INTRODUCTION
With about 740 million people infected, hookworms remain a major public health problem in large parts of the world, particularly in economically deprived populations (de Silva et al. Reference de Silva, Brooker, Hotez, Montresor, Engels and Savioli2003). The human hookworms Necator americanus and Ancylostoma duodenale are intestinal nematodes, with infection being acquired through contact with soil contaminated with infectious larval stages. Upon entering the host by skin penetration, the parasites migrate through the lungs, undergo further development and finally establish themselves as blood-feeding adult worms in the small intestine (Hotez et al. Reference Hotez, Brooker, Bethony, Bottazzi, Loukas and Xiao2004). As is the case with other helminth infections, the distribution of adult worms is highly aggregated and overdispersed (Schad and Anderson, Reference Schad and Anderson1985). Inter-host differences in exposure and/or susceptibility are assumed to play a causal role in creating such patterns. Exposure and susceptibility may be influenced by environmental conditions, behaviour, demography and socio-economic status, but also by variation in underlying genetic factors influencing host defence mechanisms (Quinnell, Reference Quinnell2003). Evidence for a role of host genetics in determining infection intensity has been reported for a variety of parasitic helminths of humans. Published studies of Ascaris lumbricoides (Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999), Trichuris trichiura (Williams-Blangero et al. Reference Williams-Blangero, McGarvey, Subedi, Wiest, Upadhayay, Rai, Jha, Olds, Guanling and Blangero2002) and species of Schistosoma (Bethony et al. Reference Bethony, Williams, Blangero, Kloos, Gazzinelli, Soares-Filho, Coelho, Alves-Fraga, Williams-Blangero, Loverde and Correa-Oliveira2002; King et al. Reference King, Blanton, Muchiri, Ouma, Kariuki, Mungai, Magak, Kadzo, Ireri and Koech2004; Ellis et al. Reference Ellis, Li, Rong, Chen and McManus2006) demonstrate significant heritable components of egg excretion, with additive genetic effects accounting for up to 44% of the phenotypic variation. In the case of hookworms, however, the situation is less clear. Predisposition to high or low hookworm burdens or worm weights has been observed (Schad and Anderson, Reference Schad and Anderson1985; Bradley and Chandiwana, Reference Bradley and Chandiwana1990; Quinnell et al. Reference Quinnell, Griffin, Nowell, Raiko and Pritchard2001), but only one study, explicitly investigating genetic determinants of infection, has been published to date (Williams-Blangero et al. Reference Williams-Blangero, Blangero and Bradley1997). The findings were consistent with genetic variation in the human host accounting for almost 40% of phenotypic variation, but it was not possible to differentiate the genetic influences from those due to shared household risk factors.
In the present study, we carried out quantitative genetic analyses using a large dataset of extended pedigrees from a study site in Papua New Guinea, to elucidate further the role of genetic factors involved in the susceptibility of humans to hookworm infections. The aims were to quantify the relative roles of additive genetic and shared household effects in determining faecal egg counts of N. americanus and, given recent reports of sex-differences in heritabilities of a broad range of quantitative traits in humans (Weiss et al. Reference Weiss, Pan, Abney and Ober2006), to explore the relative role of both factors in males and females.
MATERIALS AND METHODS
Study population and parasitology
Fieldwork was carried out in 5 villages in lowland Madang province, Papua New Guinea, an area highly endemic for N. americanus (Pritchard et al. Reference Pritchard, Quinnell, Slater, McKean, Dale, Raiko and Keymer1990). All inhabitants (at least 4 years of age) of 5 villages (Bauri, Gumaru, Haven, Mawan and Wasab) were considered eligible for inclusion in this study. Exclusion criteria consisted of refusal or withdrawal of informed consent and reported anthelminthic treatment in the 24 months prior to this study. The study was approved by the Medical Research Advisory Committee of Papua New Guinea.
Quantitative faecal egg counts were used as a measure of hookworm infection intensity (Stoll, Reference Stoll1924; Hill, Reference Hill1926). The relationship between egg production and worm burden in hookworm infection is approximately linear (Anderson and Schad, Reference Anderson and Schad1985). ‘Baseline’ parasitological examinations were carried out in May to September 1998, and people were treated orally with the anthelminthic albendazole (400 mg) (Horton, Reference Horton2000) or pyrantel pamoate (10 mg/kg) (Pritchard et al. Reference Pritchard, Quinnell, Slater, McKean, Dale, Raiko and Keymer1990; Quinnell et al. Reference Quinnell, Pritchard, Raiko, Brown and Shaw2004). Patients in 3 (Gumaru, Haven, Wasab) of 5 villages were re-examined in August to September 2001, after a period of 3 years and treated again with albendazole. At both time-points, stool examination was carried out using a quantitative McMaster salt flotation technique. Approximately 0·5 g of faeces was weighed, suspended in 25 ml of saturated salt solution and 0·3 ml of this suspension counted in a McMaster slide; the results (faecal egg counts) were expressed as eggs per gramme of faeces (epg). Adult hookworms expelled after pyrantel pamoate treatment were collected from a number of patients in 1998 and preserved in 10% formalin; 44 worms from 12 people (1–3 people from each village) were cleared in lactoglycerol and identified to species by examination of mouthparts. All hookworms were identified as N. americanus, which is the only species of hookworm previously reported from the area (Pritchard et al. Reference Pritchard, Quinnell, Slater, McKean, Dale, Raiko and Keymer1990).
