Introduction
The Iberian Peninsula has a remarkable biological diversity, harbouring more than 50% of European animal and plant species (Médail and Quézel, Reference Médail and Quézel1997; Martín et al., Reference Martín, García-Barros, Gurrea, Luciañez, Munguira, Sanz and Simón2000; Williams et al., Reference Williams, Araújo, Humphries, Lampinen, Hagemeijer, Gasc and Mitchell-Jones2000; Araújo et al., Reference Araújo, Lobo and Moreno2007; Cardoso, Reference Cardoso2008; Rueda et al., Reference Rueda, Rodríguez and Hawkins2010; López-López et al., Reference López-López, Maiorano, Falcucci, Barba and Boitani2011; Penado et al., Reference Penado, Rebelo and Goulson2016) and approximately 31% of all European endemic vertebrate and plant species (Williams et al., Reference Williams, Araújo, Humphries, Lampinen, Hagemeijer, Gasc and Mitchell-Jones2000). This high species diversity is linked with several climatic and geological changes occurring over the region since the Cenozoic period (Hsü et al., Reference Hsü, Ryan and Cita1973; Rosenbaum et al., Reference Rosenbaum, Lister and Duboz2001), when putative migration routes periodically emerged and disappeared. However, the main factor influencing the degree of endemism is most likely geographical isolation resulting from the elevation of the Pyrenees in the north-east combined with the generally mountainous topography of the peninsula, which provided a multitude of refuges during glacial periods (Gante et al., Reference Gante, Micael, Oliva-Paterna, Doadrio, Dowling and Alves2009; Hewitt, Reference Hewitt, Zachos and Havel2011).
While the species diversity of Iberian freshwater ichthyofauna is relatively low in comparison to other European regions (Kottelat and Freyhof, Reference Kottelat and Freyhof2007), the majority of species are endemic. The Peninsula hosts representatives of just a few native freshwater fish groups, with most species belonging to the Cyprinidae and Leuciscidae families [order Cyprinoidea; following the classification proposed by Schönhuth et al. (Reference Schönhuth, Vukić, Šanda, Yang and Mayden2018)]. The Leuciscidae (previously considered as Leuciscinae within Cyprinidae; Ketmaier et al., Reference Ketmaier, Bianco, Cobolli, Krivokapic, Caniglia and De Matthaeis2004; Levy et al., Reference Levy, Doadrio and Almada2009; Perea et al., Reference Perea, Böhme, Zupančic, Freyhof, Šanda, Özulug and Doadrio2010; Imoto et al., Reference Imoto, Saitoh, Sasaki, Yonezawa, Adachi, Kartavtsev, Miya, Nishida and Hanzawa2013) are represented by the monotypic genus Anaecypris, the genera Phoxinus, Iberocypris and Squalius, and by four recently erected genera belonging to Chondrostoma sensu lato: Achondrostoma, Iberochondrostoma, Parachondrostoma and Pseudochondrostoma (Kottelat and Freyhof, Reference Kottelat and Freyhof2007; Robalo et al., Reference Robalo, Almada, Levy and Doadrio2007; Schönhuth et al., Reference Schönhuth, Vukić, Šanda, Yang and Mayden2018). In contrast to the leuciscids, cyprinids are represented by just two genera: Barbus and Luciobarbus (Kottelat and Freyhof, Reference Kottelat and Freyhof2007; Gante, Reference Gante, Grillo and Venora2011; Gante et al., Reference Gante, Doadrio, Alves and Dowling2015). The distribution of a given cyprinoid species is usually confined to a specific ichthyogeographic province and the ranges of different species rarely overlap (Doadrio, Reference Doadrio1988; Gante et al., Reference Gante, Doadrio, Alves and Dowling2015), suggesting that speciation is closely linked with the formation of river basins (Zardoya and Doadrio, Reference Zardoya and Doadrio1998; Machordom and Doadrio, Reference Machordom and Doadrio2001; Doadrio et al., Reference Doadrio, Carmona and Machordom2002; Mesquita et al., Reference Mesquita, Cunha, Carvalho and Coelho2007; Casal-López et al., Reference Casal-López, Perea, Sousa-Santos, Robalo, Torralva, Oliva-Paterna and Doadrio2017; Sousa-Santos et al., Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019).
In contrast to the thorough previous and on-going research on Iberian cyprinoids, data on their helminth parasites are scarce (da Costa Eiras, Reference Da Costa Eiras2016). In previous studies focused on freshwater fishes in different regions of the northern hemisphere (e.g. Mexico and the Balkans), it has been suggested that the biogeography of fish helminth parasites reflects the historical dispersion and current distribution of their hosts (e.g. Choudhury and Dick, Reference Choudhury and Dick2001; Pérez-Ponce de León and Choudhury, Reference Pérez-Ponce de León and Choudhury2005; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). However, very few studies have been carried out on cyprinoid monogeneans in the Iberian Peninsula, by far the most thorough being those of El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992) and Šimková et al. (Reference Šimková, Benovics, Rahmouni and Vukić2017). The former study, describing seven species of Dactylogyrus from six cyprinid species (relying on morphological data only) suggested that the pattern of the geographical distribution of Dactylogyrus spp. follows the distribution of their cyprinid hosts, for which they are highly host-specific. The study by Šimková et al. (Reference Šimková, Benovics, Rahmouni and Vukić2017) focused on phylogenetic relationships between endemic Dactylogyrus from cyprinids in Iberia and Dactylogyrus from Central Europe and north-west Africa. The authors suggested multiple origins of endemic Dactylogyrus in the Iberian Peninsula as the presence of Dactylogyrus lineages in different Luciobarbus lineages was associated with specific dispersion events.
