parents fed on these host categories had similar development times (Tukey's test, z = 0·9, P = 0·65; Fig. 2). Male offspring from parents exploiting non-reproductive and pregnant hosts were significantly larger than those exploiting lactating hosts (Tukey's tests; z = 2·3 and z = 3·8, respectively, P<0·05, Fig. 3). Female offspring from fleas fed on pregnant hosts were significantly larger than those from fleas fed on both lactating and non-reproductive hosts (Tukey's tests, z = 6·3 and z = 6·2, respectively, P<0·0001 for both; Fig. 3). Male new imagoes survived under starvation the longest when their parents fed on lactating hosts and the shortest when their parents fed on pregnant hosts (Tukey's tests, z = 2·4–5·3, P<0·05 for all; Fig. 4). Fleas from parents fed on non-reproductive hosts demonstrated moderate resistance to starvation. It was significantly lower than that in fleas from lactating hosts (Tukey's test, z = 3·0, P<0·001) but significantly higher than in fleas from pregnant hosts (Tukey's test, z = 2·4, P = 0·04) (Fig. 4). Female offspring of parents that exploited lactating hosts also survived under starvation significantly longer than those that exploited non-reproductive and pregnant hosts (Tukey's tests, z = 2·7 and z = 2·9, respectively, P<0·02 for both; Fig. 4). However, there was no difference in time to death under starvation in female offspring of fleas fed on the two latter host categories (Tukey's test, z = 0·4, P = 0·93).
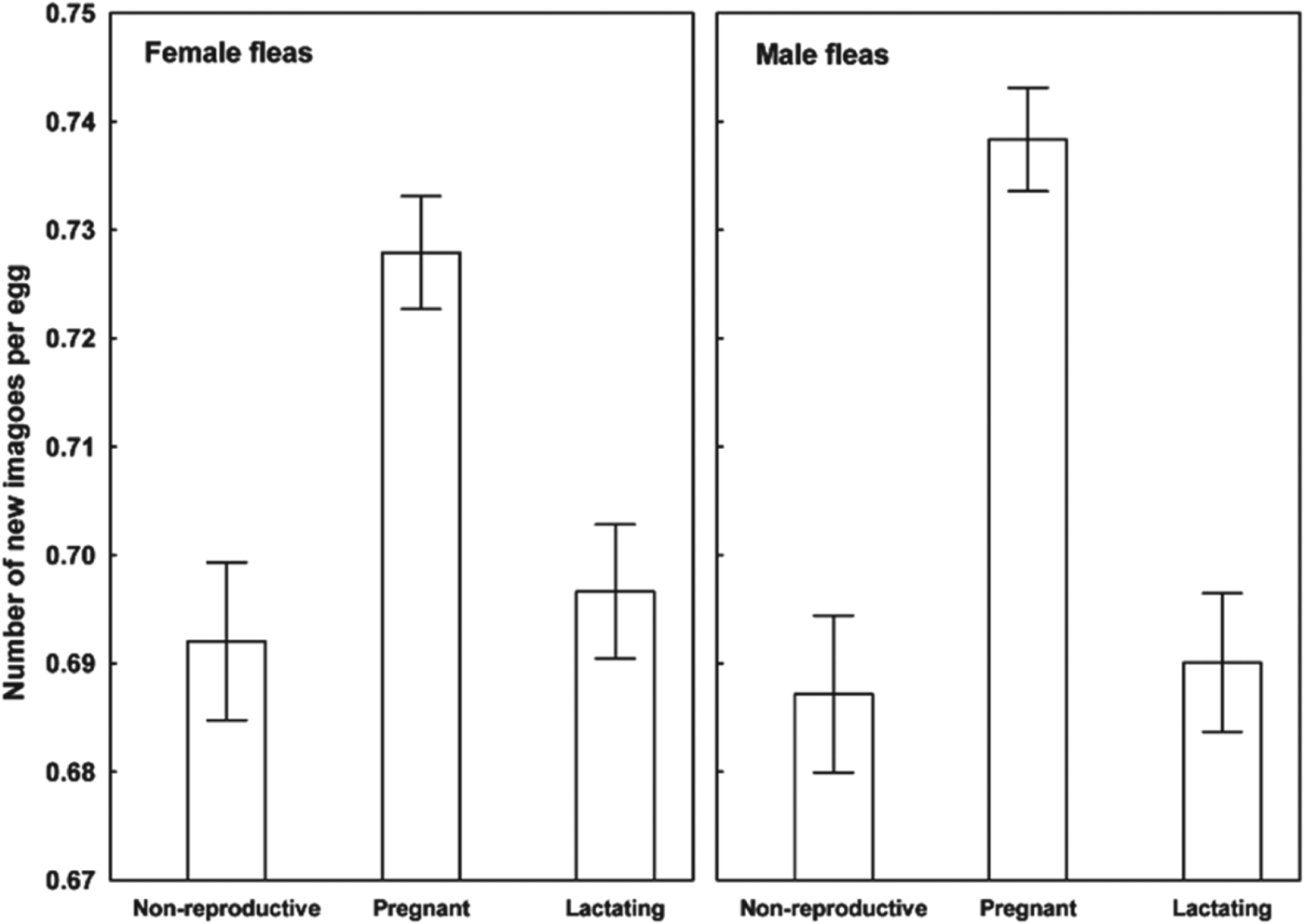
Fig. 1. Emergence success (mean±s.e. number of new imagoes per egg) of female and male offspring of X. ramesis fed on non-reproductive, pregnant or lactating female M. crassus.
