INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite that infects a wide variety of warm-blooded animals and humans. For humans, although T. gondii rarely afflicts most healthy individuals, it may cause abortion and congenital diseases in pregnant women and foetuses and lethal encephalitis in immunocompromised individuals (Kim and Weiss, Reference Kim and Weiss2004; Rorman et al. Reference Rorman, Zamir, Rilkis and Ben2006). T. gondii also causes abortion and congenital diseases in sheep and goats, with great economic losses (Haumont et al. Reference Haumont, Delhaye, Garcia, Jurado, Mazzu, Daminet, Verlant, Bollen, Biemans and Jacquet2000; Dubey, Reference Dubey2004). It was previously mentioned (Desolme et al. Reference Desolme, Mevelec, Buzoni and Bout2000) that a primary infection with T. gondii could give the host a protective immunity against re-infection, and therefore the development of effective vaccines is considered to be a realistic goal.
Attenuated live vaccine is currently available for the control of T. gondii infection in sheep, in which the parasites are unable to cause chronic infection (Buxton, Reference Buxton1993). However, it is not suitable for use in humans because of the risk of reversion to a pathogenic form (Ogra et al. Reference Ogra, Faden, Abraham, Duffy, Sun and Minor1991). The drawbacks of a live vaccine could be circumvented via the development of subunit vaccines. In this context, the search for novel antigens involved in protective immunity has mainly focused on surface antigens (SAGs), dense granule antigens (GRAs), rhoptry antigens (ROPs), and microneme antigens (MICs). Among the antigens that have been characterized, the dense granule antigen 4 (GRA4) is considered to be a strong candidate for vaccine development. The GRA4 has been shown to induce both humoral and systemic immune responses following oral infection with T. gondii (Chardes et al. Reference Chardes, Bourguin, Mevelec, Dubremetz and Bout1990). Vaccination with either the recombinant GRA4 protein or a plasmid encoding the GRA4 gene provided partial protection against toxoplasmosis in mice (Desolme et al. Reference Desolme, Mevelec, Buzoni and Bout2000; Martin et al. Reference Martin, Supanitsky, Echeverria, Litwin, Tanos, Roodt, Guarnera and Angel2004; Mevelec et al. Reference Mevelec, Bout, Desolme, Marchand, Magne, Bruneel and Buzoni2005). In addition, the protective efficacy of the GRA4 DNA vaccine has been improved by co-inoculation of a plasmid encoding a colony-stimulating factor (GM-CSF) (Desolme et al. Reference Desolme, Mevelec, Buzoni and Bout2000; Mevelec et al. Reference Mevelec, Bout, Desolme, Marchand, Magne, Bruneel and Buzoni2005).
Currently, several vaccination strategies have been reported to greatly enhance the immunogenicity of plasmid-delivered antigens (Shedlock and Weiner, Reference Shedlock and Weiner2000; Indresh et al. Reference Indresh, Margaret and Liu2003). One of the most promising strategies is the sequential delivery generically referred to as a heterologous prime-boost vaccination regime (Ramshaw and Ramsay, Reference Ramshaw and Ramsay2000; Moore and Hill, Reference Moore and Hill2004). A heterologous prime-boost vaccination regime using DNA and a vaccinia virus, both expressing the same antigen, has been shown to be effective to control Plasmodium ssp. in both animal models and humans (Vuola et al. Reference Vuola, Keating, Webster, Berthoud, Dunachie, Gilbert and Hill2005; Miao et al. Reference Miao, Li, Liu, Xue, Bujard and Cui2006). To date, no report has described the heterologous prime-boost vaccination regime against T. gondii infection.
The aim of the present study is to evaluate the feasibility of the heterologous prime-boost vaccination regime using DNA and a vaccinia virus, both expressing GRA4 against T. gondii. Our results demonstrate that the heterologous prime-boost vaccination regime can elicit both humoral and cellular immune responses and provide complete protection against acute and chronic T. gondii infections in mice.
MATERIALS AND METHODS
Parasite culture
T. gondii tachyzoites of RH and PLK/GFP (PLK expressing green fluorescence protein gene) strains were maintained in Vero cells grown in Eagle's minimum essential medium (MEM) supplemented with 8% foetal bovine serum (FBS) at 37°C in a 5% CO2 air environment. For the purification of tachyzoites, the parasites were scraped from the flask and then passed through a 27G needle and, subsequently, a 5·0 μm filter (Millipore, USA). The parasites were then washed in phosphate-buffered saline (PBS) and stored at −30°C until use.
