Introduction
Parasites are capable of imposing strong selection on host populations, often resulting in the evolution of host defence mechanisms (Thompson, Reference Thompson1994, Reference Thompson2005). Host populations can exhibit a diverse array of defences that confer increased fitness in the presence of parasites. Multiple molecular pathways enable cellular immune responses in both the adaptive (Litman et al. Reference Litman, Rast and Fugmann2010) and innate (Engelmann and Pujol, Reference Engelmann and Pujol2010; Buchon et al. Reference Buchon, Silverman and Cherry2014) immune systems of hosts. Further, hosts may also engage in behavioural defences against parasites. Behavioural defence includes medicinal behaviours that clear or impede existing infections (Chapuisat et al. Reference Chapuisat2007; Singer et al. Reference Singer, Mace and Bernays2009; Lefevre et al. Reference Lefevre2010) and parasite avoidance, wherein the host engages in actions that limit its exposure to the parasite (Taylor, Reference Taylor1954; Dudley and Mitton, Reference Dudley and Mitton1990; Feener and Moss, Reference Feener and Moss1990; Hart, Reference Hart1990, Reference Hart1994; Chang et al. Reference Chang, Paek and Kim2011; Moore, Reference Moore2012; Meisel and Kim, Reference Meisel and Kim2014). Given this diversity of host responses, many factors may shape the evolution of host defence. Do specific host traits consistently evolve to confer defence for a given host–parasite interaction, or is the evolution of host defence influenced by the context of the interaction?
Both the host mating system and host–parasite coevolutionary dynamics can significantly alter the evolutionary trajectories of host populations, which may then shape the evolution of host defence. With regard to mating systems, host outcrossing can facilitate rapid adaptation to parasites, whereas self-fertilization can impede adaptive evolution (Busch et al. Reference Busch, Neiman and Koslow2004; Morran et al. Reference Morran, Parmenter and Phillips2009, Reference Morran2011, Reference Morran2013; Teotonio et al. Reference Teotonio2012; Masri et al. Reference Masri2013; Carvalho et al. Reference Carvalho2014; Slowinski et al. Reference Slowinski2016). Selection imposed by coevolving parasites may favour host outcrossing over self-fertilization for several reasons that are not mutually exclusive. First, if coevolving parasites impose negative frequency-dependent selection, then outcrossing may generate host progeny with rare genotypes (Jaenike, Reference Jaenike1978; Hamilton, Reference Hamilton1980; Bell, Reference Bell1982). This is the basis for the Red Queen hypothesis (reviewed in Lively and Morran, Reference Lively and Morran2014). Second, outcrossing may increase the efficacy of selection on certain loci, relative to self-fertilization, by breaking linkage disequilibrium (Fisher, Reference Fisher1930; Muller, Reference Muller1932; Hill and Robertson, Reference Hill and Robertson1966; Felsenstein, Reference Felsenstein1974). Under this scenario, outcrossing should facilitate greater rates of adaptation than self-fertilisation by uniting multiple beneficial mutations into a common host genome more rapidly (reviewed in Hartfield and Keightley, Reference Hartfield and Keightley2012). Further, by increasing the efficacy of selection across the genome, outcrossing may increase the likelihood of evolving multiple host defences in response to selection acting simultaneously on different traits. Therefore, the host mating system can significantly alter the evolution of the host genome in response to selection and consequently host defence may evolve differently under different mating systems. Specifically, outcrossing populations may have a greater capacity to evolve significantly enhanced host defence, potentially via multiple mechanisms, relative to predominantly selfing populations.
Avoidance can be a very effective first line of defence against parasites (Hart, Reference Hart1990, Reference Hart1994). However, avoidance requires hosts to detect the parasite; therefore, some level of recognition, and perhaps specificity, between host and parasite populations should be critical for hosts to exhibit avoidance as a defence (Meisel and Kim, Reference Meisel and Kim2014). Local adaptation occurs when host, parasite or host and parasite populations evolve specificity towards their sympatric antagonist population, as opposed to the evolution of a response to individuals of the antagonist species in general. Antagonistic coevolutionary interactions, as opposed to transient or static interactions, can promote specificity between hosts and parasites (Ebert, Reference Ebert1994; Kaltz and Shykoff, Reference Kaltz and Shykoff1998; Lively and Dybdahl, Reference Lively and Dybdahl2000; Laine, Reference Laine2005, Reference Laine2007; Morgan et al. Reference Morgan, Gandon and Buckling2005; Greischar and Koskella, Reference Greischar and Koskella2007; Gibson et al. Reference Gibson2015). Therefore, antagonistic coevolution may favour more rapid evolution of avoidance behaviour than host interactions with transient or static parasite populations. Additionally, the evolution of specificity in host–parasite interactions can be accelerated by host outcrossing (Morran et al. Reference Morran2014). Therefore, outcrossing host populations may be more likely to evolve avoidance as a response to selection imposed by coevolving parasites than selfing hosts.
In a previous study, obligately outcrossing and mixed mating host populations of the nematode Caenorhabditis elegans were exposed to either coevolving or static (non-coevolving) populations of the bacterial parasite Serratia marcescens in the presence of their normal laboratory food source, Escherichia coli (Morran et al. Reference Morran2011). Serratia marcescens can rapidly kill C. elegans upon consumption (Schulenburg and Ewbank, Reference Schulenburg and Ewbank2004). However, paradoxically, C. elegans hosts generally prefer to feed on S. marcescens rather than some non-parasitic strains of E. coli (Zhang et al. Reference Zhang, Lu and Bargmann2005; Pradel et al. Reference Pradel2007; Glater et al. Reference Glater, Rockman and Bargmann2014). Hosts were plated directly onto a lawn of S. marcescens and were required to crawl to E. coli for survival and reproduction. After 30 generations, the host populations exhibited elevated levels of fitness in the presence of the parasites, and the obligately outcrossing populations exhibited greater rates of adaptation than the mixed mating populations (Morran et al. Reference Morran2014; Penley et al. Reference Penley, Ha and Morranunpublished result). Further, the parasites that coevolved with the obligately outcrossing populations exhibited greater host specificity than the parasites that coevolved with mixed mating hosts (Morran et al. Reference Morran2014). We hypothesized that selection to avoid the parasite may have been strong during experimental evolution, and that host defence may have evolved differently depending on the host mating system and the nature of host interactions with the parasite. Here, our goal was to determine if adaptation in the host populations was driven by the evolution of parasite avoidance. Using our experimentally evolved populations, we measured C. elegans host preference for E. coli vs S. marcescens parasite populations to test for the evolution of parasite avoidance within the context of our previous experiment. Additionally, we assessed whether the host mating system and host–parasite coevolutionary dynamics influenced the evolution of parasite avoidance as a host defence.
Methods and materials
Host populations
Host populations consisted of five mixed mating and five obligately outcrossing C. elegans populations derived from strain PX382 with an overall CB4856 background, obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, Minnesota, USA). Obligately outcrossing populations were modified with the fog-2(q71) mutation, which prevents hermaphrodites from producing sperm, requiring outcrossing for reproduction (Schedl and Kimble, Reference Schedl and Kimble1988). Each of the five replicate populations within each mating type were independently exposed to ethyl methanesulphonate (EMS) mutagenesis to infuse novel genetic variation prior the start of the experiment, and then each population was split and replicated across each treatment (Morran et al. Reference Morran2011). A subset of each population was frozen at −80 °C prior to experimentation and preserved prior to experimental evolution, the ancestral population for a given experimental population.