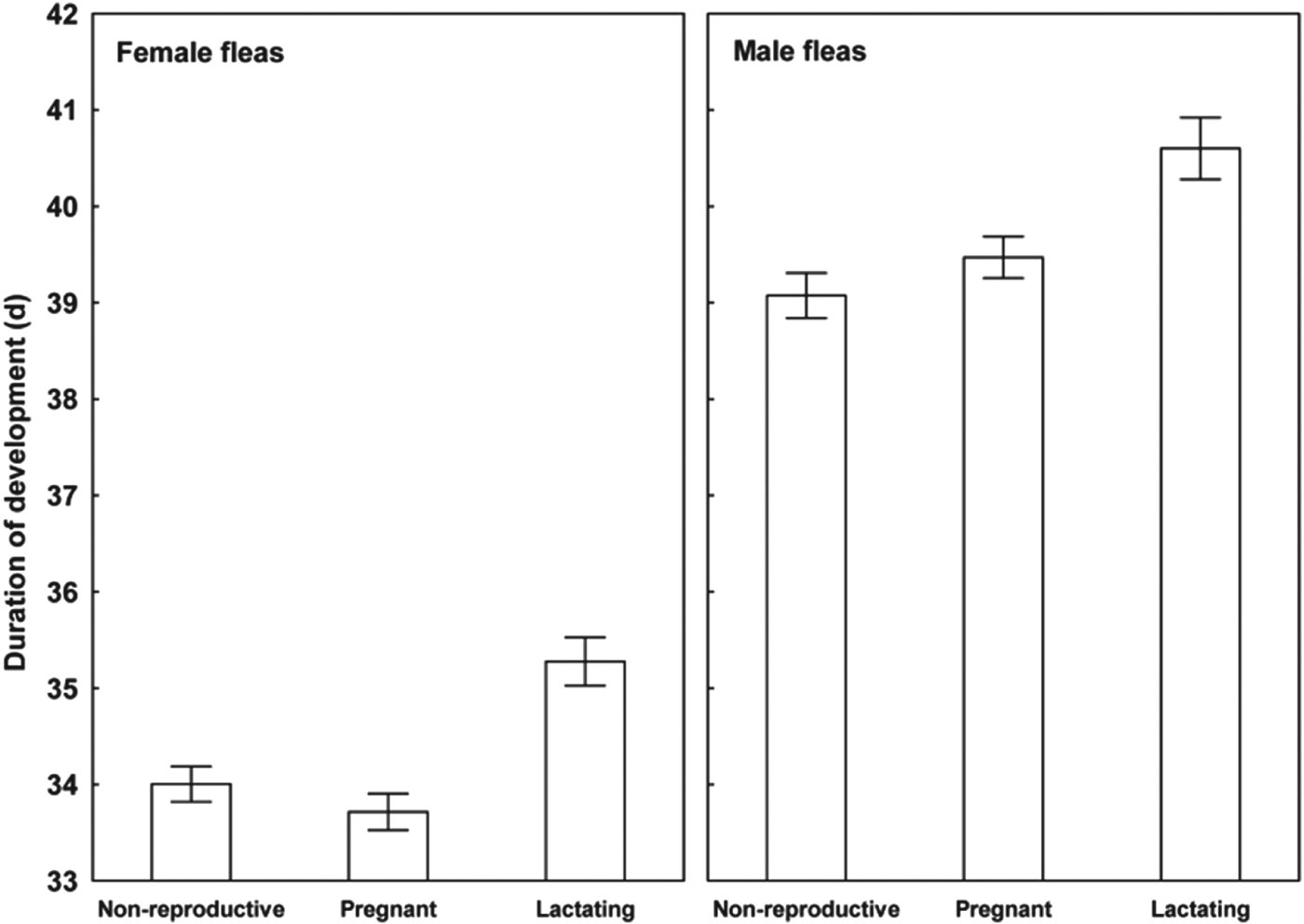
Fig. 2. Mean (±s.e.) duration of development from egg to imago in female and male offspring of X. ramesis fed on non-reproductive, pregnant or lactating female M. crassus.