Cloning and expression of the GRA4 gene
The purified T. gondii tachyzoites (1×108) of the RH strain were lysed in 0·1 m Tris-HCl (pH 8·0) containing 1% sodium dodecyl sulfate (SDS), 0·1 m NaCl, and 10 mm EDTA and then treated with proteinase K (100 μg/ml) at 55°C for 2 h. The genomic DNA was extracted by phenol/chloroform followed by ethanol precipitation. The DNA pellets were dissolved in a TE buffer (10 mm Tris-HCl, pH 8·0, and 1 mm EDTA) and used as a template DNA for PCR. The DNA fragment encoding GRA4 was amplified by PCR using oligonucleotide primers with introduced EcoRI sites (underlined), 5′-ACGAATTCTACAATGCAGGGCACT-3′ and 5′-ACGAATTCTCTTTGCGCATTCTTT-3′. The PCR product was digested with EcoRI and then cloned into the EcoRI site of the bacterial expression vector, pGEX-4T-3 (Promega, USA). The resulting plasmid was designated as pGEX/GRA4 after checking by sequencing. The GRA4 gene was expressed as a glutathione S-transferase (GST) fusion protein (rGRA4) in E. coli (BL21 strain) according to the manufacturer's instructions.
Production of anti-GRA4 mouse serum
One hundred micrograms of the rGRA4 were injected intraperitoneally into mice (ddY, 6-week-old, female) with Freund's complete adjuvant. On days 14 and 28 post-immunization, the same antigen was intraperitoneally injected with Freund's incomplete adjuvant. The anti-rGRA4 sera were collected 10 days after the last immunization.
Construction of a DNA vaccine expressing GRA4
The entire GRA4 gene was obtained from pGEX/GRA4 after digestion with EcoRI and ligated into the EcoRI site of a eukaryotic expression vector pcDNA3.1 (Invitrogen, USA) containing the CMV promoter. The resulting plasmid was designated as pcDNA3.1/GRA4 (pGRA4). The control plasmid pcDNA3.1/GFP (pGFP) was constructed by getting the GFP gene from pCX-EGFP (kindly provided by Dr Miyazaki). The plasmids were purified by using a column chromatography kit (QIAGEN) according to the instructions of the manufacturer, dissolved in a TE buffer, and stored at −20°C. Vero cells were transfected with 2 μg of pGRA4 by using the Cellfectin reagent (Invitrogen, USA) and harvesting the transfected cells after 48 h. They were then checked by IFAT (Xuan et al. Reference Xuan, Nakamura, Sato, Tuchiya, Nosetto, Ishihana and Ueda1995) and Western blot analysis (Boldbaataar et al. Reference Boldbaataar, Xuan, Kimbita, Huang, Igarashi, Byambaa, Battsetseg, Battur, Battsetseg, Batsukh, Nagasawa, Fujisaki and Mikami2001) with anti-rGRA4 mouse serum as the primary antibody.
Construction of a recombinant vaccinia virus expressing GRA4
The recombinant vaccinia virus (VV) LC16mO strain, which expresses TgGRA4 or GFP, was constructed as follows (VV/GRA4 or VV/GFP). The GRA4 gene was obtained from pGEX/GRA4 after digestion with EcoRI and blunted with the Klenow Fragment and cloned into the SalI site of the vaccinia virus transfer vector, pAK8 (Nishikawa et al. Reference Nishikawa, Iwata, Katsumata, Xuan, Nagasawa, Igarashi, Fujisaki, Otsuka and Mikami2001). For the GFP, plasmid pCX-EGFP was cut with EcoRI, and the fragment (732 bp) containing the GFP gene was blunted with the Klenow fragment and cloned into the SalI site of the vaccinia virus transfer vector, pAK8. Rabbit kidney (RK13) cells infected with a vaccinia virus (LC16mO) were transfected with the recombinant transfer vectors by using the Cellfectin reagent (Invitrogen, USA). Thymidine kinase-negative (TK−) viruses were isolated by plaque assay on 143TK− cells in the presence of 5-bromo-2′-deoxyuridine at a concentration of 100 μg/ml. The in vitro expression of GRA4 was checked by IFAT and Western blot analysis with anti-rGRA4 mouse serum as the primary antibody.