Gill monogeneans belonging to Dactylogyrus are currently the most species-diversified group within the Platyhelminthes [more than 900 nominal Dactylogyrus species, mostly described from morphology, are presently known according to the latest review by Gibson et al. (Reference Gibson, Timofeeva and Gerasev1996)]. Dactylogyrus species are strictly specific to cyprinoids and many Dactylogyrus species are specific to a single host species (Šimková et al., Reference Šimková, Verneau, Gelnar and Morand2006b). However, the degree of host specificity across Dactylogyrus species differs and, in some cases, host specificity is likely to reflect the ecology and recent distribution of their hosts (Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). Dactylogyrus species with a narrow host range are most common in regions with a high number of endemic host species. In Europe, such regions include the Balkan Peninsula, where a multitude of strictly host-specific endemic Dactylogyrus species has been documented (Dupont and Lambert, Reference Dupont and Lambert1986; Benovics et al., Reference Benovics, Kičinjaová and Šimková2017, Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018), and the Iberian Peninsula, where many Dactylogyrus endemic species have been documented for Luciobarbus (El Gharbi et al., Reference El Gharbi, Renaud and Lambert1992). It has been suggested that such a high degree of endemism in Dactylogyrus is the result of co-speciation with their hosts over long evolutionary periods in geographically isolated regions (Dupont, Reference Dupont1989). Over time, the Dactylogyrus parasites have developed an attachment organ (haptor) that is highly specialized towards their host (Šimková et al., Reference Šimková, Desdevises, Gelnar and Morand2000; Jarkovský et al., Reference Jarkovský, Morand, Šimková and Gelnar2004; Šimková and Morand, Reference Šimková and Morand2008). As such, the shape and size of monogenean haptoral sclerites are considered to be species specific and represent suitable morphological characters for species determination. Nevertheless, some species exhibit haptoral sclerites that are very similar in shape and size (see Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009); thus, species identification is often difficult from the observation of haptoral sclerotized structures only. It has been suggested, therefore, that the shape of the sclerotized parts of copulatory organs are more suitable for the identification of monogeneans to species level due to their putative faster evolutionary rate (Pouyaud et al., Reference Pouyaud, Desmerais, Deveney and Pariselle2006; Šimková et al., Reference Šimková, Verneau, Gelnar and Morand2006b; Vignon et al., Reference Vignon, Pariselle and Vanhove2011; Mendlová et al., Reference Mendlová, Desdevides, Civáňová, Pariselle and Šimková2012; Mandeng et al., Reference Mandeng, Bilong Bilong, Pariselle, Vanhove, Bitja Nyom and Agnése2015; Benovics et al., Reference Benovics, Kičinjaová and Šimková2017). Rapid morphological diversification in the monogenean copulatory organs is hypothesized to be a mechanism to avoid hybridization (Rohde, Reference Rohde1989), which is especially likely for Dactylogyrus species living on the same hosts in overlapping microhabitats (Šimková et al., Reference Šimková, Kadlec, Gelnar and Morand2002; Šimková and Morand, Reference Šimková and Morand2008).
Compared with Central Europe, Dactylogyrus communities in the southern European Peninsulas generally appear to be species poor. Cyprinoids with a wide European distribution range, such as Rutilus rutilus and Squalius cephalus, harbour up to nine Dactylogyrus species (e.g. Šimková et al., Reference Šimková, Desdevises, Gelnar and Morand2000; Seifertová et al., Reference Seifertová, Vyskočilová, Morand and Šimková2008). In contrast, a maximum of five Dactylogyrus species per cyprinoid species have been reported from the southern European Peninsulas (Dupont and Lambert, Reference Dupont and Lambert1986; El Gharbi et al., Reference El Gharbi, Renaud and Lambert1992; Galli et al., Reference Galli, Stefani, Zaccara and Crosa2002, Reference Galli, Strona, Benzoni, Crosa and Stefani2007; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018).
In comparison to other European regions, cyprinoid monogenean communities have been underexplored in the Iberian Peninsula. Thus, the main objective of the present study was to investigate the diversity of Dactylogyrus spp. parasitizing endemic cyprinoids in this geographical region. A species delimitation method was applied to assess the species status of Dactylogyrus identified in this study based on genetic variability within and among each species, and to compare these results to species defined from morphology only. Moreover, the present study investigates the evolutionary history and phylogenetic relationships between endemic Iberian Dactylogyrus and Dactylogyrus from other Peri-Mediterranean regions, including cyprinoid species with a wide European distribution range, in order to (1) shed new light on cyprinoid phylogeography, (2) infer potential historical contacts between cyprinoids from different regions, and (3) evaluate the evolution of Dactylogyrus species diversity (using both morphology and species delimitation methods).
Material and methods
Parasite collection
Fish were collected over the years 2016 and 2017 from 17 localities in Portugal and Spain (Fig. 1). In total, 257 specimens representing 19 fish species were examined for the presence of Dactylogyrus parasites (Table 1). Fish were dissected following the standard protocol described by Ergens and Lom (Reference Ergens and Lom1970). Dactylogyrus specimens were collected from the gills, mounted on slides and fixed in a mixture of glycerine and ammonium picrate (Malmberg, Reference Malmberg1957) for further identification. Determination to species level was performed on the basis of the size and shape of the sclerotized parts of the attachment apparatus (anchor hooks, marginal hooks and connective bars of the haptor) and the reproductive organs (male copulatory organ and vaginal armament) following Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). At least five specimens of each Dactylogyrus species from each host species examined were bisected using fine needles. One-half of the body (either the anterior part containing the reproductive organs or the posterior part with the attachment organ) was mounted on a slide and used for morphological identification. The other half was individually preserved in pure ethanol for subsequent DNA extraction.

Fig. 1. Map of collection localities in the Iberian Peninsula. Collection localities are marked as yellow circles. The greatest Iberian rivers are highlighted in blue. The same codes for localities are used in Table 1 as locality IDs.
Table 1. List of cyprinoid species including localities of their collection and list of collected Dactylogyrus species from respective hosts

N = number of processed fish individuals from the respective locality, ID = code corresponding with localities marked in Fig. 1 and codes in following tables, numbers in columns 18S and 28S correspond to sequence accession numbers for the respective genetic markers in GenBank; 18S = sequences of partial gene coding 18S rRNA combined with complete ITS1 region, 28S = sequences or partial gene coding 28S rRNA. Sequence not used in the present study is marked by asterisk (*) Dashes represent localities where no Dactylogyrus parasites were collected and/or missing sequences.
DNA extraction, PCR and sequencing
DNA extraction was performed using the DNeasy Blood & Tissue Kit (Quiagen, Hilden, Germany) based on the standard protocol provided by the manufacturer. Two DNA regions were amplified. The partial gene coding 18S rRNA and complete ITS1 region was amplified using the primers S1 (forward, 5′-ATTCCGATAACGAACGAGACT-3′) and Lig5.8R (reverse, 5′-GATACTCGAGCCGAGTGATCC-3′) (Šimková et al., Reference Šimková, Plaisance, Matějusová, Morand and Verneau2003; Blasco-Costa et al., Reference Blasco-Costa, Míguez-Lozano, Sarabeev and Balbuena2012). Each amplification reaction was performed in a final volume of 20 μL, the reaction mixture comprising 1.5 U Taq polymerase (Fermentas), 1× buffer, 1.5 mm MgCl2, 0.2 mm of dNTPs, 0.1 mg mL−1 BSA, 0.5 μ m of each primer and 2 μL of pure DNA (20 ng μL−1). PCR was carried out using the following steps: 3 min initial denaturation at 95 °C, followed by 40 cycles of 40 s at 94 °C, 30 s at 52 °C and 45 s at 72 °C, and 4 min of final elongation at 72°C. The second marker, a part of the gene coding 28S rRNA, was amplified using the primers C1 (forward, 5′-ACCCGCTGAATTTAAGCA-3′) and D2 (reverse, 5′-TGGTCCGTGTTTCAAGAC-3′) (Hassouna et al., Reference Hassouna, Michot and Bachellerie1984), following the PCR protocol described in Šimková et al. (Reference Šimková, Matějusová and Cunningham2006a). The PCR products were purified prior to sequencing using the ExoSAP-IT kit (Ecoli, Bratislava, Slovakia), following the standard protocol, and directly sequenced using the PCR primers and the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). Sequencing was carried out on an ABI 3130 Genetic Analyzer (Applied Biosystems). The newly generated sequences were deposited in GenBank (see Table 1 for accession numbers).