Pedigree structures were determined by interviewing 1 adult member of each household. Only data from subjects with at least 1 participating relative with phenotypic information were used for the analyses presented here. In total, 945 faecal egg counts from 704 individuals were included in the analysis, with 620 egg counts being from 1998 and 325 from 2001; 241 individuals provided samples in both years. These 704 subjects belonged to 82 extended families, with a median of 5 (range: 2–61) subjects having been sampled per pedigree. Participants resided in 188 households with up to 11 individuals sampled per household. The analyses included a total of 6686 informative relative-pairs as determined by the ‘relpairs’ routine of the software package SOLAR (Almasy and Blangero, Reference Almasy and Blangero1998). The median age of subjects in 1998 was 20 years (range: 3–70 years), with 41% of samples being from children less than 16 years old. There were 365 males and 339 females, with 135 males and 106 females providing samples in both years.
Statistical analysis
To estimate the amount of phenotypic variation explicable by genetic factors, heritability estimation was undertaken using a variance components approach (Lange et al. Reference Lange, Westlake and Spence1976). In the simplest models, the total phenotypic variance (Vtot) was broken down into variance due to additive genetic effects (Va) and residual errors (Ve), where the design matrix for the former effects consists of two times the coefficients of kinship between all the possible pairs of study subjects. An additional variance component Vc was incorporated to control for common environmental risk factors shared by individuals living in the same household (design matrix elements of 1 for individuals residing in the same household, and 0 otherwise). To avoid any inflation of Va and Vc estimates that could result from a number of subjects contributing 2 observations, a variance component Vpe was applied to subjects with repeated measurements, and was included in all models. This accounts for permanent factors beyond those covered by the other components in the model, e.g. constant environmental factors not following the household pattern or non-additive genetic effects (design matrix elements of 1 for observations from the same subject, and 0 otherwise). Thus, for the full model, the quantitative trait heritability is calculated as h 2=Va/Vtot=Va/(Va+Vc+Vpe+Ve), while the household effects are given by c 2=Vc/Vtot=Vc/(Va+Vc+Vpe+Ve). All models were fitted by restricted maximum-likelihood (REML) using the programme ASReml (Gilmour et al. Reference Gilmour, Gogel, Cullis, Welham and Thompson2002). Normal distribution of the trait values is an underlying assumption of the models used; as the distribution of egg counts was highly skewed, a generalized linear model (GLM) accounting for the overdispersed, negative-binomial distribution of the egg counts was fitted using the MASS extension (Venables and Ripley, Reference Venables and Ripley2002) of the software package R (R Development Core Team, 2006), and the residuals of this GLM were used as trait values for the subsequent variance component analysis (Smith et al. Reference Smith, Wilson, Pilkington and Pemberton1999). Similar results were obtained using log-transformed data in a single mixed model, rather than removing covariate effects first (not shown). Significant (P⩽0·1) covariate terms controlled for in the GLM were stool consistency (5-level factor), age, age2, receiving treatment in 1998, village of residence (5-level factor), and interactions of the age terms with treatment and village. Sex and its interaction terms were dropped from the model due to insignificance. The degree of predisposition to high or low hookworm infection intensity was assessed using Pearson's correlation coefficient between the residuals for hookworm egg count for samples from the same individuals taken in 1998 and 2001. Only individuals treated in 1998 were included in this analysis. Homogeneity of correlation coefficients was tested as described (Sokal and Rohlf, Reference Sokal and Rohlf1995).