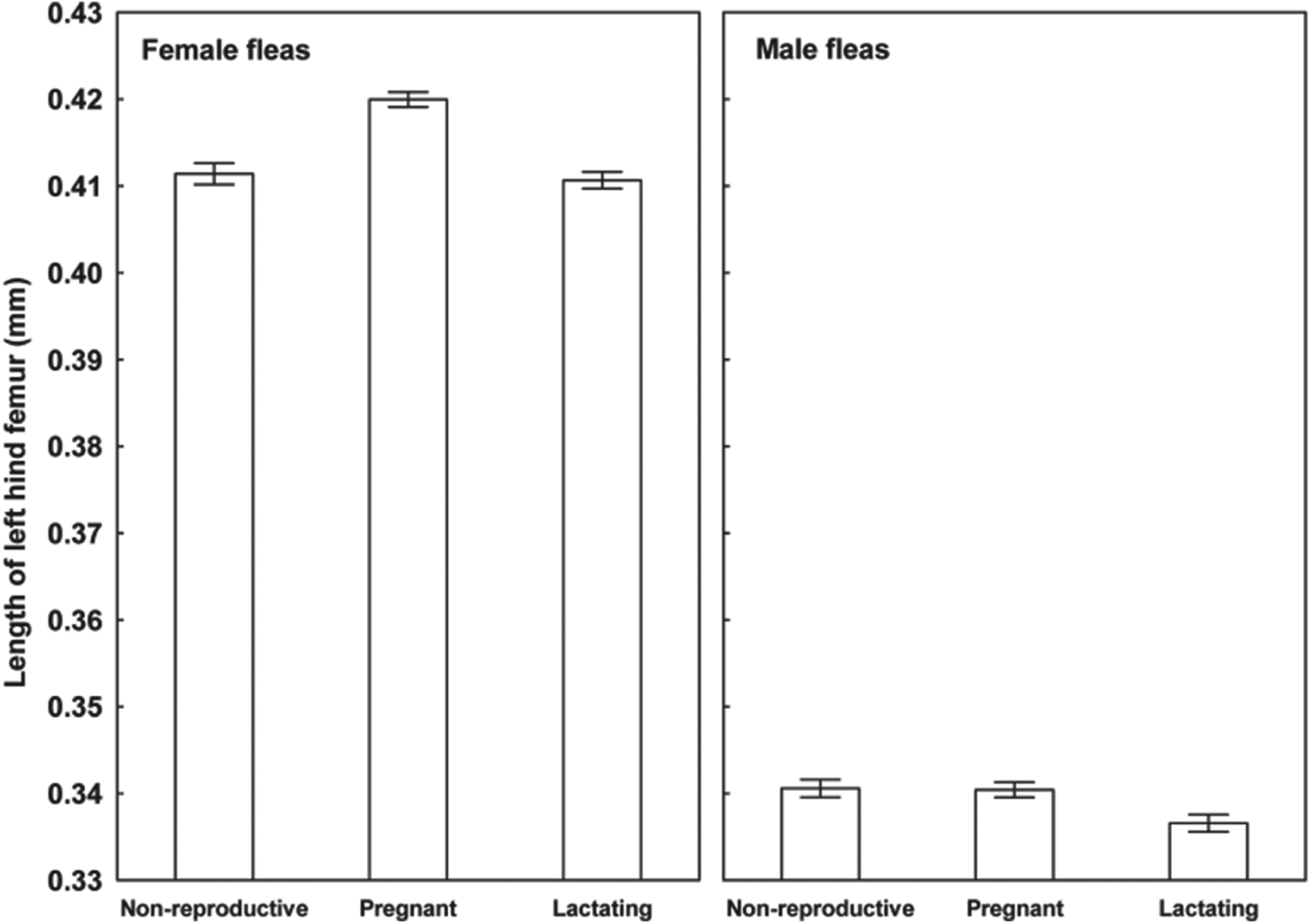
Fig. 3. Mean (±s.e.) length of left hind femur in male and female offspring of X. ramesis produced by females fed on non-reproductive, pregnant or lactating female M. crassus.