Phylogenetic and species delimitation analysis
Partial sequences coding 18S rRNA and 28S rRNA, and complete sequences of the ITS1 region were concatenated and aligned using the fast Fourier transform algorithm implemented in MAFFT (Katoh et al., Reference Katoh, Misawa, Kuma and Miyata2002) using the G-INS-i refinement method. Out of 71 DNA sequences used in the alignment, 35 were newly sequenced in this study. Sequences from 35 other Dactylogyrus species, used as representative species from different European regions, and sequences of Ancyrocephalus percae, used as an outgroup [phylogenetically closely related to Dactylogyrus according to Mendoza-Palmero et al. (Reference Mendoza-Palmero, Blasco-Costa and Scholz2015)], were obtained from GenBank (see online Supplementary Table S1 for accession numbers). Gaps, hypervariable regions and ambiguously aligned regions were removed from the alignment using GBlocks v. 0.91 (Talavera and Castresana, Reference Talavera and Castresana2007). The optimal DNA evolutionary model was selected separately for each part of the alignment corresponding to one of the three markers analysed (18S, ITS1, 28S) using the Bayesian information criterion in jModelTest v. 2.1.10 (Guindon and Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboala, Doallo and Posada2012).
Maximum likelihood (ML) analysis was conducted in RAxML v. 8.2.11 (Stamatakis, Reference Stamatakis2006, Reference Stamatakis2014), applying the general time-reversible model (GTR; Lanave et al., Reference Lanave, Preparata, Sacone and Serio1984) of nucleotide substitution. Internal node support was assessed by running 1000 bootstrap pseudoreplicates. Bayesian inference (BI) analysis was performed in MrBayes v. 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) using two parallel runs, each with four Markov chains (one cold and three heated) of 107 generations with trees sampled every 102 generations. The first 30% of trees were discarded as initial burn-in. Convergence was indicated by an average standard deviation of split frequencies per parallel run of <0.01, subsequently checked using Tracer v. 1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018). Posterior probabilities were calculated as the frequency of samples recovering particular clades.
To investigate genetic diversity in the commonly used genetic markers between well-defined endemic Dactylogyrus species, uncorrected pairwise genetic distances (p-distances) were computed for 12 selected taxa in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). Three sequence alignments were used: the partial gene coding 18S rRNA, the complete ITS1 region and the partial gene coding 28S rRNA. All positions containing gaps and missing data were removed from the final computations.
The Bayesian-implemented Poisson Tree Processes model (bPTP; Zhang et al., Reference Zhang, Kapli, Pavlidis and Stamatakis2013) was applied to the phylogram resulting from BI in order to infer putative species of Iberian Dactylogyrus. The bPTP method only requires a phylogenetic tree as its input and uses branch lengths to estimate the mean expected a number of substitutions per site between two branching events. Within species, branching events will be frequent whereas they will be rarer between species. The model implements two independent classes of the Poisson process (one describing speciation and the other describing coalescent processes) and searches for transition points between interspecific and intraspecific branching events. Potential species clusters are then determined by identifying the clades or single lineages that originate after these transition points. The computation was run for 5 × 105 generations with the first 30% of trees discarded as initial burn-in. The distant outgroup taxon was removed from the final analysis to improve delimitation in the results.
Results
Twenty-two Dactylogyrus species (identified using morphological characters, i.e. sclerotized parts of the haptor and reproductive organs) were collected from endemic Iberian cyprinoid species (Table 1). From one to five Dactylogyrus species were recorded per host species, with highest species richness found on Luciobarbus spp. (five species on L. guiraonis, four species on L. graellsii and four species on L. sclateri). Both Parachondrostoma species, Barbus haasi, Iberochondrostoma almacai and Phoxinus bigerri were parasitized by a single Dactylogyrus species. Overall, Dactylogyrus bocageii exhibited the widest host range across the Iberian Peninsula, parasitizing four Luciobarbus species. Minor genetic variation was observed between D. bocageii collected from different hosts (p-distance ⩽ 0.002 in the partial gene for 28S rRNA, p-distance ⩽ 0.020 in the ITS1 region; Tables 2 and 3).