Variation in heritability between the sexes can be investigated by fitting separate variance components for each sex (Towne et al. Reference Towne, Blangero and Siervogel1993, Reference Towne, Siervogel and Blangero1997). A sex-specific model allowing the variance components to vary between the two sexes can be considered a special case of a bivariate trait model, in which no subject has observations for both traits, e.g. male and female egg counts. Thus, standard multivariate mixed model extensions can be applied (Lange and Boehnke, Reference Lange and Boehnke1983). Here, we fitted various models allowing one or more variance components (Va, Vc, Vpe and Ve) to vary between the sexes. For individuals of different sexes, all variance components and the covariance term for additive genetic and household effects were unconstrained. This approach allows variance components to be negative, and genetic and household correlations between sexes are not constrained to be between −1 and +1. Negative estimates of variance components are expected to occur by chance where a variance component is near 0, and fitting an unconstrained model provides the least biased estimates of other variance components. For some models, where a variance component estimate was negative, model fits were repeated with variance components constrained to be non-negative, and relevant covariances constrained to zero. Regarding Vpe as the constant part of the uncorrelated errors otherwise covered by Ve, we constrained the corresponding males:females covariance term to zero. The significance of differences between model hypotheses was determined by likelihood ratio tests (LRT) with the degrees of freedom (d.f.) equalling the difference in numbers of freely varying parameters between 2 nested models. The test for a single additional variance component presents a special case in which the test statistic follows a ½:½ mixture of χ2 distributions with 0 and 1 d.f. (Stram and Lee, Reference Stram and Lee1994). Corresponding P-values are obtained by carrying out a LRT with 1 d.f. and dividing the resulting P-value by 2. Variation in heritability between age classes was analysed similarly, dividing individuals into children (<16 years old in 1998) and adults (16 or more years old).
RESULTS
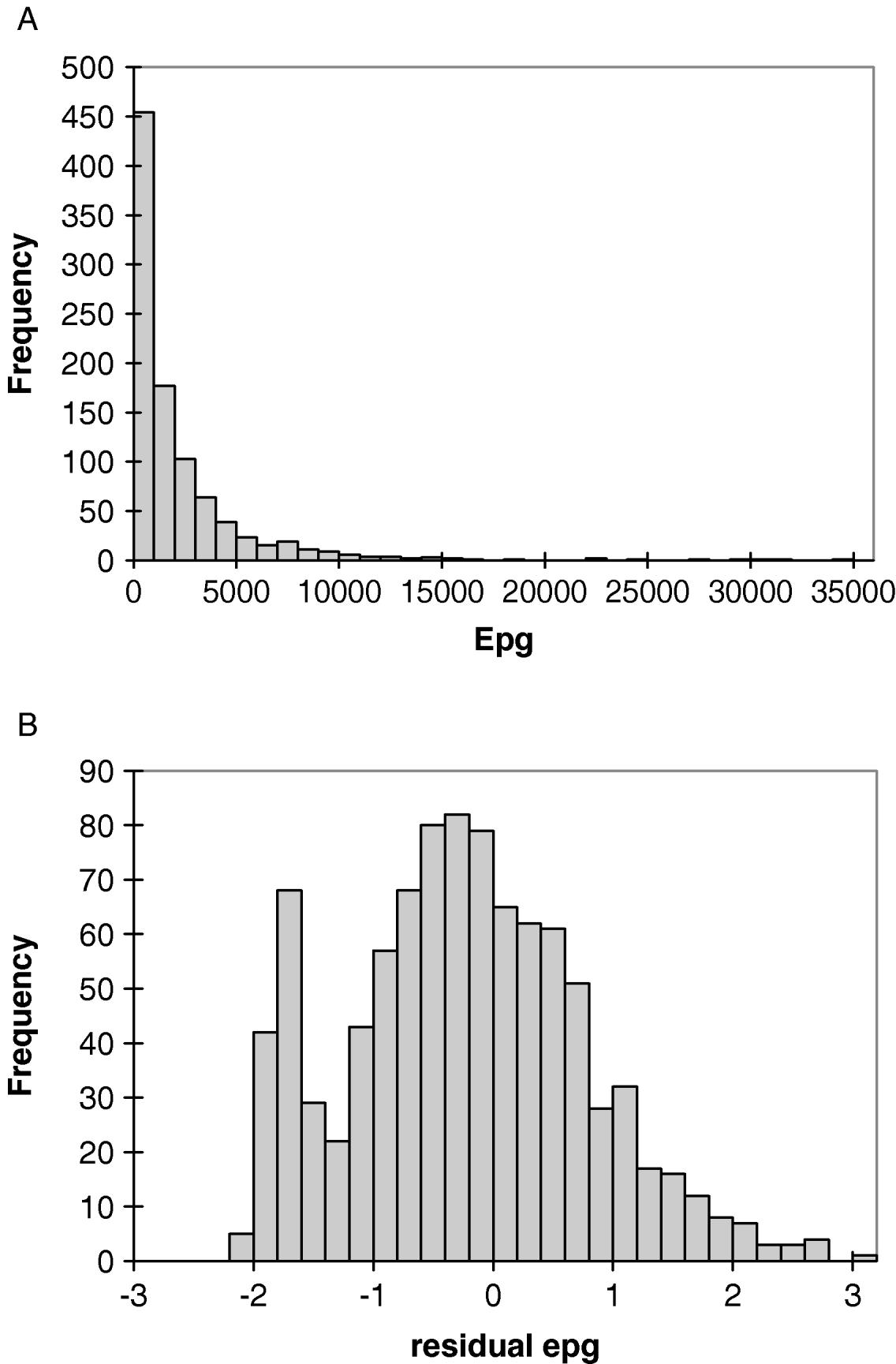
The prevalence of hookworm infection (95% CI) among 704 subjects eligible for the genetic analyses was 81% (78–84%) in 1998 and 90% (86–93%) in 2001, with geometric mean egg counts of 395 epg (n=620) and 495 epg (n=325), respectively. The age profile of infection intensity, assessed by epg, was monotonic pre-treatment, with a rapid increase in early childhood to a plateau being reached in young adults. Intensity was similar in males and females, with geometric means of 505 versus 303 epg in 1998 and 465 versus 534 epg in 2001. As expected, the frequency distribution of faecal egg counts was highly overdispersed (Fig. 1A). In contrast, the distribution of residuals was much closer to normal (skewness=0·30, kurtosis=0·16) (Fig. 1B).