Parasite populations
Serratia marcescens (Sm2170) is a gram-negative bacterium and virulent parasite of C. elegans that causes systemic infection and ~80% mortality within 24 h upon consumption (Schulenburg and Ewbank, Reference Schulenburg and Ewbank2004). Sm2170 can impose strong selective pressure against C. elegans (Morran et al. Reference Morran, Parmenter and Phillips2009). Serratia marcescens can be recovered from the C. elegans gut and cultured, facilitating experimental coevolution (Morran et al. Reference Morran2011).
Experimental evolution
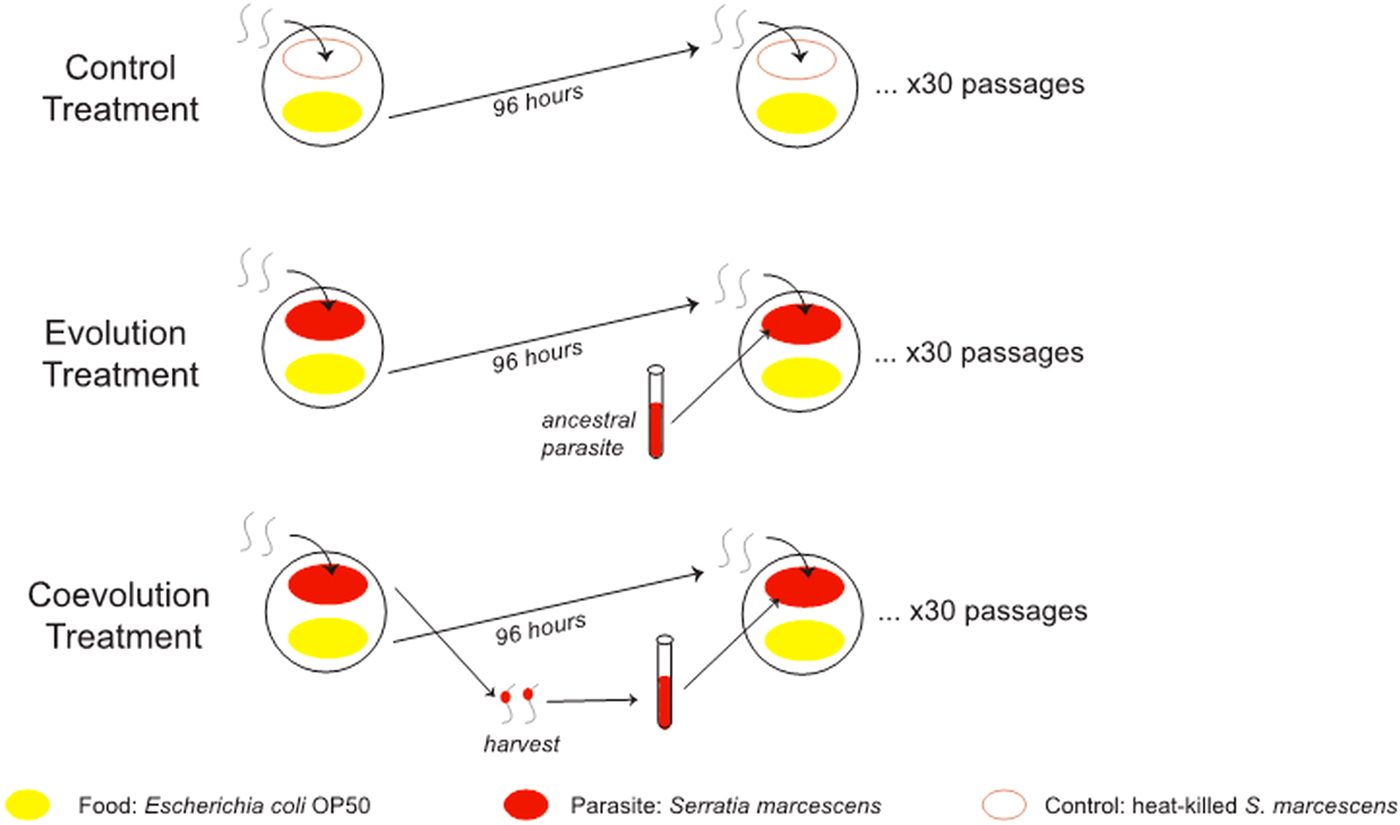
Each host population was split across three treatments for experimental evolution: evolution, coevolution and control for 30 generations on Serratia Selection Plates (SSP) following methods outlined in Morran et al. (Reference Morran2011) (Fig. 1). Briefly, SSPs were constructed by spreading S. marcescens on 1/3 of the NGM-Lite (US Biological, Swampscott, Massachusetts, USA) agar of a 100 mm Petri dish, E. coli OP50 on the opposite 1/3 and ampicillin (200 µg mL−1) in the middle 1/3 of the agar. Caenorhabditis elegans were plated onto the S. marcescens side of the plate and required to survive parasite exposure and travel to the E. coli side in order to reproduce and be passaged. The evolution treatment consisted of exposure to a static, non-evolving S. marcescens strain Sm2170, whereas the coevolution treatment consisted of exposure to coevolving S. marcecens, and control treatment groups were exposed to heat killed Sm2170. Host and parasite populations in the experimental coevolution treatment were passaged under the potential for reciprocal selection. Specifically, parasites were required to kill hosts for passage to the next round of selection, whereas hosts were required to survive parasite exposure and reproduce for their offspring to be passaged. This coevolution treatment differed from the evolution treatment in that within the evolution treatment, only the hosts were under selection to survive and reproduce. Importantly, S. marcescens strain 2170 served as both the ancestral parasite strain in the coevolution treatment and the static parasite strain in the evolution treatment. A subset of each experimental host population and coevolving S. marcescens was frozen and stored at −80 °C at generations 0, 10, 20 and 30.