Table 2. Uncorrected pairwise genetic distances between individuals from clade B (Fig. 2) collected from different Barbus and Luciobarbus species in the Iberian Peninsula

Table 3. Uncorrected pairwise genetic distances between individuals from clade B (Fig. 2) collected from different Barbus and Luciobarbus species in the Iberian Peninsula

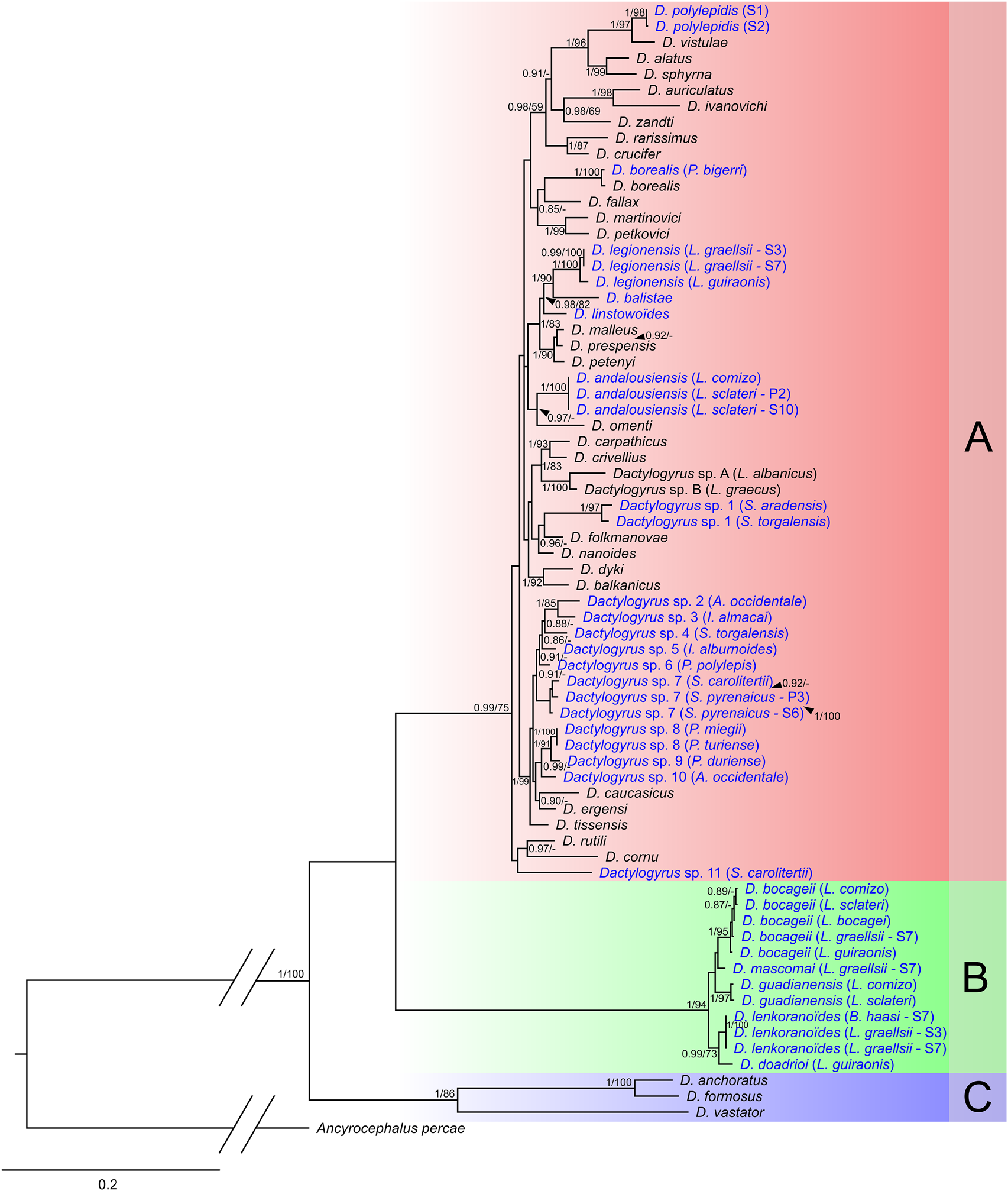
Fig. 2. Phylogenetic tree of 70 Dactylogyrus haplotypes reconstructed by Bayesian inference (BI). The tree is based on combined parts of genes coding 18S and 28S rRNA, and the complete ITS1 region. Values between branches indicate posterior probabilities from BI and bootstrap values from ML analysis. Values below 0.80 (BI) and 50 (ML) are shown as dashes (–). The letters A–C represent specific well-supported lineages, as described in the Results section.
The final concatenated alignment of partial genes for 18S rRNA, 28S rRNA and the ITS1 region included 71 sequences and contained 1533 unambiguous nucleotide positions. The most suitable evolutionary models were TrNef + I + G, TPM2uf + G and GTR + I + G for the partial genes coding 18S rRNA, the ITS1 region and part of the gene for 28S rRNA, respectively. Both ML and BI analyses produced trees with congruent topologies varying only in some support values for individual nodes (Fig. 2). Phylogenetic analysis divided all taxa into three strongly supported clades.
The first group (Clade A; Fig. 2) included the majority of Dactylogyrus species from Europe, and especially the species parasitizing Leuciscidae. In addition, several Dactylogyrus species from Barbus and Luciobarbus (Cyprinidae) were also placed in this clade (i.e. Dactylogyrus of Barbus spp. and Luciobarbus spp. from Central Europe and the Balkans, and D. balistae, D. legionensis, D. linstowoïdes and D. andalousiensis of Iberian Luciobarbus spp.). Dactylogyrus from Iberian cyprinoids were divided into seven lineages within Clade A. Dactylogyrus polylepidis of Achondrostoma arcasii was in a well-supported sister position to the morphologically similar D. vistulae. Dactylogyrus from European cyprinids formed three well-supported groups within Clade A. Dactylogyrus legionensis, D. balistae and D. linstowoïdes were grouped in a sister position to common Dactylogyrus species from Central European Barbus spp. (D. malleus, D. prespensis and D. petenyi). The second group contained D. andalousiensis from two Iberian Luciobarbus species, and D. omenti from Aulopyge huegelii (Balkan endemic species). The third group contained D. carpathicus and D. crivellius (two common species of Barbus spp.) and two yet undescribed endemic Dactylogyrus species of endemic Balkan Luciobarbus species (L. albanicus and L. graecus). The phylogenetic position of Dactylogyrus sp. 1 from S. aradensis and S. torgalensis (morphologically identical but genetically slightly different; p-distance = 0.010) was not fully resolved and its sister position to D. folkmanovae was only supported by BI. The majority of Iberian Dactylogyrus species (Dactylogyrus sp. 2 to Dactylogyrus sp. 10) formed a well-defined phylogenetic lineage that also included D. caucasicus, D. ergensi and D. tissensis. The three latter species and the Iberian Dactylogyrus in this lineage all have the same or very similarly shaped male copulatory organs commonly classified as ‘ergensi’ of the ‘chondrostomi’ type (see Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). Generalist Dactylogyrus species within Clade A (i.e. D. legionensis, D. polylepidis, Dactylogyrus sp. 1, Dactylogyrus sp. 7 and Dactylogyrus sp. 8) exhibited intraspecific genetic variability. The second major group (Clade B) comprised five Dactylogyrus species specific to Iberian Luciobarbus. Where intraspecific genetic variability was documented, all genetic variants formed well-supported clades (i.e. D. bocageii, D. guadianensis and D. lenkoranoïdes). The last strongly supported group (Clade C) encompassed Dactylogyrus species host specific to Carassius spp. and/or Cyprinus carpio distributed across the Europe and Asia.
In general, no pattern was observed in phylogenetic relatedness of individual Dactylogyrus species reflecting their geographic distribution. However, the phylogenetic relationships between genetic variants of single Dactylogyrus species (e.g. three genetic variants for D. legionensis, or Dactylogyrus sp. 7) were in congruence with the geographic distribution of their respective hosts (i.e. two genetic variants collected from hosts belonging to different species, but collected from geographically proximal localities, or the same river basin, were phylogenetically closer to each other, rather than to other genetic variants of the same Dactylogyrus species).