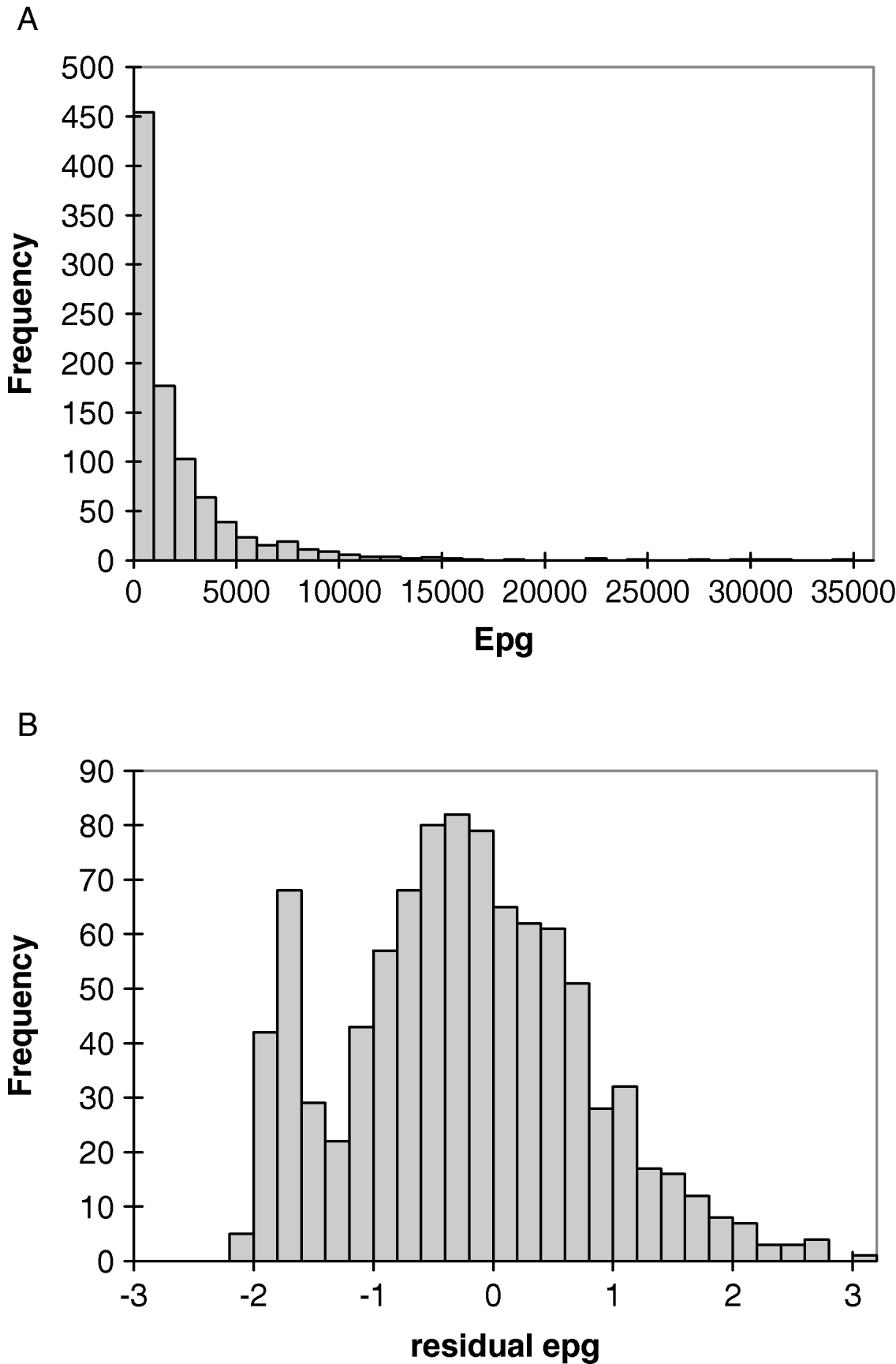
Fig. 1. Frequency distribution of (A) hookworm epg and (B) deviance residuals of epg for the Papua New Guinea study population.