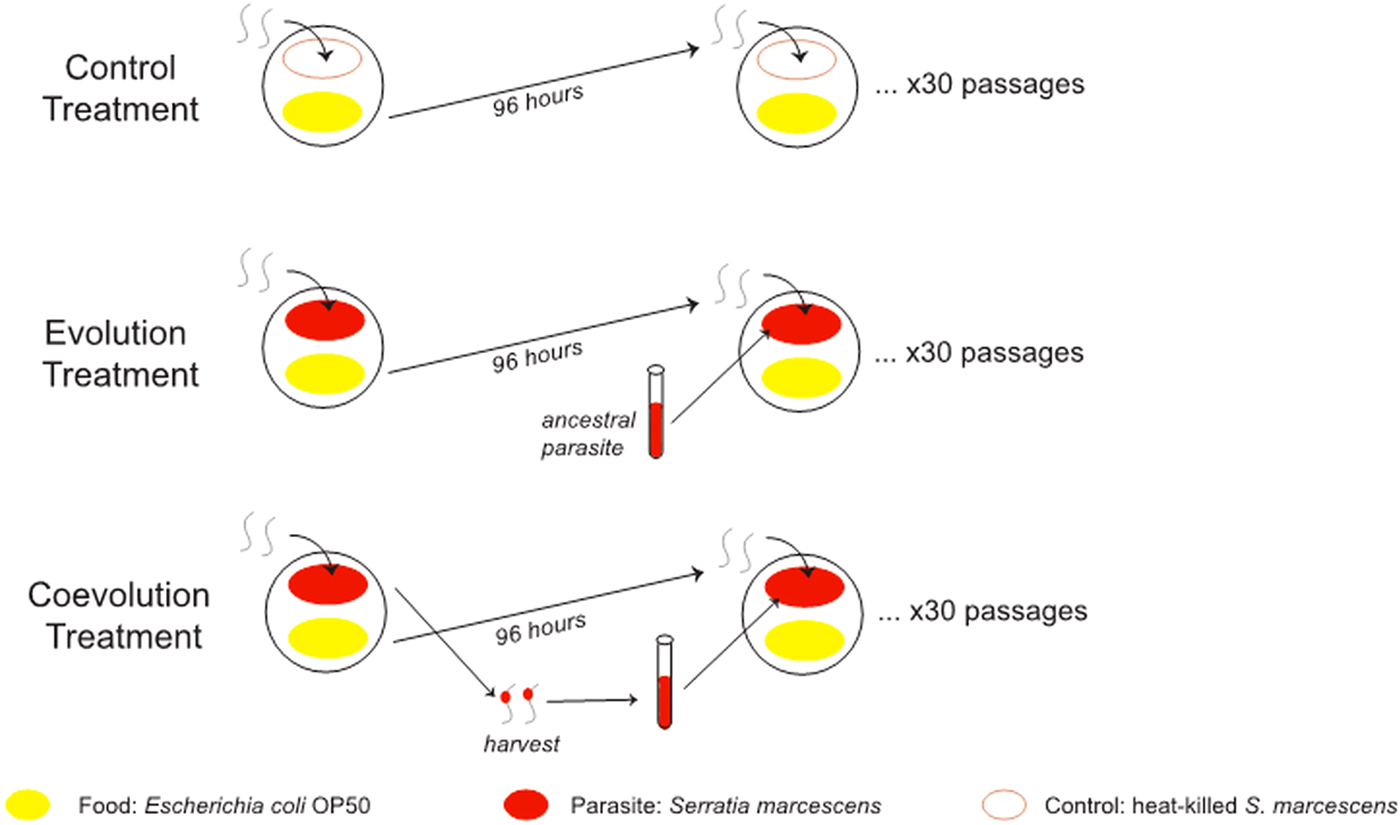
Fig. 1. Experimental evolution treatments. In a previous experiment, five replicate populations of mixed mating Caenorhabditis elegans hosts and five replicate populations of obligately outcrossing C. elegans hosts were passaged for 30 generations on three treatments: control, evolution and coevolution. For each replicate population, parental hosts were placed on the Serratia marcescens side of the Serratia Selection Plate. Then, after 4 days of parasite exposure, the host offspring were collected on the Escherichia coli portion and transferred to another plate. The hosts were exposed to heat-killed parasites in the control. The hosts were exposed to the same strain of live S. marcescens each generation in the evolution treatment. Live S. marcescens parasites were copassaged with the hosts in the coevolution. Parasites were extracted from dead hosts after 24 h of exposure and then used to seed the Serratia Selection Plate for the next generation of hosts.
Bacterial choice assay
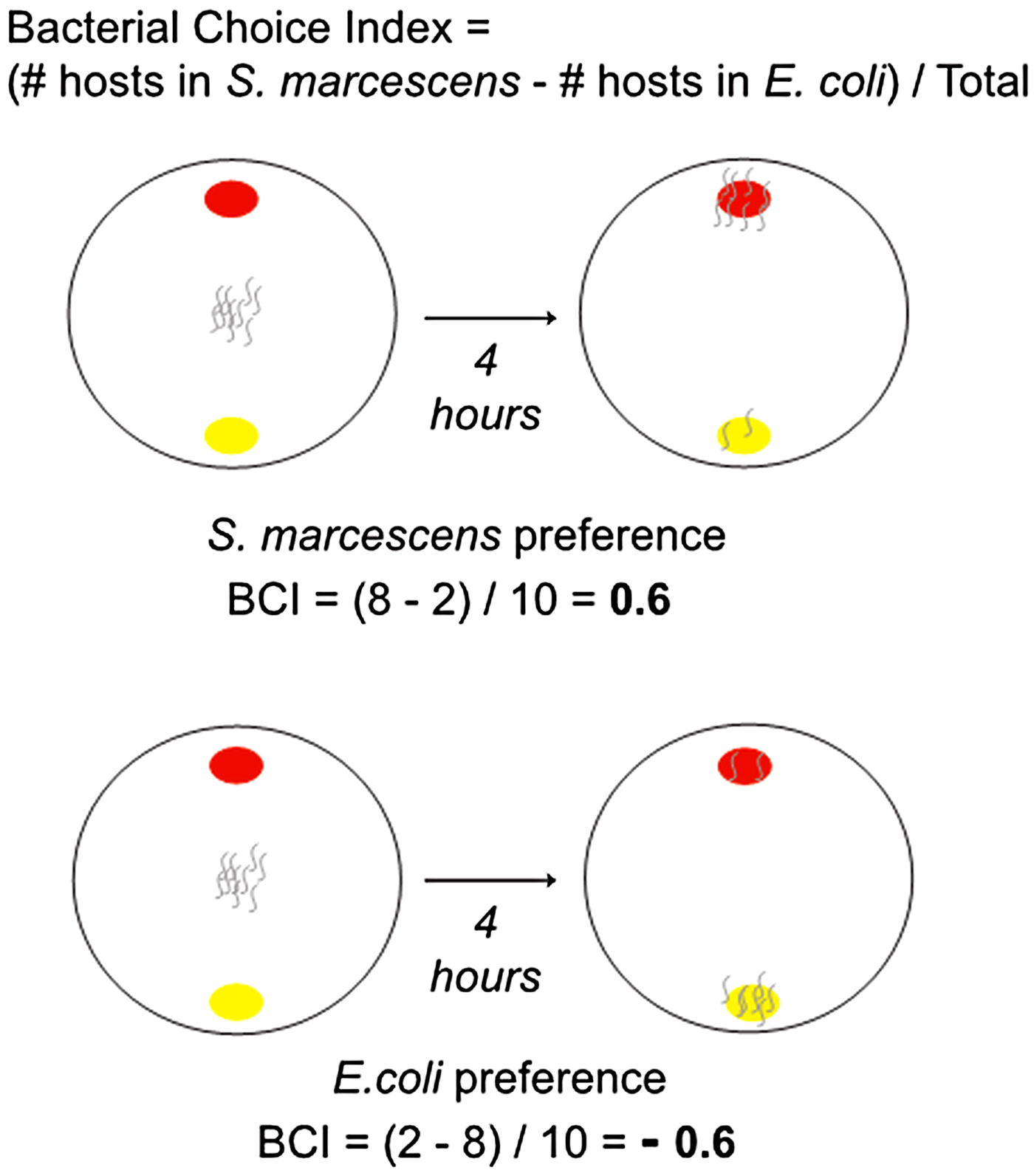
Bacterial choice assays were performed using methods modified from Zhang et al. (Reference Zhang, Lu and Bargmann2005) and Glater et al. (Reference Glater, Rockman and Bargmann2014) (Fig. 2). Choice assays were set up in 100 mm petri dishes containing 30 mL of NGM-Lite. A 25 µL of S. marcescens (strain Sm2170 or coevolved) and E. coli were spotted onto opposite far sides of the plate and allowed to incubate at room temperature for 5 h. Host populations from the coevolution treatment were paired with their respective sympatric parasite population, which is the S. marcescens population with which they coevolved. Host populations were bleach synchronised and ~200 L4 individuals in M9 buffer were introduced onto the middle of the assay plate. Petri dishes were left ajar at room temperature for ~30 min until the M9 buffer had completely evaporated, at which point the lids were closed and dishes were moved into the 20 °C incubator. Four hours after plating the hosts, assay plates were scored by counting the number of host individuals in each bacteria spot and on the rest of the plate. We assayed populations after 4 h because we observed that 4 h was the minimum amount of time required for >75% of the hosts to exhibit choice. Each choice assay was replicated six times.