Genetic distances were computed between morphologically similar species from Clade B (Fig. 2). Three alignments of 12 sequences representing five Dactylogyrus species of group B were analysed to compare intra- and interspecific genetic variability calculated using genetic markers commonly used in monogeneans. The alignments comprised 486 nucleotide positions for the partial gene coding 18S rRNA combined, 716 nucleotide positions for the ITS1 segment and 807 nucleotide positions for the partial gene coding 28S rRNA. The lowest genetic variability was observed for the partial gene coding 18S rRNA. No intraspecific/inter-population genetic variability was observed (p-distance = 0.000) and interspecific pairwise nucleotide diversity varied from 0.002 to 0.010 (Table 4). Low pairwise interspecific diversity was also observed for the partial gene coding 28S rRNA (0.006–0.020); however, minor intraspecific genetic variability was observed in this gene (p-distance ⩽0.002). Slight genetic distance in part of the gene for 28S rRNA was observed between different populations of D. bocageii (0.001–0.002) and between individuals from different populations of D. guadianensis (p-distance = 0.001). The highest genetic diversity was observed in the ITS1 region, in which intraspecific distances varied from 0.000 (D. lenkoranoïdes) to 0.020 (D. bocageii). The pairwise interspecific diversity in the ITS1 region varied from 0.031 between D. doadrioi and D. guadianensis to 0.135 between D. doadrioi and D. mascomai.
Table 4. Uncorrected pairwise genetic distances between individuals from clade B (Fig. 2) collected from different Barbus and Luciobarbus species in the Iberian Peninsula

The species status of Dactylogyrus collected from endemic Iberian cyprinoids was investigated using the bPTP method, with the addition of Dactylogyrus species parasitizing cyprinoids in other parts of Europe used as a reference of previously delimited species (Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). The results of the bPTP analysis were largely consistent with the species previously described on the basis of morphology (Fig. 3), though the ML solution suggested a higher species diversity. Based on ML results, D. legionensis encompasses two species, each being host-specific (one to L. graellsii and the other to L. guiraonis), as well as Dactylogyrus sp. 1 (S. aradensis and S. torgalensis). Both BI- and ML-supported solutions, obtained from bPTP analysis, suggested a generalist status for D. andalousiensis, D. bocageii, D. lenkoranoïdes and D. guadianensis (i.e. there were no host-specific parasites within these delimited species). A potentially new species, Dactylogyrus sp. 7, was also supported by the species delimitation analysis as a generalist, parasitizing both S. carolitertii and S. pyrenaicus. This analysis also suggested that D. borealis, determined using morphological characters, is a common parasite of Phoxinus spp. in other parts of Europe and is also found on P. bigerri in the Iberian Peninsula. bPTP analysis also suggested that Parachondrostoma miegi and P. turiense are both parasitized by a single Dactylogyrus species (Dactylogyrus sp. 8) that is morphologically similar and phylogenetically close to Dactylogyrus sp. 9, parasitizing P. duriense. Finally, species delimitation analysis supported the discovery of at least 11 unknown Dactylogyrus species in the Iberian Peninsula, as all other Iberian genetic variants were identified as individual host-specific species.

Fig. 3. Results of species bPTP delimitation analysis applied to clades comprising endemic Dactylogyrus. Brackets at the terminal branches indicate different species, as suggested by BI and ML analyses.
Discussion
Parasite diversity and distribution
The Iberian Peninsula harbours a high diversity of cyprinoids that have been the subject of extensive research; nevertheless, the species diversity of their host-specific parasites is still underexplored, especially in areas with a high diversity of endemic cyprinoids. Following previous research on the Dactylogyrus (or Monogenea in general) of Iberian cyprinids (El Gharbi et al., Reference El Gharbi, Renaud and Lambert1992; Lacasa-Millán and Gutiérrez-Galindo, Reference Lacasa-Millán and Gutiérrez-Galindo1995; Gutiérrez-Galindo and Lacasa-Millán, Reference Gutiérrez-Galindo and Lacasa-Millán2001), this study is the first to investigate the overall diversity of Iberian Dactylogyrus, including molecular data for both cyprinoid fish and their host-specific Dactylogyrus.
The present study revealed the presence of several potentially new Dactylogyrus species to science, all of which were well supported by the bPTP species delimitation method. This strongly suggests that endemic Iberian cyprinoid species harbour an endemic Dactylogyrus fauna, as previously suggested for Iberian Luciobarbus species by El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992). In contrast to the Balkan and Apennine Peninsulas (Dupont and Lambert, Reference Dupont and Lambert1986; Dupont and Crivelli, Reference Dupont and Crivelli1988; Dupont, Reference Dupont1989; Galli et al., Reference Galli, Stefani, Zaccara and Crosa2002, Reference Galli, Strona, Benzoni, Crosa and Stefani2007; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018), Iberian Dactylogyrus spp. appear to exhibit a higher degree of host specificity as the majority of Dactylogyrus species from Leuciscidae were restricted to a single host species. Benovics et al. (Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018) proposed that southern European endemic cyprinoids harbour species-poor Dactylogyrus communities compared with European cyprinoids with a wide distribution range (e.g. R. rutilus, S. cephalus). The same pattern was also observed in the Iberian Peninsula, where one to five Dactylogyrus species were found on a single cyprinoid host species. It should be noted, however, that parasite community composition may be strongly influenced by seasonal abiotic factors (e.g. González-Lanza and Alvarez-Pellitero, Reference González-Lanza and Alvarez-Pellitero1982; Lux, Reference Lux1990; Appleby and Mo, Reference Appleby and Mo1997; Šimková et al., Reference Šimková, Sasal, Kadlec and Gelnar2001b; Poulin and Morand, Reference Poulin and Morand2004; Zhang et al., Reference Zhang, Yan, Wang, Gibson and Yang2015; Sinaré et al., Reference Sinaré, Boungou, Ouéda, Gnémé and Kabré2016). Until now, knowledge of Dactylogyrus diversity in southern European Mediterranean Peninsulas has been based on studies taking place in summer only (Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018, this study) as the Dactylogyrus diversity is expected to be highest during this period (Šimková et al., Reference Šimková, Sasal, Kadlec and Gelnar2001b).