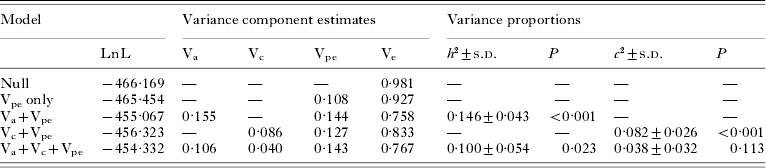
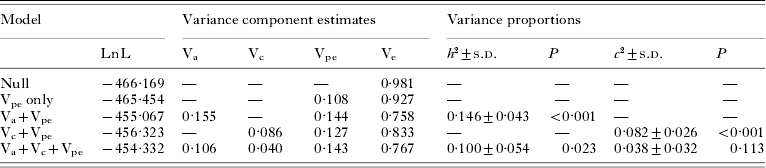
There was significant predisposition to high or low hookworm infection intensity in the study population, with a significant positive correlation between faecal egg counts from the same individuals in each year for all subjects treated in 1998 and re-examined in 2001 (r=0·216, 95% CI: 0·090–0·335, P<0·001, 232 d.f.). The parameter estimates obtained by variance component modelling for the entire population are presented in Table 1. The polygenic effects of quantitative trait loci on N. americanus infection intensity, as measured by the narrow-sense heritability of eggs per gramme of faeces, were clearly significant in the polygenic model, with heritability (h 2)=0·15±0·04. Likewise, household effects (Vc) were significant in the household-only model, with c 2=0·08±0·03. When jointly estimating Va and Vc, only genetic effects were significant (P=0·023). In this saturated model, the heritability and household effects were estimated to total h 2=0·10±0·05 and c 2=0·04±0·03 of the overall phenotypic variance, respectively.
Table 1. Variance components, heritability (h 2) and household effects (c 2) of hookworm infection intensity in Papua New Guinea
(Parameter estimates from REML models fitted to a total of 945 egg counts are presented. P-values refer to likelihood ratio tests for one additional variance component.)
Analysis of sex-specific trait determination
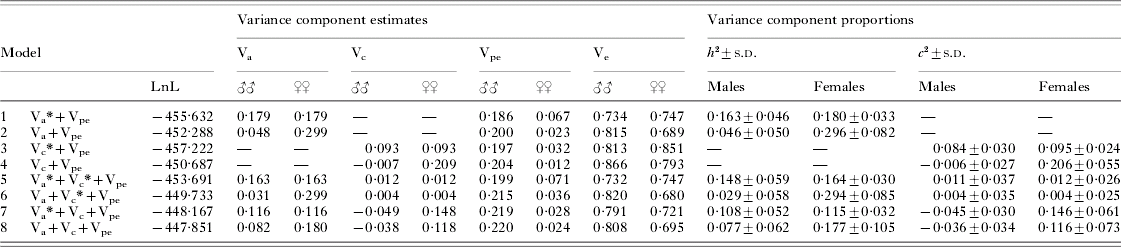
The degree of predisposition to hookworm infection in males and females was similar, with r(males)=0·171 (95% CI: −0·001–0·333) and r(females)=0·263 (95% CI: 0·073–0·435) and homogeneity of the two correlation coefficients not being rejected (P=0·5). Results from model fits estimating sex-specific variance components are presented in Table 2. Details of important LRT referred to in the following paragraphs are shown in Table 3. It should be noted that, since Vpe and Ve are free to vary in a sex-specific manner, Vtot and thus h 2 and c 2 may slightly differ between males and females even when Va and Vc are constrained to be equal in the two sexes.
Table 2. Sex-specific variance component analysis of heritability (h 2) and household effects (c 2) of hookworm infection intensity in Papua New Guinea
(Parameter estimates from models with variance components constrained to be equal in both sexes (indicated by an asterisk) or allowed to vary between males and females. For hypothesis testing see Table 3.)