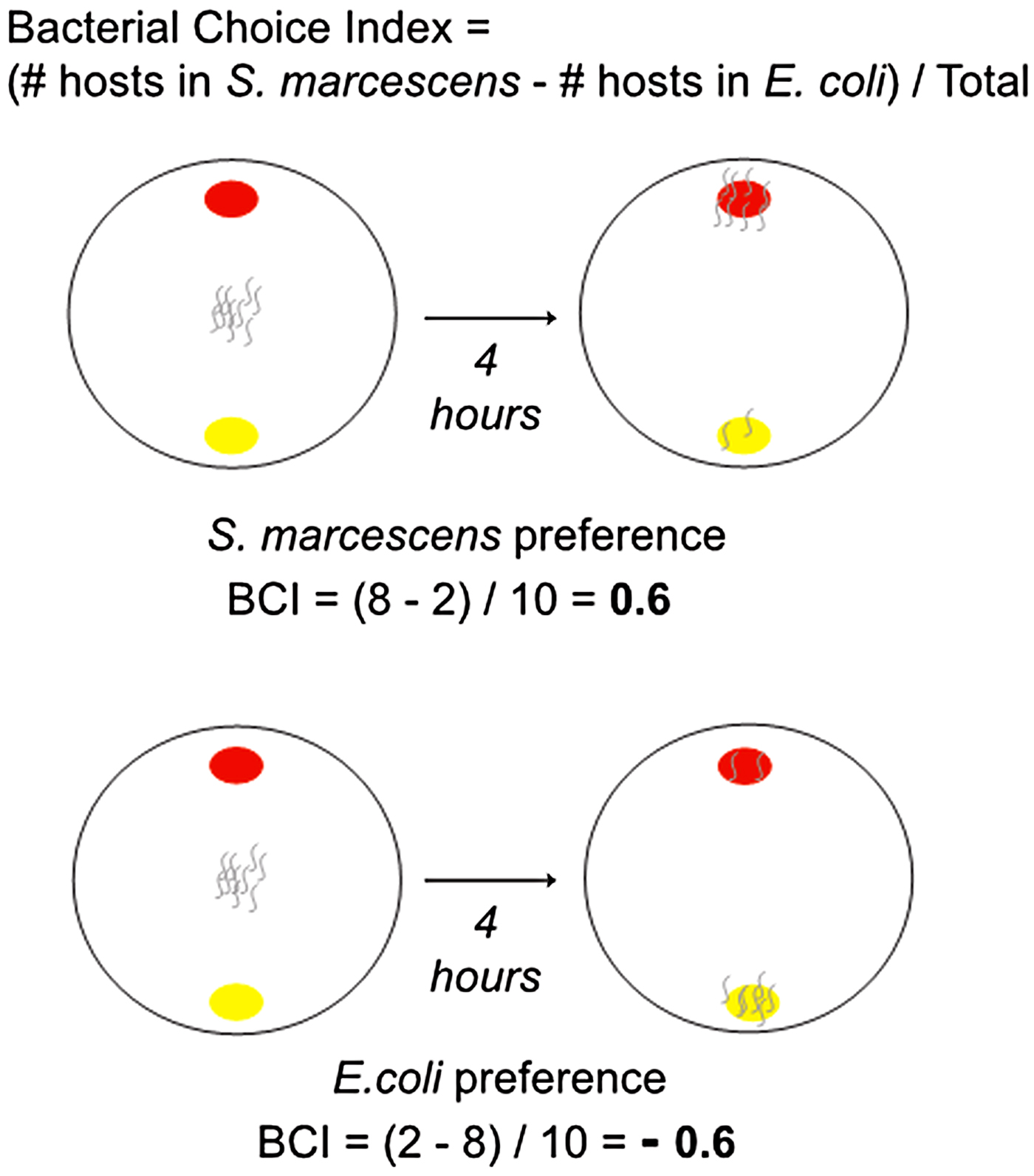
Fig. 2. BCI schematic. Caenorhabditis elegans hosts were placed between patches of Serratia marcescens and Escherichia coli, then their location was recorded after 4 h. These data were used to calculate the BCI for the hosts. BCI, bacterial choice index.
Ancestral host populations, as well as host populations from the coevolution, evolution and control treatments were assayed for choice between E. coli OP50 and Sm2170. Further, host populations from generation 20 and generation 30 of the coevolution treatment were assayed for their avoidance of the ancestor parasite (Sm2170) and the generation 20 coevolved parasite. We assayed generation 20 hosts with their contemporary parasite from the coevolution treatment to assess parasite avoidance in the midst of the arms race. We assayed generation 30 hosts with their parasites from the recent past to determine if there is a time lag in the host response. We were unable to revive two of the five obligately outcrossing host populations from the coevolution treatment at generation 30 from frozen stock. Therefore, our analysis included only three replicate populations for this particular treatment by mating system combination.
The following equation, modified from Glater et al. (Reference Glater, Rockman and Bargmann2014), was used to calculate the host bacterial choice index. We modified their equation by dividing the difference between the number of hosts on S. marcescens and the number on E. coli by the total number of hosts, rather than dividing the number of hosts that specifically chose either S. marcescens or E. coli. Thus, ‘Total # hosts plated’ indicates total number of hosts individuals assayed, regardless of where they were located upon scoring the assay. In each replicate assay, regardless of the host or parasite population, multiple host individuals were not in either bacterial spot at the end of the assay. Our method of calculating the bacterial choice index is a more precise measure of preference as a whole in that the absolute value of choice index scores are reduced by individuals that do not explicitly choose a bacterial spot. Further our method is more applicable in the context of our experiment because host fitness was dependent on both avoiding S. marcescens and feeding on E. coli, as opposed to only avoiding the parasite.
 $${\eqalign {\rm Bacterial}\;{\rm choice}\;{\rm index} \cr\qquad = \displaystyle{{(\# \;{\rm hosts}\;{\rm in}\;S. \,marcescens - \#\,{\rm hosts}\;{\rm in}\;E.{\rm}\,coli)} \over {{\rm Total}\,\#\,{\rm hosts}\;{\rm plated}}}$$
$${\eqalign {\rm Bacterial}\;{\rm choice}\;{\rm index} \cr\qquad = \displaystyle{{(\# \;{\rm hosts}\;{\rm in}\;S. \,marcescens - \#\,{\rm hosts}\;{\rm in}\;E.{\rm}\,coli)} \over {{\rm Total}\,\#\,{\rm hosts}\;{\rm plated}}}$$A choice index >0 indicates a preference for the parasite S. marcescens. A choice index <0 indicates preference for E. coli (Fig. 2).