Animals and immunizations
Female inbred 6-week-old C57BL/6 mice were used for immunization (n=10, Table 1). The gene gun vaccination was performed as described previously (Saito et al. Reference Saito, Aosai, Rikihisa, Mun, Norose, Chen, Kuroki, Asano, Ochiai, Hata, Ichinose and Yano2001). The Helios Gene Gun System (Nippon Bio-Rad Laboratories, Japan) was used in accordance with the company's manual. Briefly, plasmid pGRA4 or pGFP was affixed onto gold particles (1·0 μm diameter) at a rate of 2 μg DNA per 1 mg of gold by the addition of 1 m CaCl2 in the presence of 0·05 m spermidine. Then, a gene gun vaccination was performed on the shaved ventral skin of the mice using the hand-held He-pulse gun at discharge pressures (400 psi), and each mouse received 2 shots (approximately 2 μg of DNA per mouse). Mice were immunized 3 times at 3-week intervals. The recombinant vaccinia virus (VV/GRA4 or VV/GFP, 106 PFU virus/mouse) immunization was performed 2 weeks after the last gene gun vaccination.
Table 1. The immunization regimes

Measurement of humoral responses
The total anti-rGRA4 antibodies (IgG) in the sera from the sixth week post-immunization were measured by ELISA with the rGRA4 as the antigen. Briefly, 96-well microtitre plates (Nunc, Denmark) were coated with 250 ng of rGRA4. Mouse sera were diluted 1:100 with PBS containing 3% skim milk and applied to the wells, followed by goat anti-mouse IgG-HRP (Bethyl Laboratories, USA) conjugate as a secondary antibody. After incubation in a substrate solution, the absorbance was measured at 415 nm.
In vitro spleen cell proliferation
Three mice from each group were sacrificed just before challenge, and their spleens were removed and single-cell suspensions obtained by teasing the spleens apart. The erythrocytes in the spleen cell suspension were removed by RBC lysing buffer (Sigma, USA), and the remaining cells were washed and suspended in an RPMI 1640 medium (Sigma, USA) supplemented with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin. The cell suspension was adjusted to 5×106 cells/ml and 100 μl per well were seeded in triplicate in flat-bottomed 96-well microtitre plates. One hundred μl of antigen (rGRA4 with a final concentration of 10 μg/ml) or 5 μg/ml concavanalin A (Con A) had previously been added to the plates. The plates were incubated for 72 h in 5% CO2 at 37°C. Supernatants were then stored at −70°C for cytokine quantification.
Measurement of cytokine production
Cell-free supernatants were harvested as described above and assayed for interleukin-4 (IL-4) and gamma interferon (IFN-γ) activity at 72 h. The IL-4 and IFN-γ concentrations were evaluated using a commercial ELISA kit according to the manufacturer's instructions (BioSource, USA). The cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IL-4 and IFN-γ.
Challenge infection
Three weeks after the boosting, mice were challenged intraperitoneally with 20 000 T. gondii tachyzoites of PLK/GFP, and the survival rates of mice were monitored every day. Forty-five days after challenge, whole brains from the surviving mice were harvested and homogenized in 2 ml of PBS. Brain cysts and free parasites were counted as follows: 10 μl of brain suspension was placed on a glass slide and mounted with a cover-slip. Since the parasites used for challenge are carrying the GFP gene, the fluorescent parasites can be recognized and counted easily under the fluorescent microscope (Nikon microphot FXA, Japan) at ×40 magnification. The total number of cysts and free parasites were calculated in the whole brain.
Statistical analysis
The statistical significance of the differences for the IFN-γ production and brain-free parasite loads between groups was calculated with one-way analysis of variance (ANOVA) by using S-Plus 6 software (Insightful Co., USA). P values less than 0·05 (P<0·05) were considered significant.