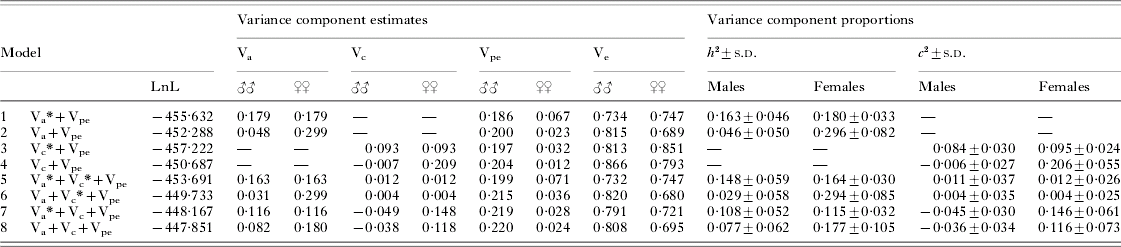
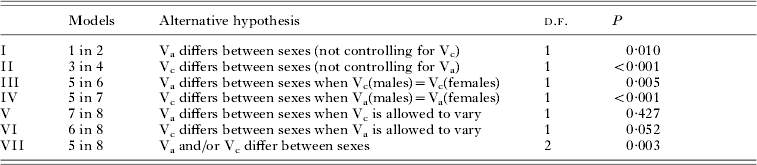
Table 3. Likelihood ratio tests for selected models presented in Table 2
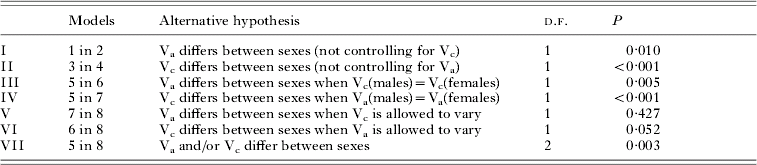
The estimates for both Va and Vc showed pronounced sex-differences in models allowing these parameters to freely vary between the sexes, with estimates being much higher in females, whereas Vpe was higher in males. In the saturated model (model 8), heritability in males was 0·08±0·06, compared to 0·18±0·11 in females, whereas the proportion of variation accounted for by household effects was −0·04±0·03 in males and 0·12±0·07 in females. Refitting the saturated model with c 2 constrained to be non-negative in males produced similar estimates of sex-specific heritability, with h 2 of 0·05±0·05 in males and 0·19±0·11 in females. Similar patterns were shown for models fitting only additive genetic or household effects (models 2 and 4, Table 2). Comparing these models with corresponding ones, forcing the respective component to be equal across sexes, indicated a significantly improved model fit of the sex-specific polygenic as well as household models (LRT I and II). The significance of these sex differences was further strengthened when controlling for a general household or polygenic effects component (LRT III and IV). In the saturated model, sex-differences in neither heritability (LRT V) nor household effects (LRT VI) were significant on their own, but a joint test of differences in either variance component was highly significant (P=0·003) (LRT VII). Thus, there are some ambiguities in clearly identifying the sex-specific determinants of the trait, likely a consequence of fitting increasingly complex models in a dataset of somewhat limited size. Nevertheless, it is clear that allowing sex-specific parameter estimation significantly improved the general, saturated model. Based on this sequence of results, the sex-specific saturated model incorporating additive genetic and household effects components (model 8) emerged as the most realistic reflection of the underlying structure of the trait.
Analysis of age-specific trait determination
The degree of predisposition to hookworm infection in children (<16 years old) and adults (16+ years old) was similar, with r(children)=0·206 (95% CI: 0·001–0·394) and r(adults)=0·223 (95% CI: 0·060–0·374) and homogeneity of the two correlation coefficients not being rejected (P=0·90). When the variance components were allowed to vary between children and adults, there was no evidence for significantly different heritabilities in any of the models (data not shown). In the saturated model, equivalent to model 8 above, heritability (h 2) was 0·15±0·09 in children and 0·12±0·07 in adults, with corresponding household effects (c 2) of 0·02±0·06 in children and 0·02±0·04 in adults; with no evidence for significant differences in either (LRT VII, P=0·69).
DISCUSSION
Quantitative genetic analysis of hookworm infection intensity (assessed by epg) in Papua New Guinea showed significant heritability of 0·15±0·04. The influence of additive genetic effects on infection intensity remained significant with heritability of 0·10±0·05, when controlling for household effects. More detailed modelling allowing for sex-specific trait structures revealed striking differences between males and females regarding heritability and other sources of inter-individual variation. The results indicated that, at least in females, the intensity of infection with N. americanus in the study population in Papua New Guinea is controlled, to some degree, by additive genetic factors, which account for approximately one fifth of the phenotypic variation. In contrast, additive genetic effects and household effects were much less important in males.