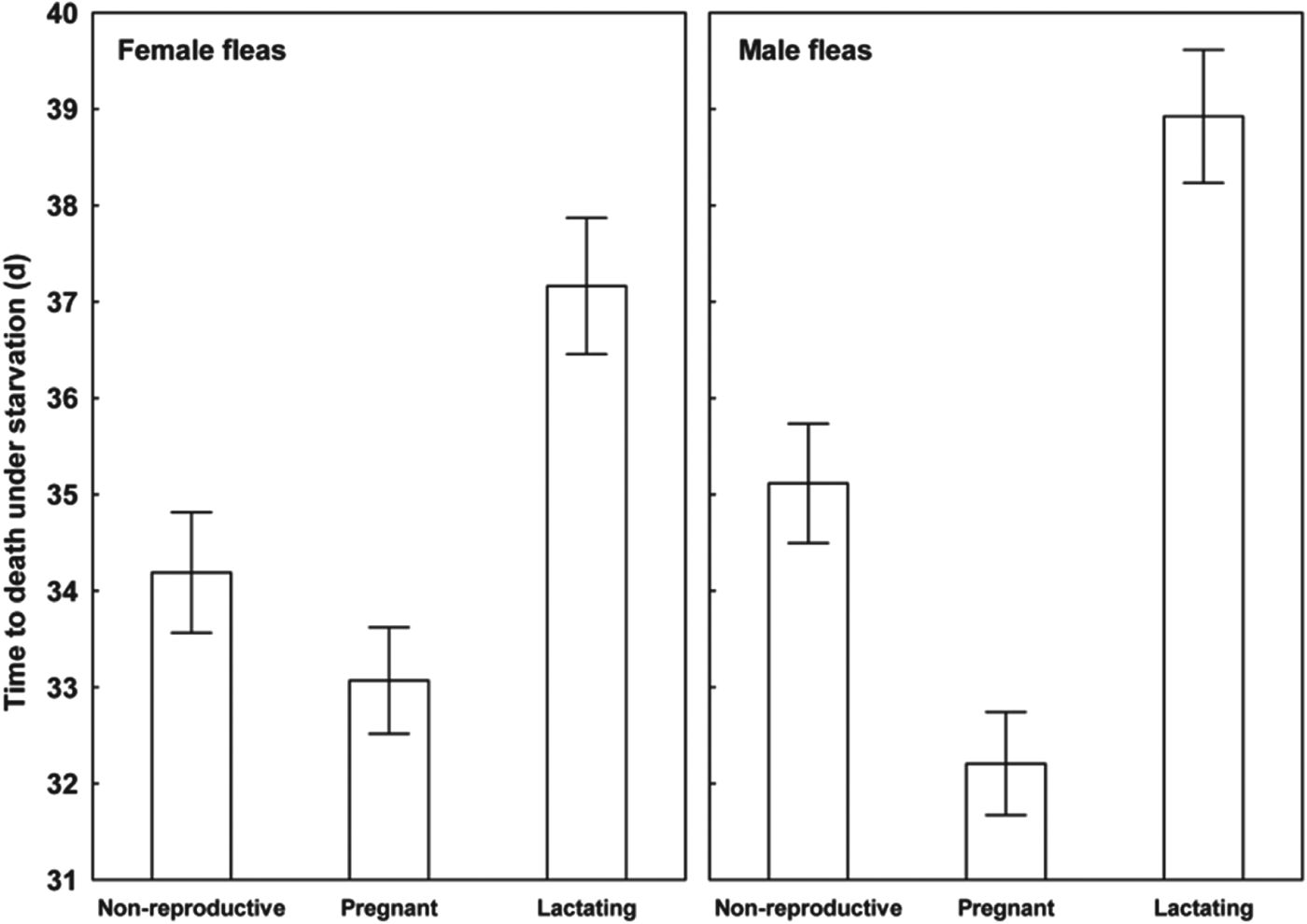
Fig. 4. Mean (±s.e.) time of survival from emergence under starvation in female and male offspring of X. ramesis fed on non-reproductive, pregnant or lactating female M. crassus.