RESULTS
In vitro expression of GRA4
The expression of GRA4 in mammalian cells was investigated. Vero cells were transfected with pGRA4 by using Cellfectin reagent and, after 48 h, the transfected cells were checked by IFAT and Western blot analysis with anti-rGRA4 mouse serum as the primary antibody. RK13 cells were infected with VV/GRA4, and, after 48 h, the cells were harvested. IFAT and Western blot analysis with anti-rGRA4 mouse serum as the primary antibody was performed with both sets of transfected cells. The specific fluorescence was seen on both Vero cells transfected with pGRA4 and RK13 cells infected with VV/GRA4 (Fig. 1). In the Western blot analysis, a specific band with a molecular mass of 40 kDa, which is similar to that of the native protein in Toxoplasma lysed antigen (TLA), was detected in both Vero cells transfected with pcDNA/GRA4 and RK13 cells infected with VV/GRA4 (Fig. 2). In addition, the GRA4 expressed in Vero and RK13 cells was reacted with T. gondii-infected mice sera (data not shown).

Fig. 1. The expression of GRA4 in vitro was confirmed by IFAT with mouse anti-GRA4 serum followed by Alexa Fluor-594-conjugated secondary antibodies (Molecular Probes, USA). (A) Vero cells transfected by pcDNA; (B) Vero cells transfected by pcDNA/GRA4; (C) RK13 cells infected by VV/GFP; (D) RK13 cells infected by VV/GRA4. (B and D) Specific red fluorescence detected on the cells.

Fig. 2. Western blot analysis of the in vitro expression of GRA4 using anti-rGRA4 serum. M, molecular weight; lane 1, VV/GRA4-infected RK13 cells; lane 2, VV/GFP-infected RK13 cells; lane 3, T. gondii tachyzoites; lane 4, pGRA4-transfected Vero cells; lane 5, with pGFP-transfected Vero cells.
Humoral immune responses induced by heterologous prime-boost vaccination regimes
In order to evaluate the vaccine efficacy of GRA4, C57BL/6 mice were vaccinated with pGRA4 priming using a gene gun followed by recombinant vaccinia virus VV/GRA4 boosting. Sera from immunized mice were collected prior to challenge and analysed by ELISA for specific anti-rGRA4 responses. A strong IgG antibody response was found in all the groups of immunized mice from the sixth week after the first immunization, and the response was found to increase until challenge (Fig. 3). To examine whether a Th1 and/or Th2 was elicited in immunized mice, the distribution of IgG subtypes was analysed against rGRA4. A predominance of anti-T. gondii IgG1 over IgG2a was observed in sera from the mice with 3 vaccination regimes: pGRA4+pGRA4, pGRA4+VV/GRA4, and VV/GRA4+pGRA4, suggesting that vaccination with the 3 vaccine regimes elicited a humoral Th2-dominant response (Fig. 4).

Fig. 3. Specific anti-GRA4 IgG antibody response induced in mice vaccinated with prime-boost regimes. Serum samples were taken from the 6th week after the first immunization and analysed by ELISA for the detection of IgG to rGRA4. DNA, pGRA4; VV, VV/GRA4; control DNA, pGFP; control VV, VV/GFP.

Fig. 4. Specific anti-GRA4 IgG1 and IgG2a antibody responses induced in mice vaccinated with prime-boost regimes. Serum was taken from the 11th week after the first immunization and analysed by ELISA for detection of IgG1 or IgG2a to rGRA4. DNA, pGRA4; VV, VV/GRA4; control DNA, pGFP; control VV, VV/GFP.
Cellular immune responses induced by heterologous prime-boost vaccination regimes
To examine the cellular immune responses elicited in mice with the prime-boost vaccination regimes, we quantified the production of the cytokines released from splenocytes from immunized mice that were restimulated with rGRA4 in vitro. The production of IFN-γ was observed in the spleen cell cultures from vaccinated mice (Fig. 5). The statistical significance of the difference for the IFN-γ production between group means was calculated with one-way analysis of variance (ANOVA) with S-Plus 6 software, the P value was equal to 0·0025, which is highly significant, therefore we conclude that the vaccination regimes affected the production level of IFN-γ. In addition, by using the Bonferroni method, the production level of IFN-γ in the mice vaccinated with DNA priming followed by VV/GRA4 boosting was significantly higher than those in control group and pGRA4+pGRA4 vaccinated group (Fig. 5, P<0·05). There was no detectable IL-4 production in the spleen cell cultures from mice with all heterologous prime-boost vaccination regimes.