There has been limited previous study of the human genetic control of hookworm infection. The heritability of hookworm epg in a study in Zimbabwe (Williams-Blangero et al. Reference Williams-Blangero, Blangero and Bradley1997) was 0·37±0·09, without including household effects. More recently, data from Brazil suggest that host genes determine about a quarter of the variance in hookworm egg counts when controlling for household effects (Brooker et al. Reference Brooker, Bethony and Hotez2004). Significant narrow-sense heritability has been reported for a variety of other parasitic helminths of humans, with heritabilities varying from 0 to 0·42 (Quinnell, Reference Quinnell2003; Bethony and Quinnell, Reference Bethony and Quinnell2008). The present results are generally in accordance with these reports, although both the heritability and household effects in Papua New Guinea were relatively low. Heritability, as a function of several variance components, is a population-specific measure, and also depends on the quality of pedigree and phenotypic data and information on potentially confounding covariates. Furthermore, sample size and pedigree and household structures will influence the contributions of the various variance components, and the feasibility to statistically separate them in a given study population. Here, the median pedigree size was relatively low compared with other studies of humans, which will reduce power and may indicate missing pedigree information. More generally, more reliable estimates of heritability for helminth infection in humans will depend on improved knowledge of the environmental covariates of exposure (Bethony et al. Reference Bethony, Williams, Blangero, Kloos, Gazzinelli, Soares-Filho, Coelho, Alves-Fraga, Williams-Blangero, Loverde and Correa-Oliveira2002). Due to the tendency of more closely related individuals to share common environmental risk factors, not accounting for these risk factors will generally inflate heritability. Using household effects as a surrogate measure of shared environmental factors is unlikely to be accurate (Bethony et al. Reference Bethony, Williams, Blangero, Kloos, Gazzinelli, Soares-Filho, Coelho, Alves-Fraga, Williams-Blangero, Loverde and Correa-Oliveira2002), and partitioning of variance between these two overlapping random effects is difficult. Moreover, some genetic effects, particularly dominance effects, are very hard to separate from the shared household environment. Increased knowledge of the spatial, environmental and socio-economic risk factors for hookworm infection (Saathoff et al. Reference Saathoff, Olsen, Sharp, Kvalsvig, Appleton and Kleinschmidt2005; Brooker et al. Reference Brooker, Alexander, Geiger, Moyeed, Stander, Fleming, Hotez, Correa-Oliveira and Bethony2006) should allow more realistic variance components models to be analysed. The use of worm counts, rather than faecal egg counts, as a phenotype should also improve the accuracy of heritability estimates, as errors in the measurement of egg count are large (Anderson and Schad, Reference Anderson and Schad1985).
Both mean faecal egg counts, and their variances, were similar in males and females. However, when fitting models in which the variance components were allowed to vary between the sexes, estimates for additive genetic and shared household effects were consistently higher in females than males. The full model, with all variance components allowed variation between sexes, gave heritability in males and females of 0·08 and 0·18, and household effects of −0·04 and 0·12 respectively. The significance of the sex-differences varied from model to model. Under the most complex model specification, allowing both the additive genetic and household components to vary freely between the sexes, neither difference was significant in isolation, though a joint test was significant. However, as household effects themselves were not significant, retaining this parameter is conservative and potentially a model over-specification, and sex-differences in heritability were significant when household effects were constrained to be equal in males and females, or not included. Separate analyses of males and females showed neither significant h 2 nor c 2 in males, but significant h 2 in females when controlling for c 2 (data not shown). In summary, this analysis shows clearly sex-specific differences in genetic and household effects combined, with both being more important in females, and strongly suggests that heritability differs between males and females.
Interestingly, the variance attributable to permanent environment effects was consistently higher in males than in females. Thus, permanent factors contributing to male phenotype determination in the present study population apparently exist, but do not obey the structure of additive genetic or household effects. One caveat to the permanent effect analysis is that the number of subjects contributing 2 egg counts was low (n=241), leading to large confidence intervals for Vpe. However, these results are consistent with the sex-specific predisposition analysis, which showed comparable predisposition to infection in males and females, confirming results of earlier work in Papua New Guinea (Quinnell et al. Reference Quinnell, Slater, Tighe, Walsh, Keymer and Pritchard1993, Reference Quinnell, Griffin, Nowell, Raiko and Pritchard2001). In contrast, sex-dependent variation in predisposition to hookworm infection has been reported from other populations, with higher predisposition either in males (Bradley and Chandiwana, Reference Bradley and Chandiwana1990) or females (Haswell-Elkins et al. Reference Haswell-Elkins, Elkins, Manjula, Michael and Anderson1988). Besides differences in the genetic trait determination, behavioural differences between males and females leading to sex-specific patterns of exposure to risk factors could be responsible for such a phenomenon. Little is known about any such behavioural differences in the present study population in Papua New Guinea, though it is reasonable to hypothesize that women spend a larger proportion of their time in the proximity of the house. This proposal would be consistent with the sex-differences of household effects in our models, and suggests that males may acquire most infection away from the household, with consistent differences in exposure between males over a 3-year period. Interestingly, a previous longer term study in Papua New Guinea showed that predisposition in males, but not females, had declined to zero by 8 years after treatment (Quinnell et al. Reference Quinnell, Griffin, Nowell, Raiko and Pritchard2001). Thus, the permanent environmental effects for males appear not to be consistent in the longer term. The results emphasize that very different factors may lead to a similar degree of predisposition, and care must be taken when heterogeneous samples of individuals are examined together.