In this study, a higher number of Dactylogyrus species was observed on Luciobarbus species. While the overall species richness on these fish was in accordance with the observations of El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992), the species composition in the present study differed slightly from their data. In line with the study of El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992), D. bocageii was the most common species (occurring on five Luciobarbus species), though its distribution range was wider, as proposed by Lambert and El Gharbi (Reference Lambert and El Gharbi1995), stretching via Zujar and Torgal rivers to the south-western part of the peninsula (south-west Iberian province; Filipe et al., Reference Filipe, Araújo, Doadrio, Angermeier and Collares-Pereira2009). Interestingly, unlike other European regions, the only endemic representative of the genus Barbus in Iberia, B. haasi, harbours Dactylogyrus species typical of Luciobarbus spp. In the Balkans, endemic Barbus spp. are parasitized by common Dactylogyrus species for this fish genus (e.g. D. dyki and D. crivellius), while Luciobarbus spp. are parasitized by different, strictly host-specific species (Benovics et al., Reference Benovics, Kičinjaová and Šimková2017, Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). In accordance with our own findings, El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992) showed that B. haasi is a common host of D. bocageii, D. mascomai and D. lenkoranoïdes, while D. dyki and D. carpathicus (commonly distributed on European Barbus spp.) were only found in previous studies on B. haasi × B. meridionalis hybrids in the north-eastern part of the Peninsula. Nevertheless, Gutiérrez-Galindo and Lacasa-Millán (Reference Gutiérrez-Galindo and Lacasa-Millán1999) also reported the latter two Dactylogyrus species from B. haasi in the River Llobregat (north-east Spain). However, the fish hosts from this study could potentially also be hybrids, as the presence of the B. haasi × B. meridionalis hybrids was previously documented in Llobregat basin (Machordom et al., Reference Machordom, Berrebi and Doadrio1990). In contrast to the aforementioned studies, only D. lenkoranoïdes was collected from B. haasi in this study (Uldemo River; Ebro basin). This low parasite diversity may be linked with the seasonal fluctuation in parasite communities previously documented among Iberian Dactylogyrus [e.g. D. legionensis (González-Lanza and Alvarez-Pellitero, Reference González-Lanza and Alvarez-Pellitero1982) or D. balistae (Simón-Vicente, Reference Simón-Vicente1981)]. In addition to the common parasitization of Iberian Barbus by Dactylogyrus parasites typically recognized as specific to Luciobarbus, several cases of infection by Dactylogyrus species common for Barbus were also reported in Iberian Luciobarbus species. Gutiérrez-Galindo and Lacasa-Millán (Reference Gutiérrez-Galindo and Lacasa-Millán2001) also reported that L. graellsii was parasitized by D. dyki and D. extensus (host-specific parasites of Barbus spp. and C. carpio, respectively). However, the presence of D. dyki on Luciobarbus spp. may result from non-detected instances of hybridization, as hybrids of cyprinoid species are usually parasitized by Dactylogyrus specific for each of the parental species (Šimková et al., Reference Šimková, Dávidová, Papoušek and Vetešník2013; Krasnovyd et al., Reference Krasnovyd, Vetešník, Gettová, Civáňová and Šimková2017). Hybridization between Iberian Luciobarbus spp. (potentially also between Luciobarbus and Barbus; Gante et al., Reference Gante, Doadrio, Alves and Dowling2015) appears to be quite common, especially between congeners living in sympatry (e.g. Luciobarbus spp.; Almodóvar et al., Reference Almodóvar, Nicola and Elvira2008; Sousa-Santos et al., Reference Sousa-Santos, Pereira, Branco, Costa, Santos, Ferreira, Lima, Doadrio and Robalo2018). Thus, host-switching is possible, most likely occurring between species from phylogenetically close genera (i.e. Barbus and Luciobarbus; Yang et al., Reference Yang, Sado, Hirt, Pasco-Viel, Arunachalam, Li, Wang, Freyhof, Saitoh, Simons, Miya, He and Mayden2015) in north-eastern Iberian drainages where the distribution ranges of Central European barbels [e.g. B. meridionalis; see Kottelat and Freyhof (Reference Kottelat and Freyhof2007) for its distribution range] and Iberian barbels overlap.
Despite the presence of high numbers of endemic Dactylogyrus species in Iberia, P. bigerri was parasitized by D. borealis, a common species on European Phoxinus spp. (Moravec, Reference Moravec2001; Šimková et al., Reference Šimková, Morand, Jobet, Gelnar and Verneau2004; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). The presence of this common European Dactylogyrus species is in contrast to the expected high degree of endemism in south European peninsulas (Williams et al., Reference Williams, Araújo, Humphries, Lampinen, Hagemeijer, Gasc and Mitchell-Jones2000; Hewitt, Reference Hewitt, Zachos and Havel2011). Other common European Dactylogyrus species are absent from Iberia; for example, D. vistulae, which parasitizes the highest number of cyprinoid species across Europe, is absent from Iberia, and only the closely related D. polylepidis is found on Iberian cyprinoids. These findings suggest that either (1) D. borealis was only recently introduced into the Iberian Peninsula with another Phoxinus species coming from different European areas (see Corral-Lou et al., Reference Corral-Lou, Perea, Aparicio and Doadrio2019), or (2) D. borealis represent an extremely slowly evolving species, meaning that the Iberian lineage would be morphologically and genetically similar to D. borealis from other European areas. In the present study, D. polylepidis, originally described from Pseudochondrostoma polylepis (Alvarez-Pellitero et al., Reference Alvarez-Pellitero, Simón-Vicente and González-Lanza1981), was found for the first time on three host species (all members of the Leuciscidae). The wider host range recorded for D. polylepidis indicates that this species represents a true generalist parasite, probably endemic to this region. In contrast to D. polylepidis, the morphologically similar and phylogenetically closely related D. vistulae is a typical generalist in Europe (except Iberia) and Asia, parasitizing a multitude of cyprinoid species and genera (Moravec, Reference Moravec2001; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018). Dactylogyrus polylepidis and D. vistulae share remarkably similar morphological traits, including an enlarged seventh pair of marginal hooks, large anchor hooks and a similar size and shape of the copulatory organs (see Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). It has previously been hypothesized that large attachment structures (or structures with variable size and shape) in monogeneans increases the probability of switching to fish species of different body sizes, which is in accordance with the low degree of host specificity observed in D. vistulae (e.g. Šimková et al., Reference Šimková, Desdevises, Gelnar and Morand2001a; Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018) and D. polylepidis (this study). Compared to endemic cyprinids, endemic leuciscids harbour species-poor Dactylogyrus communities, though leuciscid Dactylogyrus species exhibit a higher degree of host specificity, with most species harbouring at least one specific Dactylogyrus species. The majority of new species recorded are morphologically similar, with Dactylogyrus sp. 2 and Dactylogyrus sp. 10, for example, sharing the ‘ergensi’ type of male copulatory organ but differing in the shape and size of the haptoral hard parts. Phylogenetic analyses and species delimitation analyses supported their species identities, i.e. nine new species were recognized. Species delimitation has received much attention recently, and numerous methods have now been developed that help identify species by using molecular data in a rigorous framework alongside morphological examination (Carstens et al., Reference Carstens, Pelletier, Reid and Satler2013; Zhang et al., Reference Zhang, Kapli, Pavlidis and Stamatakis2013; Grummer et al., Reference Grummer, Bryson and Reeder2014). DNA-based delimitation methods have also been used to confirm or invalidate morphologically determined species, to identify cryptic species or highlight significant intraspecific genetic variability. The aforementioned diversity in haptoral part shape and size appears to be common in Dactylogyrus spp. and was previously hypothesized to be the result of adaptations to specific microhabitats (i.e. specific positions on fish gills; Šimková et al., Reference Šimková, Desdevises, Gelnar and Morand2001a; Jarkovský et al., Reference Jarkovský, Morand, Šimková and Gelnar2004). Thus, minor morphological variabilities in the attachment organs may be observed in species with ongoing speciation parasitizing phylogenetically distant hosts, as is the case in the Iberian Peninsula.