Fig. 5. IFN-γ production in splenocytes taken from mice vaccinated with prime-boost regimes. The supernatants were assayed for the presence of IFN-γ after stimulating with rGRA4. Each bar represents the IFN-γ production of splenocytes from 3 mice. The differences of IFN-γ production between experimental groups were analysed by ANOVA and that a P value of less than 0·05 was considered significant. DNA, pGRA4; VV, VV/GRA4; control DNA, pGFP; control VV, VV/GFP.
Protective efficacy of heterologous prime-boost vaccination regimes in mice
To evaluate the protective effect of heterologous prime-boost vaccination regimes, all mice were challenged by intraperitoneal injection with 20 000 T. gondii tachyzoites of the PLK/GFP strain after 3 weeks of boosting. The mice vaccinated with pGRA4 priming using a gene gun and boosting with VV/GRA4 (pGRA+VV/GRA4) were completely protected from the T. gondii challenge infection (Fig. 6). On the other hand, the mice vaccinated with pGRA4+pGRA4 and VV/GRA4+pGRA4 regimes were moderately protected from the T. gondii challenge infection (Fig. 6). In contrast, the mice vaccinated with VV/GRA4+VV/GRA4 and the control vectors had a very low level of protection (Fig. 6). To evaluate the inhibitory effect on T. gondii cyst development in the chronic phase of infection, the surviving mice were sacrificed at 45 days after infection, and the numbers of free parasites and cysts in the brain were determined microscopically. In the 2 mice surviving in the control DNA+VV group, the cyst numbers were 400 and 600, respectively, and the cyst number in the only mouse surviving in the VV+VV vaccinated group was 400. In contrast, the formation of cysts was inhibited in the mice vaccinated with pGRA4+VV/GRA4, pGRA4+pGRA4, and VV/GRA4+pGRA4 regimes (Table 2). The number of free parasites in mice vaccinated with the pGRA4+VV/GRA4 regime was the lowest load as compared to other groups, and it was significantly lower than all groups tested except the VV/GRA4+pGRA4 group (Fig. 7, P<0·05).

Fig. 6. Protective effects of prime-boost regimes against Toxoplasma gondii infection in C57BL/6 mice. The mice vaccinated with prime-boost regimes were challenged with 20 000 PLK/GFP tachyzoites 3 weeks after the boosting (n=7). DNA, pGRA4; VV, VV/GRA4; control DNA, pGFP; control VV, VV/GFP.

Fig. 7. The number of free parasites in the brain of surviving mice. Free parasites were determined in the brains of surviving mice 1 month post-challenge infection (*, P<0·05). DNA, pGRA4; VV, VV/GRA4; control DNA, pGFP; control VV, VV/GFP.
Table 2. The cyst numbers in the whole brain of surviving mice
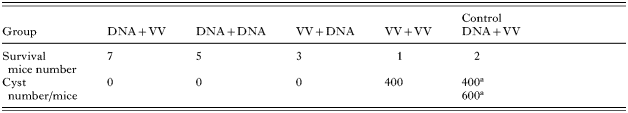
a There are 2 mice surviving in the control DNA+VV group, the cyst numbers are 400 and 600, respectively.
DISCUSSION
In the present study, we have demonstrated that the heterologous prime-boost vaccination regime using DNA and a vaccinia virus, both expressing GRA4 of T. gondii, could induce strong specific humoral and cellular immune responses in mice. The immunity acquired during the vaccination is capable of protecting the mice from T. gondii challenge infection with a lethal dose. To our knowledge, this is the first report showing that the heterologous prime-boost vaccination regime is useful for controlling acute and chronic T. gondii infections in mice.