Sex-differences in the genetic determination of quantitative traits can result from, for example, sex-linked genes, sex:gene interactions and sex-specific gene:environment interactions. A number of studies have reported sex differences in the heritability of human quantitative traits, ranging from immunological (Ober et al. Reference Ober, Pan, Phillips, Parry and Kurina2006; Weiss et al. Reference Weiss, Pan, Abney and Ober2006) to physiological and psychological traits (Towne et al. Reference Towne, Blangero and Siervogel1993; Cho et al. Reference Cho, Guo, Iritani and Hallfors2006; Pilia et al. Reference Pilia, Chen, Scuteri, Orru, Albai, Dei, Lai, Usala, Lai, Loi, Mameli, Vacca, Deiana, Olla, Masala, Cao, Najjar, Terracciano, Nedorezov, Sharov, Zonderman, Abecasis, Costa, Lakatta and Schlessinger2006; Scurrah et al. Reference Scurrah, Byrnes, Hopper and Harrap2006). We are not aware of any previous analyses of sex-specific heritability in humans in the context of helminth infections, although heritability of nematode epg in a free-ranging sheep population was demonstrated to be similar in males and females (Coltman et al. Reference Coltman, Pilkington, Kruuk, Wilson and Pemberton2001). More generally, sex differences in susceptibility to parasitic infection are well-known, with males typically being more susceptible to parasites of several taxa, which may reflect stronger immune responses in females (Klein, Reference Klein2004). A number of immune responses are known to be involved in protective immunity to hookworms, including interleukin-5 and anti-hookworm IgE (Pritchard et al. Reference Pritchard, Quinnell and Walsh1995; Quinnell et al. Reference Quinnell, Pritchard, Raiko, Brown and Shaw2004; Bethony et al. Reference Bethony, Loukas, Smout, Brooker, Mendez, Plieskatt, Goud, Bottazzi, Zhan, Wang, Williamson, Lustigman, Correa-Oliveira, Xiao and Hotez2005). Such Th2 responses are also involved in the pathogenesis of asthma, such that it is of interest that sex-differences in genetic linkage and association patterns have been found in a study of asthma-related traits, with loci for various phenotypes reaching genome-wide significance either in females or males only, including a female total serum IgE locus not being found in males (Ober et al. Reference Ober, Pan, Phillips, Parry and Kurina2006). Studies of the heritability of potentially protective immune responses in hookworm-infected individuals would be informative. Protective immunity to helminth infections can increase with exposure and thus age, and it might be expected that heritability of egg counts would also vary with age. Increasing heritability of faecal egg count with age has been reported in sheep with Teladorsagia circumcincta infection, and may be linked to the development of IgA responses influencing worm length (Bishop et al. Reference Bishop, Bairden, McKellar, Park and Stear1996; Stear et al. Reference Stear, Bairden, Duncan, Holmes, McKellar, Park, Strain, Murray, Bishop and Gettinby1997). In contrast, the present study found no evidence for differences in heritability between adults and children (defined as <16 years old). However, the ability to estimate age-specific genetic parameters is limited here by available sample sizes, and it may not be appropriate to rule out age effects.
In conclusion, we have presented the first detailed heritability study for hookworm infection in humans that shows a significant influence of additive genetic factors on infection intensity when shared environmental (household) effects are controlled for. However, genetic and household factors were much more important in females compared to males in our Papua New Guinean study population. The results show that different factors can be important in determining infection intensity in males and females, even where mean infection intensities and levels of predisposition do not differ, and highlight the need to account for sex effects in future genetic linkage or association studies.
We are indebted to the patient participants of our study. Fieldwork was supported by a Medical Research Council Career Development Award to R. J. Q. L. P. B. received funding within the Leeds Marie Curie EST Programme ‘Advanced Genetic Analysis in the Post-Genomic Era’, and A. J. W. was funded by the Natural Environment Research Council. We thank Moses Bockarie, Michael Alpers and John Reeder for facilities in Papua New Guinea, and Stuart Davidson, Kay Nolan, Nandau Tarongka, Kerry Lorry and Chris Kum for assistance with fieldwork. Loeske Kruuk provided valuable comments on the manuscript.