Table 1. Summary of linear mixed-effects modelling of the effect of host (Meriones crassus) reproductive status [non-reproductive, pregnant (P) or lactating (L)] on variables describing quality of female (f) and male (m) offspring of a flea (Xenopsylla ramesis). ES, emergence success; DD, duration of development from an egg to a new imago; FL, length of left femur used as a proxy for body size; and SS, time of survival under starvation. Individual host number and the day an offspring was laid as an egg were included in the models as random effects (day nested within individual host). The reference level for the categorical independent variable was non-reproductive

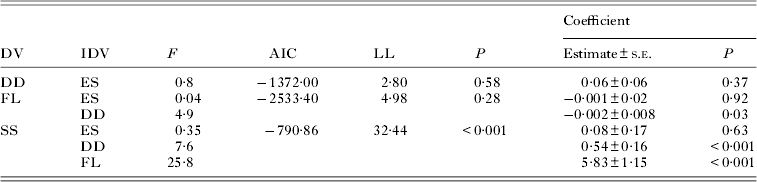
Two of the four variables describing the quality of the offspring were affected by the reproductive effort of parent females (Table 2). Coefficient estimates indicated that emergence success and duration of development of fleas decreased significantly with an increase in mean number of eggs laid by their mothers (Table 2). The only significant effects among the offspring quality variables revealed after controlling for confounding effects of gender and host reproductive status were that larger fleas that had longer developing times lived significantly longer under starvation (Table 3; see illustrative example with femur size in Fig. 5). Although the coefficient estimate of the duration of development in its effect on body size of new imagoes was significantly negative, the model itself was not (Table 3).

Fig. 5. Relationship between time of survival under starvation and body size (measured via length of a left hind femur) in male (a) and female (b) newly emerged X. ramesis produced by mothers fed on non-reproductive (bold line, closed circles), pregnant (solid line, open circles) or lactating (dashed line, closed triangles) M. crassus.
Table 2. Summary of linear mixed-effects modelling of the relationships between reproductive effort of parent fleas (mean number of eggs produced per female; independent variable) and emergence success (ES), duration of development (DD), body size measured as length of a left hind femur (FL), or the time to death under starvation (SS) (dependent variable) in newly emerged Xenopsylla ramesis. To control for the confounding effects of flea sex and reproductive status of a host, the original values were substituted with their residual deviations from the linear mixed-effects models of the effect of flea sex and/or rodent reproductive status on each of the variables (see text for details). AIC, Akaike Information Criterion; LL, log-likelihood ratio χ2
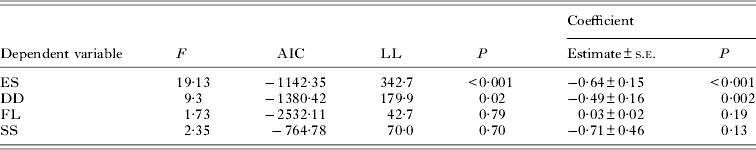
Table 3. Summary of linear mixed-effects modelling of the relationships among emergence success (ES), duration of development (DD), body size measured as length of a left hind femur (FL) and the time to death under starvation (SS) in newly emerged Xenopsylla ramesis. A dependent variable (DV) represents an earlier event, while independent variable(s) (IDV) represent(s) later event(s) (see text for explanations). To control for the confounding effects of flea sex and reproductive status of a host, the original values were substituted with their residual deviations from the linear mixed-effects models of the effect of flea sex and/or rodent reproductive status on each of the variables (see text for details). AIC, Akaike Information Criterion; LL, log-likelihood ratio χ2
DISCUSSION
The results of this study supported the assumption that the main cause of a trade-off between quantity and quality of offspring may be condition-dependent physiological constraints. Variation in flea performance among hosts of different reproductive status in terms of quantity of offspring did not translate directly into variation in performance in terms of quality of offspring. Across hosts of different reproductive status, some measures of offspring quality demonstrated the same trend as offspring quantity, whereas the opposite was true for other quality traits. For example, low egg production in fleas fed on pregnant hosts (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014) was accompanied by high survival (Fig. 1) and relatively short duration of pre-imaginal development of the pre-imago (Fig. 2), but also by low resistance to starvation of newly emerged adults (Fig. 4). These results indicate a quantity vs quality trade-off in fleas feeding on hosts of different reproductive status. However, this trade-off was trait-dependent, being observed for some but not other quality variables, which suggests that the ability of mothers to reliably predict the offspring environment (Parker and Begon, Reference Parker and Begon1986) may not always be a necessary condition for the negative relationship between quantity and quality of offspring.