Phylogeny of endemic Dactylogyrus
Phylogenetic reconstruction of Dactylogyrus parasitizing Iberian cyprinoids suggests that Iberian Dactylogyrus belong to two well-supported phylogenetic lineages (Clade A and Clade B; Fig. 2). One of these clades contains Dactylogyrus from endemic Cyprinidae only (representatives of five Luciobarbus species and B. haasi), while the second includes Dactylogyrus endemic to Iberian cyprinoids (both Cyprinidae and Leuciscidae) and Dactylogyrus parasitizing cyprinoids from other parts of Europe. This was previously reported by Šimková et al. (Reference Šimková, Benovics, Rahmouni and Vukić2017) following the analysis of phylogenetic relationships between Dactylogyrus from north-west Africa and those from the Iberian Peninsula, the authors suggesting multiple origins for Dactylogyrus from both Mediterranean areas in association with the historical biogeography of their cyprinid hosts. Clade B comprises Dactylogyrus species described by El Gharbi et al. (Reference El Gharbi, Renaud and Lambert1992), using morphological characteristics of the haptor and reproductive organs. According to their study (also supported by our own morphometric data), all these species achieve a small body size and display remarkably similar morphological features (i.e. sclerotized parts of attachment and copulatory organs), in accordance with their phylogenetic proximity. Previously, their description was based on small differences in the shape and size of sclerotized parts only (e.g. spiralization of the male copulatory organ and the size of haptoral sclerites). However, as has been previously documented, such variability may be present within single species and is common in the different monogenean taxa (e.g. Rohde and Watson, Reference Rohde and Watson1985; Boeger and Kritsky, Reference Boeger and Kritsky1988; Vignon and Sasal, Reference Vignon and Sasal2010), including Dactylogyrus (Rahmouni et al., Reference Rahmouni, Řehulková, Pariselle, Rkhami and Šimková2017). Nonetheless, the species status of each taxon in Clade B was supported by phylogenetic and species delimitation analyses, which was in concordance with their morphological determination. According to Šimková et al. (Reference Šimková, Benovics, Rahmouni and Vukić2017), Iberian Dactylogyrus species of this lineage are phylogenetically close to Dactylogyrus from north-west African Carasobarbus fritschii, suggesting different historical origins of Dactylogyrus in Clade B and Clade A. According to previous reports and the data presented here, each Dactylogyrus species within Clade B parasitizes several endemic Luciobarbus species. Considering the monophyletic origin of Iberian Luciobarbus (Yang et al., Reference Yang, Sado, Hirt, Pasco-Viel, Arunachalam, Li, Wang, Freyhof, Saitoh, Simons, Miya, He and Mayden2015), its probable historical dispersion via northern Africa (Bianco, Reference Bianco1990; Doadrio, Reference Doadrio1990; Zardoya and Doadrio, Reference Zardoya and Doadrio1998), and the phylogenetic relatedness of Dactylogyrus from Clade B with north-west African Dactylogyrus (Šimková et al., Reference Šimková, Benovics, Rahmouni and Vukić2017), we may postulate that these species originated on the Luciobarbus ancestor, and may have host-switched in the past to endemic north-west African Carasobarbus, subsequently dispersing to the Iberian Peninsula during its historical connection with North Africa. The high number of morphologically similar species exhibiting a low molecular divergence (e.g. D. bocageii, D. mascomai, D. guadianensis, D. lenkoranoïdes and D. doadrioi) suggests subsequent rapid speciation, most likely linked with the radiation of Luciobarbus across individual river basins within the Iberian Peninsula (Doadrio, Reference Doadrio1988; Zardoya and Doadrio, Reference Zardoya and Doadrio1998; Doadrio et al., Reference Doadrio, Carmona and Machordom2002; Mesquita et al., Reference Mesquita, Cunha, Carvalho and Coelho2007; Gante et al., Reference Gante, Doadrio, Alves and Dowling2015; Casal-López et al., Reference Casal-López, Perea, Sousa-Santos, Robalo, Torralva, Oliva-Paterna and Doadrio2017). Addition of Dactylogyrus species from Asian Capoeta (phylogenetically sister group to Iberian Luciobarbus; Yang et al., Reference Yang, Sado, Hirt, Pasco-Viel, Arunachalam, Li, Wang, Freyhof, Saitoh, Simons, Miya, He and Mayden2015) to phylogenetic reconstruction and assessing coevolutionary scenarios involving these parasites and their hosts may shed more light into the origin of the Dactylogyrus of Iberian Luciobarbus and finally resolve the phylogenetic relationships within this group of Dactylogyrus.
In contrast to Dactylogyrus from Clade B, the phylogenetic proximity of Iberian Dactylogyrus within Clade A to Central European and Balkan Dactylogyrus species supports their European origin. In accordance with the phylogeny proposed by Šimková et al. (Reference Šimková, Benovics, Rahmouni and Vukić2017), Dactylogyrus species from Iberian Luciobarbus form two well-supported lineages within Clade A, and cluster with Dactylogyrus from European Barbus. Two species within Clade A, D. balistae and D. legionensis, have a large body size, large haptoral sclerites and are missing the haptoral connective ventral bar (see El Gharbi et al., Reference El Gharbi, Renaud and Lambert1992). These species form a well-supported clade in sister position with another Iberian species, D. linstowoïdes. This clade is closely related to D. malleus, D. prespensis and D. petenyi, all host-specific parasites to European Barbus. In contrast to D. legionensis and D. balistae, these three species have a small body size, similarly shaped small haptoral elements and a ventricular ventral bar (see Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). Based on the morphology, D. linstowoïdes represents the transient form between these two lineages, with the haptoral sclerites resembling Dactylogyrus of European Barbus and copulatory organs morphologically similar to Iberian species. Our results support a common origin for these species, with D. balistae, D. legionensis and D. linstowoïdes possibly evolving in Iberia from a common ancestor and thereafter switching to Luciobarbus, following which D. balistae and D. legionensis secondarily lost their haptoral connective ventral bar.