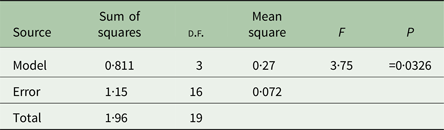
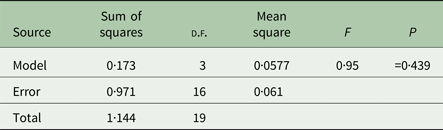
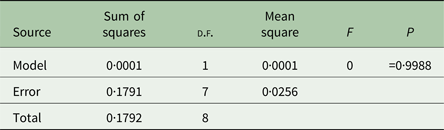
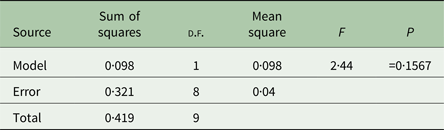
We performed analyses of variance (ANOVAs) in JMP 12 (SAS Institute, Cary, North Carolina, USA) on transformed mean bacterial choice index values for each replicate host population. Mean bacterial choice index values were square root transformed after adding a value of 1 to each mean to ensure that all values were positive prior to transformation. The transformed mean values did not violate the ANOVA assumptions of normality and equal variances according to the Shapiro–Wilk and Levene's tests. We performed separate ANOVAs for the mixed mating (Table 1) and obligately outcrossing (Table 2) populations exposed to Sm2170. In both analyses, we tested the main effect of host treatment (ancestral, control, evolution and coevolution) on mean bacterial choice index values. We also used least squares mean contrast tests to assess differences between treatments within the mixed mating analysis. Then, we performed separate ANOVAs for the mixed mating (Table 3) and obligately outcrossing (Table 4) hosts from the coevolution and control treatments from generations 20 and 30 exposed to coevolved parasites from generation 20. In both analyses, we tested the main effects of host treatment (control and coevolution) and host generation (generations 20 and 30) and the treatment by generation interaction, on the transformed mean bacterial choice index values.
Table 1. Mixed mating host ancestral bacterial choice index table

Table 2. Obligately outcrossing host ancestral bacterial choice index table

Table 3. Mixed mating host coevolved bacterial choice index table

Table 4. Obligately outcrossing host coevolved bacterial choice index table
Results
Host bacterial food preference: ancestral and static parasite populations
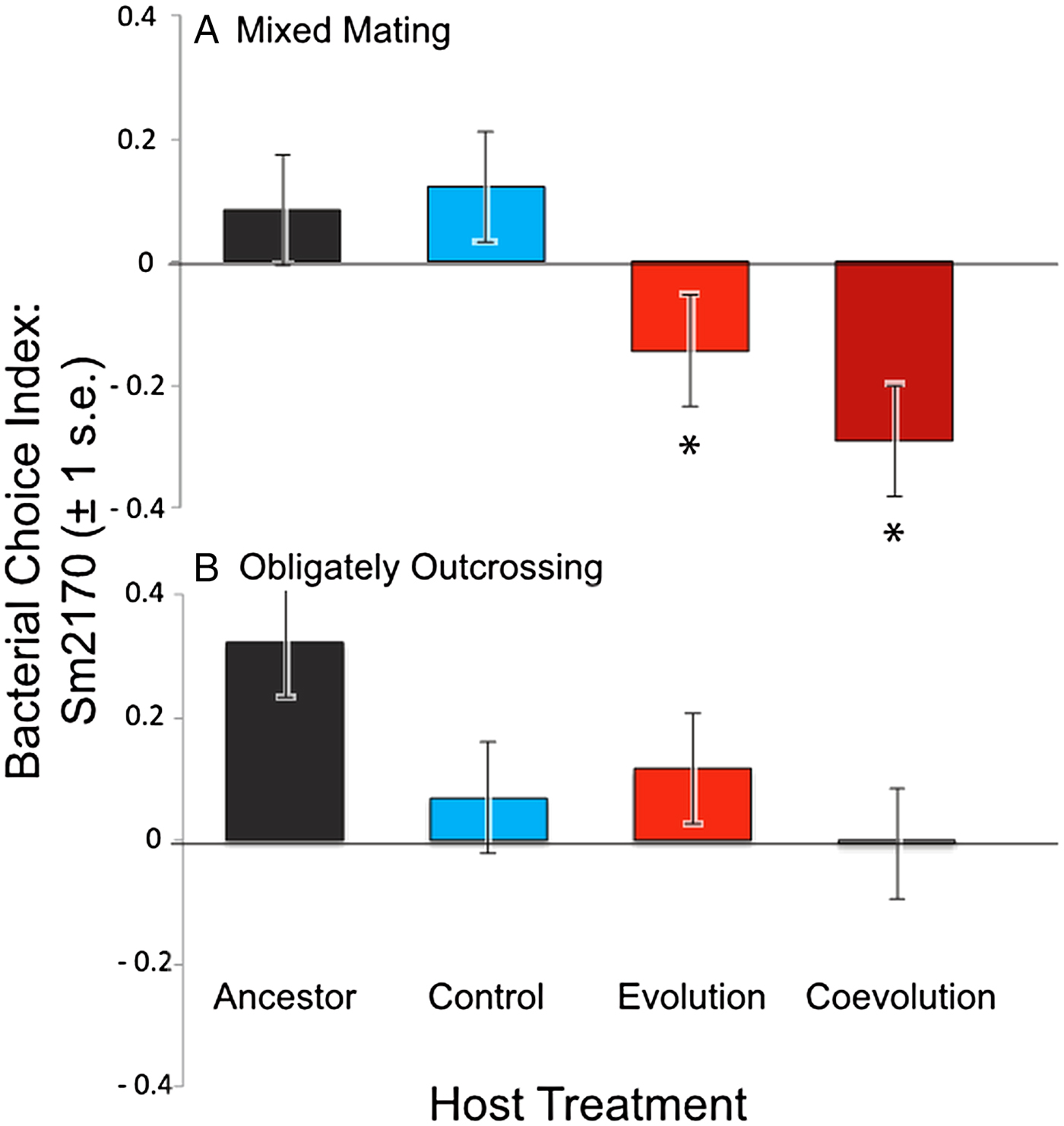
We determined bacterial choice index scores for mixed mating and obligately outcrossing host populations presented with the choice between E. coli OP50 and the parasite strain Sm2170 (Fig. 2). Sm2170 served as both the ancestral strain to the parasite populations from the coevolution treatment and was also the static parasite strain for the evolution treatment throughout experimental evolution (Fig. 1). We assayed ancestral host populations and host populations after 30 generations of exposure to the control, evolution or coevolution treatments, for both the mixed mating and obligately outcrossing hosts. The mixed mating populations from the evolution and coevolution treatments exhibited significantly greater preference for E. coli, relative to the control populations (Fig. 3A and online Supplementary Fig. S1). Specifically, mean bacterial choice index scores were significantly reduced in mixed mating hosts from the evolution (Table 1 and Fig. 3A; F 1,16 = 4·89, P = 0·042) and coevolution (Table 1 and Fig. 3A; F 1,16 = 9·41, P = 0·0074) treatments, relative to control populations. Further, the populations from the evolution and coevolution treatments exhibited an overall mean preference for E. coli, as opposed to Sm2170 (Fig. 3A). However, we did not observe a significant change in bacterial preference (mean choice index scores) in the obligately outcrossing host populations, regardless of treatment (Table 2, Fig. 3B and online Supplementary Fig. S1). Therefore, mixed mating hosts evolved parasite avoidance behaviour in the presence of Sm2170, while obligately outcrossing hosts did not.