The in vitro expression of GRA4 in eukaryotic cells by either a DNA vaccine or a vaccinia virus was confirmed with anti-rGRA4 sera and T. gondii-infected mouse sera. GRA4 expressed in eukaryotic cells has been shown to have a similar molecular mass to that of native GRA4 from T. gondii. These results are consistent with previous reports (Desolme et al. Reference Desolme, Mevelec, Buzoni and Bout2000; Mevelec et al. Reference Mevelec, Bout, Desolme, Marchand, Magne, Bruneel and Buzoni2005). The C57BL/6 mice vaccinated with DNA vaccine pGRA4 priming and followed by vaccinia virus VV/GRA4 boosting (pGRA4+VV/GRA4 regime) produced a strong IgG antibody response against rGRA4. In addition, the mice vaccinated with pGRA4+pGRA4 and VV/GRA4+pGRA4 regimes also produced a comparable IgG antibody response. However, the mice vaccinated with VV/GRA4+VV/GRA4 induced detectable but not significant IgG antibody response. These results indicate that VV/GRA4 alone might not be effective to induce humoral immunity in C57BL/6 mice.
It is well known that the primary obstacle in developing vaccines for the control of T. gondii infection is the ability to induce strong and long-lasting cell-mediated immunity associated with IFN-γ (Yap and Sher, Reference Yap and Sher1999; Kobayashi et al. Reference Kobayashi, Aosai, Hata, Mun, Tagawa, Iwakura and Yano1999). In this study, the mice vaccinated with pGRA4+VV/GRA4 showed higher IFN-γ secretion than mice vaccinated with pGRA4+pGRA4 and VV/GRA4+pGRA4 regimes. These results indicate that the pGRA4+VV/GRA4 regime is the most effective method to produce IFN-γ secretion. Interestingly, the mice vaccinated with VV/GRA4+VV/GRA4 also showed significantly higher IFN-γ secretion than mice vaccinated with empty vectors, although this regime has been shown to be ineffective to induce an antibody response, as mentioned above.
The survival rate of vaccinated mice against a lethal T. gondii challenge infection is thought to be a most direct parameter for evaluating a vaccine candidate. In this study, all the mice vaccinated with pGRA4+VV/GRA4 regime survived the lethal-dose infection challenge with T. gondii. In addition, we observed a partial protective effect from the mice vaccinated with pGRA4+pGRA4 and VV/GRA4+pGRA4 regimes. These results were correlated with the levels of IFN-γ produced as described above. The results of IFN-γ production and the survival rate suggest that the order of the immunization is very important. The regime with DNA vaccine priming followed by vaccinia virus boosting was better than that with vaccinia virus priming followed by DNA vaccine boosting or homologous prime-boost regimes. This observation was consistent with previous reports (Irvine et al. Reference Irvine, Chamberlain, Shulman, Surman, Rosenberg and Restifo1997; Moore and Hill, Reference Moore and Hill2004; Vuola et al. Reference Vuola, Keating, Webster, Berthoud, Dunachie, Gilbert and Hill2005). To evaluate the vaccine effect on cyst development in the chronic stage, the cysts or free tachyzoites in brains from survived mice were investigated. The cysts or free tachyzoites were significantly reduced in brains from mice vaccinated with the pGRA4+VV/GRA4 regime. Taken together, these results indicate that the heterologous prime-boost vaccination regime with DNA vaccine priming followed by vaccinia virus boosting, both expressing GRA4, is the most effective method to improve the protective ability of GRA4 for the control of acute infection as well as chronic infection in mice.
Previous studies have shown that a DNA vaccine with the GRA4 gene could induce Th1-dominant immunity with a higher level of IgG2a or IgG2b than IgG1 (Desolme et al. Reference Desolme, Mevelec, Buzoni and Bout2000; Mevelec et al. Reference Mevelec, Bout, Desolme, Marchand, Magne, Bruneel and Buzoni2005). In the present study, the mice vaccinated with the heterologous prime-boost regime using a DNA vaccine followed by a vaccinia virus, both expressing GRA4, had Th2-dominant immunity with a higher level of IgG1 than IgG2a. The conflict between previous and present results might be due to the use of a gene gun in the latter. It is known that gene gun vaccination triggers Th2-dominant immunity (Weiss et al. Reference Weiss, Scheiblhofer, Freund, Ferreira, Livey and Thalhamer2002; Kristina et al. Reference Kristina, Rao and Alving2003). In addition, the heterologous prime-boost regime with GRA4 induced a high level of IFN-γ production. These results suggest that induction of humoral and cellular immunity plays a crucial role in the control of murine toxoplasmosis.
This work was supported by a grant from the 21st Century COE program (A-1) and a Grant-in-Aid for Scientific Research, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.