In this study, Leuciscids generally harboured poorer Dactylogyrus species communities than cyprinids. However, due to the higher species richness of this fish family in the Iberian Peninsula, a remarkably high species diversity was observed among their Dactylogyrus parasites, and specifically among Dactylogyrus parasitizing Squalius spp. and the genera erected from Chondrostoma s.l.. Almost each genetic variant was supported as a species by the species delimitation analysis. Dactylogyrus from Iberian leuciscids formed three major phylogenetic lineages. The first comprised Dactylogyrus sp. 1 only, collected from two endemic Squalius species, S. torgalensis and S. aradensis. Previous molecular phylogenetic studies suggested that these sister species have a basal position to other representatives of Squalius in Iberia (Sanjur et al., Reference Sanjur, Carmona and Doadrio2003; Waap et al., Reference Waap, Amaral, Gomes and Coelho2011; Perea et al., Reference Perea, Vukić, Šanda and Doadrio2016; Sousa-Santos et al., Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019). The distribution of S. torgalensis and S. aradensis is limited to the south-western extremity of the Iberian Peninsula, and the same distribution range was found for Dactylogyrus sp. 1. Extrapolating from the phylogenetic reconstruction, Dactylogyrus sp. 1 is phylogenetically close to common Dactylogyrus species from European Squalius spp., i.e. D. folkmanovae and D. nanoides [hypothesized to be genus specific according to Šimková et al. (Reference Šimková, Morand, Jobet, Gelnar and Verneau2004) and Benovics et al. (Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018)], and probably represents an ancestral Dactylogyrus lineage that has coevolved in Iberia with its endemic Squalius hosts.
The majority of endemic leuciscid Dactylogyrus formed a well-supported clade, with D. caucasicus from Alburnoides spp. and D. ergensi from Chondrostoma spp. in sister position. Benovics et al. (Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018) have previously suggested that D. caucasicus originated from the ancestor of D. ergensi by host-switching to Alburnoides. The species delimitation analysis suggested the existence of nine potentially new species (Dactylogyrus sp. 2 to Dactylogyrus sp. 10) phylogenetically related to D. ergensi (the species with the widest distribution range across Europe), which may indicate that endemic Dactylogyrus sp. 2 to Dactylogyrus sp. 10 also share a common ancestor with D. ergensi. As suggested by Robalo et al. (Reference Robalo, Almada, Levy and Doadrio2007), the ancestor of Chondrostoma s.l. could have dispersed into Iberia prior to the Messinian period, when the host-specific ancestral Dactylogyrus species associated with these hosts most likely colonized Iberia. Our data suggest that the rapid radiation of Chondrostoma-related species promoted the speciation of their host-specific Dactylogyrus. Even if parasite phylogeny is not fully congruent with that of their hosts, all Iberian Dactylogyrus species, excluding Dactylogyrus sp. 8 [collected from Parachondrostoma species only distributed in rivers of the Mediterranean slope (Kottelat and Freyhof, Reference Kottelat and Freyhof2007)], parasitize leuciscids in river basins of the Atlantic slope [distribution according to Kottelat and Freyhof (Reference Kottelat and Freyhof2007); Robalo et al. (Reference Robalo, Almada, Levy and Doadrio2007); Sousa-Santos et al. (Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019)]. Considering that the distribution of cyprinoid species in Iberia is almost non-overlapping, the incongruence between host and parasite phylogenies could be the result of secondary contacts between fish host species, as recently documented in some Iberian rivers (e.g. Doadrio, Reference Doadrio2001; Sousa-Santos et al., Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019). Dactylogyrus sp. 7, for example, was collected from two separate species, S. pyrenaicus and S. carolitertii. Sousa-Santos et al. (Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019) and Waap et al. (Reference Waap, Amaral, Gomes and Coelho2011) suggested that S. pyrenaicus consists of two different species, each associated with different river basins. Previous multilocus phylogenetic analyses (Sousa-Santos et al., Reference Sousa-Santos, Jesus, Fernandes, Robalo and Coelho2019) have supported that S. pyrenaicus is paraphyletic, as genetic variants of this species from the Tagus and Colares basins were both grouped with S. carolitertii. Exactly the same pattern was observed among genetic variants of Dactylogyrus sp. 7, with individuals collected from S. pyrenaicus being in paraphyly and individuals from the River Colares grouped with individuals from S. carolitertii. A similar situation has also been observed in Dactylogyrus spp. from the Balkans, where the phylogenetic positions of two populations of D. vistulae within the D. vistulae clade (i.e. paraphyly) and molecular dissimilarity between the two populations (Benovics et al., Reference Benovics, Desdevises, Vukić, Šanda and Šimková2018) supported the existence of two different Alburnoides species, as previously proposed by Stierandová et al. (Reference Stierandová, Vukić, Vasil'eva, Zogaris, Shumka, Halačka, Vetešník, Švátora, Nowak, Stefanov, Koščo and Mendel2016).
In general, Dactylogyrus species diversity within the Iberian Peninsula appears to be associated with the historical dispersion of their cyprinoid hosts, with subsequent adaptive radiation following the peninsula's geographical isolation due to the elevation of the Pyrenees (Muñoz et al., Reference Muñoz, Martinez and Vergés1986; Puigdefàbregas et al., Reference Puigdefàbregas, Muñoz, Vergés and McClay1992; Stange et al., Reference Stange, Van Balen, Garcia-Castellanus and Cloetingh2016). At least two historical origins can be inferred for Iberian Dactylogyrus, each associated with the different dispersion routes proposed for cyprinoids (Banarescu, Reference Banarescu and Holcik1989, Reference Banarescu1992; Doadrio, Reference Doadrio1990; Doadrio and Carmona, Reference Doadrio and Carmona2003; Perea et al., Reference Perea, Böhme, Zupančic, Freyhof, Šanda, Özulug and Doadrio2010). Despite well-supported delineation between a multitude of endemic Dactylogyrus species, the phylogenetic relationships between Dactylogyrus species do not fully correspond to the phylogeny of their hosts, suggesting secondary contacts and host-switching between endemic Iberian cyprinoids.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020000050
Acknowledgements
We are grateful to Kateřina Čermáková, Jaroslav Červenka, Maria Lujza Červenka Kičinja and Tomáš Pakosta for their help with fish processing and parasite collection. Sampling was approved by the responsible governmental authorities in Portugal and in Spain (Andalusia, Extremadura, Aragon, Cataluña, Valencia and Castilla y Leon). Moreover, we are grateful to Ana Pereira, Jesus Hernandez and Pilar Risueño for their help with fieldwork. We kindly thank Kevin Roche for English revision of the final draft.
Financial support
This study was financially funded by the Czech Science Foundation (project number 15-1938S).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed. This study was approved by the Animal Care and Use Committee of the Faculty of Science, Masaryk University in Brno (Czech Republic).