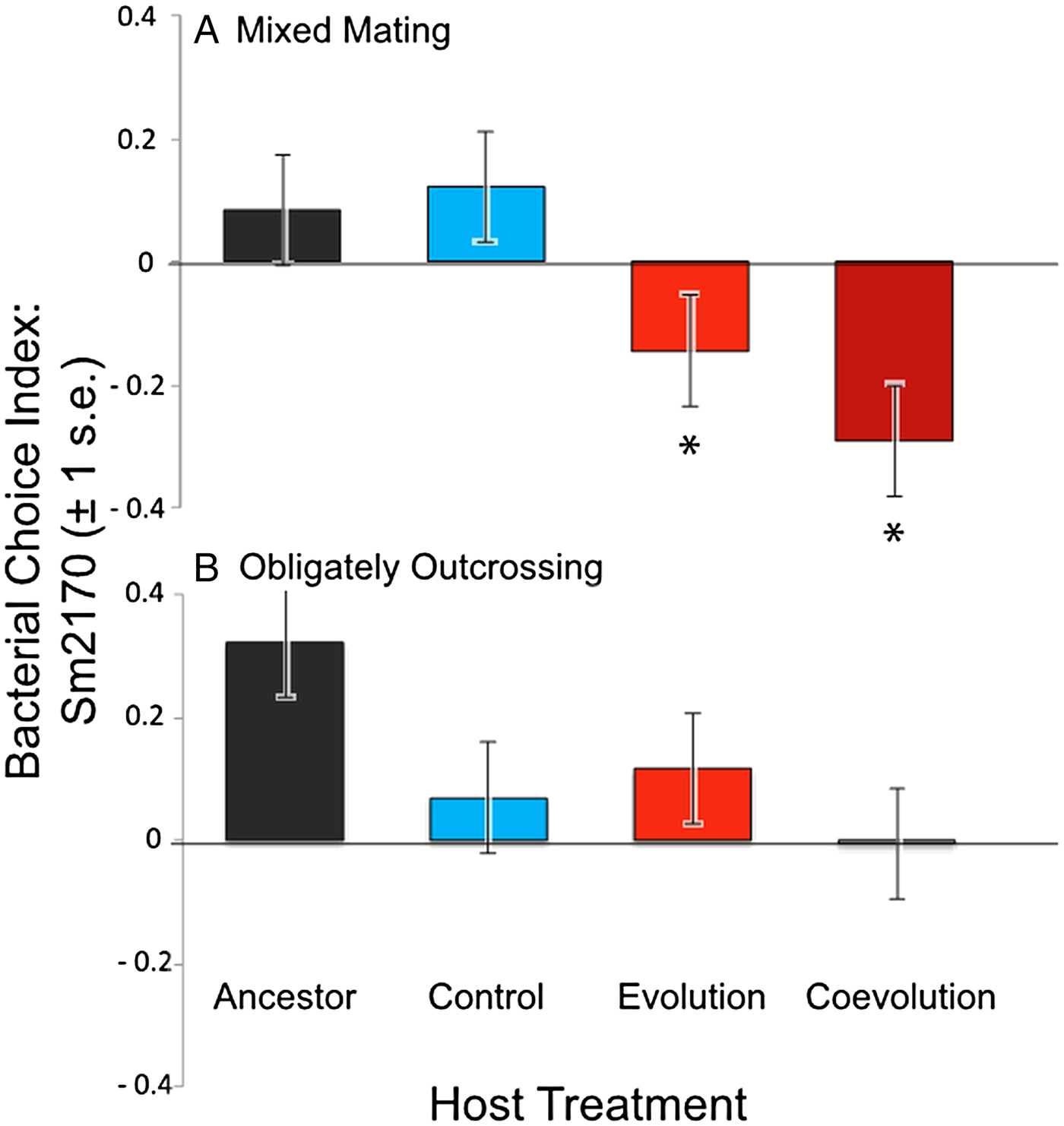
Fig. 3. Mean bacterial choice index scores on Sm2170 parasites. Positive choice index scores are indicative of host preference for Serratia marcescens Sm2170, while negative choice index scores indicate host preference for Escherichia coli OP50. (A) Mixed mating hosts from the evolution and coevolution treatments exhibit significantly increased preference for E. coli, relative to control and ancestral hosts; (B) obligately outcrossing host populations from the evolution and coevolution treatments do not exhibit altered preference relative to the control and ancestral host populations. Asterisks indicate significant difference from the control and ancestral populations.