From an evolutionary perspective, adjustment of offspring quality is thought to be adaptive and superior to fixed strategies of offspring quality in cases of (a) reliable environmental cues; (b) similar resource availability between environments; and (c) variation of offspring survival between environmental states (Taborsky, Reference Taborsky2006; Fischer et al. Reference Fischer, Taborsky and Kokko2011). Considering conspecific hosts belonging to different cohorts (for example, animals of different age or reproductive status) and their burrows as environments for fleas and their offspring (including pre-imaginal stages), conditions ‘b’ and ‘c’ hold. Indeed, resource availability in terms of blood (for adults) and organic debris in the burrow substrate (for larvae) are likely similar among hosts of, for example, different age, reproductive status and/or body conditions. Offspring survival has been shown to vary greatly among such hosts (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2013; this study). However, condition ‘a’ does not seem to fit because association of a flea with a host belonging to one of these cohorts is often short-term and may be even shorter than the time a flea needs to produce new offspring (about a month for X. ramesis; Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001). For example, offspring of a mother that exploits a juvenile or a pregnant host would not encounter a juvenile or pregnant host too, but rather a sub-adult host or lactating host (accompanied by multiple juvenile hosts), respectively. Nevertheless, it can be argued that, in a case of fleas feeding on hosts of different reproductive status, condition ‘a’ might hold to some extent, so that the environment of offspring might be predictable. Indeed, given the length of a flea life cycle and duration of pregnancy and lactation of M. crassus (see above), offspring of fleas exploiting pregnant rodents will likely face juvenile rodents, that is, numerous hosts with underdeveloped anti-parasitic defences (see Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2013). Offspring of fleas exploiting lactating females may experience host unavailability due to dispersal of young rodents and burrow switch by a mother rodent (which is a characteristic spatial behaviour of M. crassus; Krasnov et al. Reference Krasnov, Shenbrot, Khokhlova, Degen and Rogovin1996). Consequently, an advantageous strategy for a flea feeding on a pregnant host will be to produce fewer offspring that develop faster although would be weakly resistant to starvation. For a flea feeding on a lactating host, it will be advantageous to produce more offspring that develop more slowly but would be highly resistant to starvation. Both these patterns have been observed in our experiments (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014 and this study). However, this explanation is weakened by the fact that although fleas exploiting non-reproductive hosts produced as many eggs as those exploiting lactating hosts (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014), the duration of the offspring development was as short and their resistance to starvation (at least, in females) was as weak as of those from pregnant hosts (Figs 2 and 4, respectively).
Recent theoretical studies demonstrated that the level of adaptive plasticity in offspring quality, in particular in body size, is expected to increase with an increase in the reliability of environmental cues used by a mother (Fischer et al. Reference Fischer, Taborsky and Kokko2011). However, results of our study suggest that the negative relationship between offspring quantity and quality resembling a trade-off may arise due to a variety of reasons unrelated to any adaptive strategy (but see reservations above). This is especially likely in species with ontogenesis represented by a chain of discrete events (for example, holometabolous insects such as fleas) because of a variety of factors affecting the offspring quality during each stage of ontogenesis (Werner and Gilliam, Reference Werner and Gilliam1984; Fischer et al. Reference Fischer, Taborsky and Kokko2011). Indeed, fleas fed on pregnant rodents produced a relatively small number of eggs (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014) which resulted in a small number of larvae. These larvae unlikely competed for food due to their small number (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005), so the majority survived to adulthood. In contrast, fleas fed on non-reproductive or lactating females produced many eggs (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014). Larvae hatched from these large egg bundles might experience strong food competition, so their emergence success was relatively low (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005).