Host bacterial food preference: coevolved parasite populations
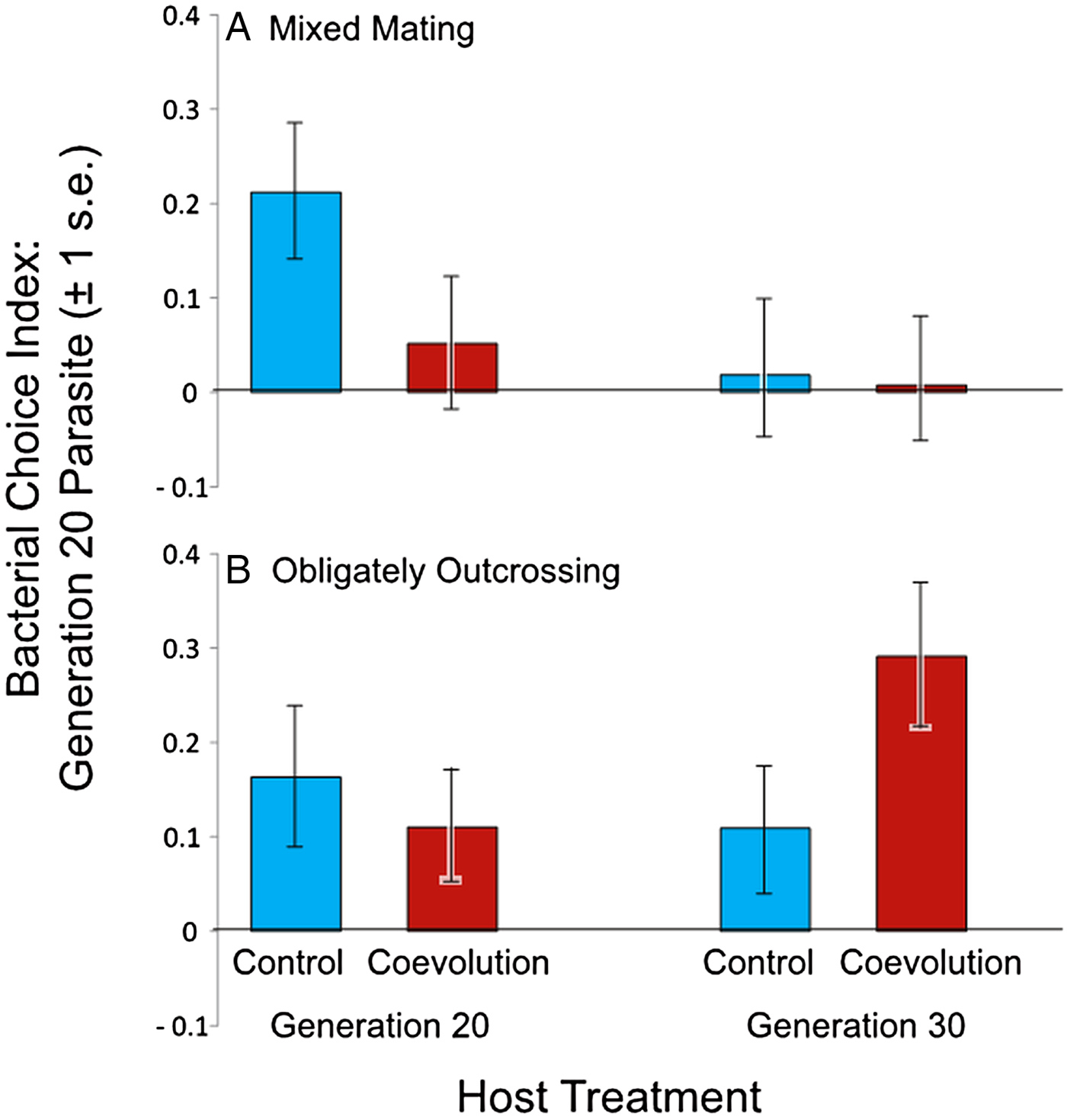
Next, we assessed host and parasite interactions from the coevolution treatment to determine if bacterial preference evolved during antagonistic coevolution. We measured bacterial choice index scores for mixed mating and obligately outcrossing host populations from the coevolution and control treatments at generations 20 and 30 of experimental evolution. Hosts were presented with a choice between E. coli OP50 and their sympatric parasite population, the specific parasite population with which the hosts were passaged, from generation 20 of coevolution. Host populations from both generations 20 and 30 of the experiment were tested to account for a potential time lag in the response of the hosts to the generation 20 parasites. Relative to controls, we observed no significant difference in the bacterial choice index scores of the mixed mating hosts (Table 3, Fig. 4A, online Supplementary Figs S2 and S3), nor the obligately outcrossing hosts (Table 4, Fig. 4B, online Supplementary Figs S2 and S3), in the presence of the coevolved parasites. Further all of the mixed mating and obligately outcrossing hosts exhibited a mean preference for the coevolved parasites after both 20 and 30 generations (Fig. 4). Therefore, at least after 20 generations of experimental evolution, hosts did not evolve parasite avoidance in response to their sympatric coevolving parasites.
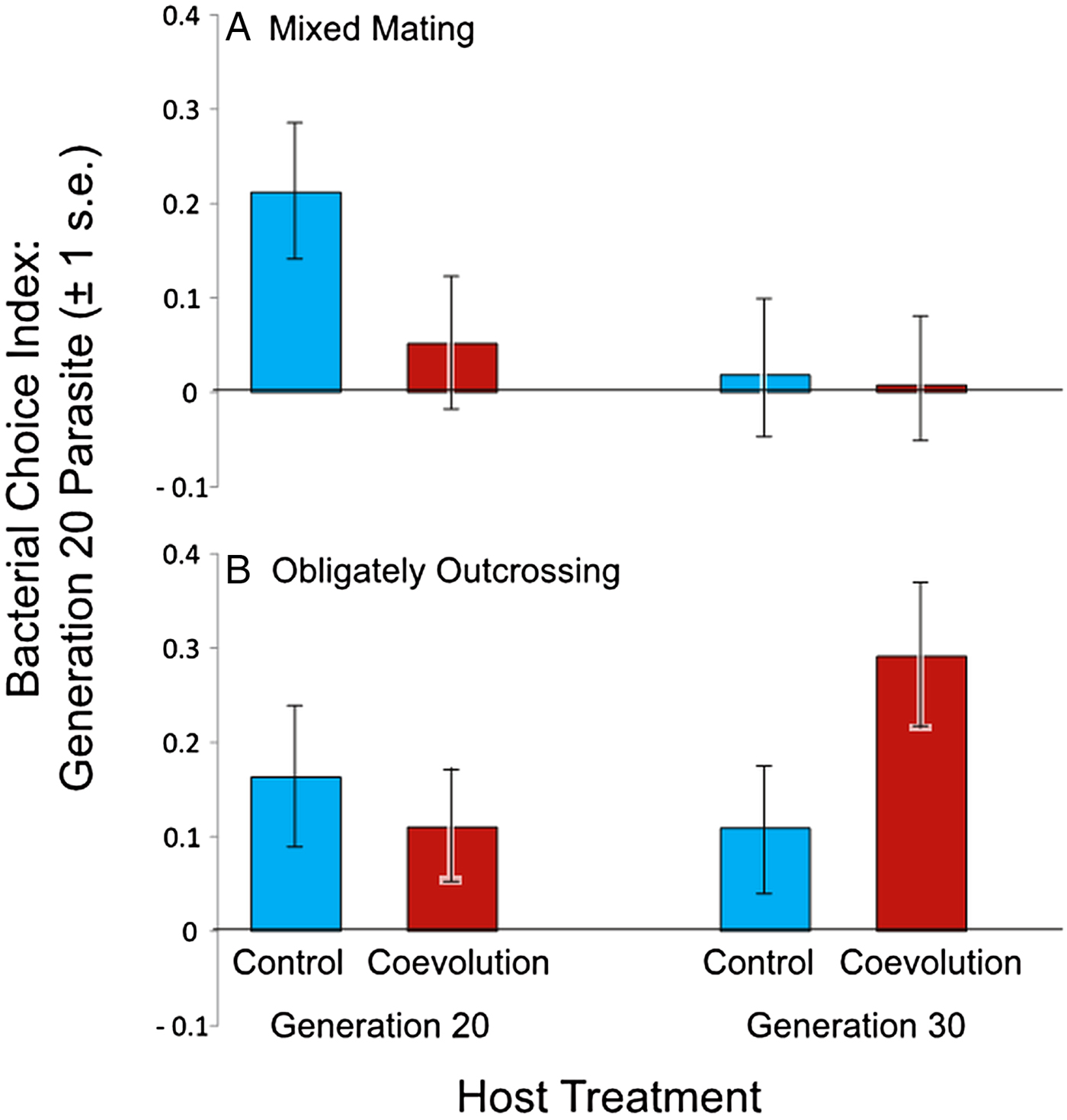
Fig. 4. Mean bacterial choice index scores on coevolved parasites. Positive choice index scores indicate preference for Serratia marcescens from the coevolution treatment after 20 generations of coevolution, while negative choice index scores indicate preference for Escherichia coli OP50. Coevolution treatment hosts were paired with their sympatric parasite population, while control treatment hosts were paired with the sympatric parasite population matching their respective coevolution treatment replicate population. (A) Mixed mating hosts from the coevolution treatment at generations 20 and 30 did not significantly differ in preference from control treatment populations at generations 20 and 30; (B) obligately outcrossing hosts from the coevolution treatment at generations 20 and 30 did not exhibit significant differences in bacterial preference relative to control populations.
Discussion
We found that some of the experimentally evolved populations of C. elegans evolved greater preference for E. coli when presented with a choice between E. coli OP50 and S. marcescens. This response to selection is particularly striking, given that C. elegans naturally prefer S. marcescens to E. coli (Zhang et al. Reference Zhang, Lu and Bargmann2005; Pradel et al. Reference Pradel2007; Glater et al. Reference Glater, Rockman and Bargmann2014). Our results suggest that this preference for E. coli is driven by active avoidance of S. marcescens, as opposed to increased preference for E. coli. The mixed mating hosts that were passaged with reciprocally evolving S. marcescens only exhibited E. coli preference in the presence of the ancestral Sm2170 strain (Fig. 3A), and not the coevolved populations of S. marcescens (Fig. 4A). Therefore, the hosts’ evolved preference for E. coli is conditional on the parasite strain present and not a general increase in preference for E. coli. Nonetheless, the evolution of increased E. coli preference, as opposed to direct avoidance of S. marcescens, would also function as parasite avoidance in the context of our experiment (Morran et al. Reference Morran2011). In the previous experiment, hosts were directly exposed to S. marcescens and crawled to E. coli to feed and reproduce (Fig. 1). Therefore, the evolution of host preference for E. coli would result in parasite avoidance as hosts exited the S. marcescens lawn to feed on E. coli. Regardless of the specific behaviour that evolved, we found that experimental evolution in the presence of either static or coevolving parasite populations resulted in the evolution of parasite avoidance in mixed mating host populations.
Interestingly, the mixed mating host populations that coevolved with S. marcescens populations exhibited parasite avoidance in the presence of the ancestral parasite populations (Fig. 3A), but did not avoid their contemporary coevolved parasites or coevolved parasites from the recent past (Fig. 4A). These results suggest that the coevolving parasite populations may have evolved to counteract avoidance by the host. The parasites in the coevolution treatment were required to kill hosts to gain fitness within the context of our experiment (Morran et al. Reference Morran2011). Therefore, avoidance on the part of the host would be particularly costly for the parasites. It is perhaps not surprising that parasite avoidance appears to have been negated when coevolving parasites had the ability to respond to the hosts. The parasite populations may have evolved a greater ability to attract the hosts to counteract avoidance by the hosts. However, the coevolved parasites are not more attractive to the control host populations (Figs 3A and 4A); therefore, any increased attractance would need to be very specific between sympatric hosts and parasites. This scenario is plausible, given that these coevolved parasite populations exhibited local adaptation with regard to their sympatric host populations (Morran et al. Reference Morran2014).
In general, both the mixed mating and obligately outcrossing host populations evolved increased fitness in the presence of static and coevolving parasites after 30 generations of experimental evolution (Morran et al. Reference Morran2011, Reference Morran2014; Penley et al., Reference Penley, Ha and Morranunpublished result). Here, we found that only the mixed mating populations evolved parasite avoidance as a mechanism of defence. However, avoidance cannot solely account for the increased fitness exhibited by mixed mating host populations from the coevolution treatment. These hosts evolved increased fitness in the presence of their coevolved sympatric parasites (Morran et al. Reference Morran2014), yet did not exhibit avoidance behaviour in the presence of those parasites (Fig. 4A). Therefore, it appears that the mixed mating populations from the coevolution treatment evolved multiple mechanisms to facilitate adaptation to parasites. Our results suggest avoidance evolved early during experimental evolution, because the hosts exhibited avoidance in the presence of the ancestral parasites (Fig. 3A), but did not avoid contemporary coevolving parasites or coevolved parasites from the past (Fig. 4A). Therefore, another form or forms of host defence likely evolved during antagonistic coevolution to confer greater host fitness to the reciprocally evolving parasites.
In contrast to the mixed mating populations, the obligately outcrossing populations did not evolve avoidance behaviour to the parasites, regardless of the treatment (Figs 3B and 4B). This is somewhat surprising because we expect the efficacy of selection to be stronger in the obligately outcrossing populations (Hill and Robertson, Reference Hill and Robertson1966; Felsenstein, Reference Felsenstein1974; Hodgson and Otto, Reference Hodgson and Otto2012), and thus their potential to evolve multiple mechanisms of defence could be greater than mixed mating populations. However, greater efficacy of selection in the obligately outcrossing populations may have simply favoured a different form of defence, like resistance or tolerance, which conferred greater overall fitness than avoidance behaviour. Given that the obligately outcrossing populations evolved greater fitness relative to the mixed mating populations (Morran et al. Reference Morran2011, Reference Morran2014; Penley et al., Reference Penley, Ha and Morranunpublished result), this is a plausible scenario. Further work is required to identify the other mechanisms of defence that evolved during experimental evolution. Additionally, mapping the loci underlying the evolved defence traits may allow us to better discern the cause of the different evolutionary trajectories in the mixed mating and obligately outcrossing populations.
It is currently unclear why C. elegans tends to prefer a bacterial parasite relative to a generally benign food source, given that the dietary preference of C. elegans can evolve in response to selection from parasites. Caenorhabditis elegans may only rarely encounter virulent strains of S. marcescens in nature, so selection for avoidance could be quite weak. Conversely, natural populations of C. elegans may be adapted to local populations of S. marcescens and the small amount of laboratory assays conducted failed to detect this phenomenon. Few C. elegans strains have been tested for avoidance behaviour, while those tested were exposed to a very narrow subset of S. marcescens strains, so the current data available are not sufficient for detecting local adaptation. Indeed there is much yet to learn about C. elegans interactions with parasites in nature, and C. elegans ecology in general (Frézal and Félix Reference Frézal and Félix2015).
Despite the seeming discrepancy between natural populations of C. elegans and our experimentally evolved populations, experimental evolution is a powerful tool for determining what can happen in nature. Overall, we found that both the host mating system and host–parasite coevolutionary dynamics can significantly alter the interactions and reciprocal adaptations between the hosts and parasites. This work demonstrates that the specific traits that evolve to confer host defence are not necessarily uniform for a particular host–parasite interaction. Instead, different host populations, or perhaps even different hosts within the same population, can exhibit varying forms of host defence against the same parasite. Although many factors likely contribute to the large variety of host defence mechanisms that exist in nature, we found that the host mating system and evolutionary history with parasites can shape the evolution of specific defence mechanisms within a host population.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017000804.
Acknowledgements
The authors thank Giang Ha and Sarah Hesse for logistical assistance. They also thank the members of the Morran laboratory, the GerMo HideR laboratories and two anonymous reviewers for comments that improved this work. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.