The relationships between the variables describing quality of new adult fleas suggested some trade-offs and provided explanation for the trait-dependence of the relationship between the number of eggs produced by fleas and quality of new adults. That is, the direction of the relationship between quantity and quality depended on which quality variable was considered. For example, after controlling for flea gender and host reproductive status, we found that fleas that were larger and had longer development times lived significantly longer under starvation. We assumed that larger body size and faster development of flea offspring were associated with their higher quality from an ecological perspective (see elaborations above). However, a larger body size (that is, higher quality) can be a consequence of a longer development (that is, lower quality) (Esperk and Tammaru, Reference Esperk and Tammaru2010) because the latter provides larva with more time for foraging and, consequently, growth. A larger larva will develop into a larger new adult. Insect body size is determined by many factors (see Nijhout, Reference Nijhout2003 for review). The size of a new imago correlates positively with the size of a larva of the last instar, which grows until the onset of the secretion of ecdysteroids (the steroid hormones initiating the moulting cycle; Nijhout, Reference Nijhout1994). This onset is associated with the time when a larva reaches a particular critical mass (Nijhout, Reference Nijhout1979) which, in turn, depends on larva provisioning (Davidowitz et al. Reference Davidowitz, D'Amico and Nijhout2003) among other factors. The time between attaining critical weight and secretion of ecdysteroids may also affect the final body mass (Nijhout et al. Reference Nijhout, Davidowitz and Roff2006). In other words, larger individuals compensate for longer development with their body size. However, the larger body size may be advantageous not only in relation to mating opportunity (Hanks et al. Reference Hanks, Millar and Paine1996; but see Burton-Chellew et al. Reference Burton-Chellew, Sykes, Patterson, Shuker and West2007) and higher mobility, providing a flea with better opportunities to locate a host (Krasnov et al. Reference Krasnov, Burdelov, Khokhlova and Burdelova2003), but also with larger fat reserves, allowing them to endure starvation longer (Arrese and Soulages, Reference Arrese and Soulages2010).
As mentioned above, in our earlier study (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014) reduction in egg production from fleas fed on pregnant hosts but not lactating hosts was found. This occurred even though lactation and not pregnancy is the most energetically demanding period faced by reproducing mammalian females (Degen, Reference Degen1997; Speakman, Reference Speakman2008), so that the reproductive response of fleas exploiting lactating females should be expected. These results did not allow us to propose an energy-based explanation for variation in flea egg production in relation to host reproductive status, but instead suggested that the difference in egg production was due to changes in the hormonal and/or immune status of the host (Dlugosz et al. Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014). However, in the present study, fleas responded to host lactation by substantially increasing duration of development of their offspring. It is possible that energy-challenged lactating hosts may somehow (e.g. via changes in blood composition and its energetic value) transfer this challenge to blood-sucking parasites leading to, for example, poor provisioning of their eggs. Larvae hatching from these eggs may be small (Giron and Casas, Reference Giron and Casas2003) and forced to compensate for this ‘bad start’ by prolonging development (Metcalfe and Monaghan, Reference Metcalfe and Monaghan2001). No evidence, however, is available to support this explanation and it warrants further investigation. Nevertheless, taken together, the lowest egg production in fleas fed on pregnant females and the longest duration of development in offspring of fleas fed on lactating females suggest that the host energetic state may also be important in its effect on parasite performance and that the effect of host reproductive status on parasite reproduction is associated with a complex interplay between energetic, hormonal and immunological factors. In addition, results of this study and of Dlugosz et al. (Reference Dlugosz, Downs, Khokhlova, Degen and Krasnov2014) demonstrate that the assessment of reproductive performance in haematophagous ectoparasites, as well as in many other species, has to be based not only on egg production but also on various estimates of offspring quality.
ACKNOWLEDGEMENTS
Experimental procedures complied with the laws of the State of Israel. This is publication no. 825 of the Mitrani Department of Desert Ecology.
FINANCIAL SUPPORT
This study was supported by the Israel Science Foundation (Grant no. 26/12 to BRK and ISK). EMD and CJD received financial support from the Blaustein Center for Scientific Cooperation (BSCS), the Kreitman School of Advanced Graduate Studies and the Swiss Institute for Dryland Environmental and Energy Research (SIDEER).