INTRODUCTION
The mode of reproduction of a species is a major factor influencing its genetic structure and therefore its evolutionary trajectory (Lymbery et al. Reference Lymbery, Constantine and Thompson1997). In hermaphroditic organisms, individuals can either reproduce by self-fertilization (i.e. selfing: fusion of male and female gametes from the same individual) or cross-fertilization (i.e. out-crossing). These two different reproductive strategies can have drastic effects on the genetic structure of populations. For example, studies of plants have shown that, compared to species using out-crossing, predominantly selfing species show significant heterozygote deficiencies and linkage disequilibrium (Loveless and Hamrick, Reference Loveless and Hamrick1984; Heywood, Reference Heywood1991). Hermaphroditism is also widespread in animals but the frequency with which self-fertilization is preferred over cross-fertilization is unknown for most hermaphrodite species (Jarne, Reference Jarne1995).
While selfing can be seen as advantageous by removing the need for a sexual partner, it is also a severe case of inbreeding: both male and female reproductive cells originate from the same parent. The main cost of this strategy is thought to be genetic deficiencies arising from inbreeding depression and inducing fitness costs in offspring produced by self-fertilization (Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987). Because selfing increases homozygosity, inbreeding depression results from the accumulation of deleterious recessive alleles and/or the lower performance of homozygotes compared to heterozygote individuals (Trouvé et al. Reference Trouvé, Renaud, Durand and Jourdane1996). Genetically homogeneous offspring may also be less likely to survive fluctuating environments (Christen and Milinski, Reference Christen and Milinski2003). Thus, the lower fitness of inbred progeny should strongly select against self-fertilization and for the maintenance of reproduction via mating (Milinski, Reference Milinski2006). Whatever the causes of inbreeding depression, it is possible that its effects have played a part in the evolution of breeding systems avoiding, or at least reducing the occurrence of self-fertilization in natural populations. The benefits of out-crossing, as opposed to selfing, are thought to have even wider implications for life-history strategies of both plants and animals. For example, they may have lead to the evolution and maintenance of complex life cycles in parasites (Brown et al. Reference Brown, Renaud, Guegan and Thomas2001; Christen et al. Reference Christen, Kurtz and Milinski2002).
From simple beginnings, parasitic helminths have evolved complex life cycles so challenging that they impose strong selective pressures on these parasite species (Poulin and Cribb, Reference Poulin and Cribb2002; Poulin, Reference Poulin2007). For example, the typical life cycle of digenean trematodes involves 3 different hosts and as many transmission events. First, eggs are released by adult worms within the definitive host and later hatch into free-swimming larvae (miracidia) which must find and penetrate the mollusc first intermediate host. Second, miracidia develop and multiply into structures (sporocysts) producing free-living cercariae that emerge from the mollusc host to infect the next intermediate host where they encyst as metacercariae (Combes et al. Reference Combes, Bartoli, Théron, Lewis, Campbell and Sukhdeo2002). Third, metacercariae must be ingested, along with their intermediate host, by the appropriate definitive host, which is always a vertebrate, for the life cycle to be completed. Complex life cycles also provide several advantages to the parasites such as longer life span, greater adult body size, higher fecundity (Parker et al. Reference Parker, Chubb, Ball and Roberts2003) and, more importantly, greater access to sexual partners (Brown et al. Reference Brown, Renaud, Guegan and Thomas2001); facilitating cross-fertilization by concentrating conspecific individuals in the same predator definitive host (Poulin and Cribb, Reference Poulin and Cribb2002; Rauch et al. Reference Rauch, Kalbe and Reusch2005).
However, some trematodes have developed a radical adaptation with 1 host (sometimes 2), most often the vertebrate definitive host, being dropped from the cycle (Lefebvre and Poulin, Reference Lefebvre and Poulin2005a). The resulting abbreviated life cycle should be easier to complete as the number of transmission events is decreased (Poulin and Cribb, Reference Poulin and Cribb2002). Shorter life cycles are likely to be favoured in parasites faced with low or highly variable probabilities of transmission. In fact, life-cycle abbreviations from 3 to 2 hosts have occurred independently in numerous trematode lineages (Poulin and Cribb, Reference Poulin and Cribb2002; Lefebvre and Poulin, Reference Lefebvre and Poulin2005a, Reference Lefebvre and Poulinb). This is usually achieved via progenesis: metacercariae mature precociously while still inside the second intermediate host and produce viable offspring by selfing without the need to reach the definitive host. Given that progenesis could serve as a reproductive insurance against failed transmission or even extinction (Bush and Kennedy, Reference Bush and Kennedy1994), it is surprising that shorter life cycles are not more widespread among trematodes (Lefebvre and Poulin, Reference Lefebvre and Poulin2005c). It is possible that fitness costs associated with shorter life cycles offset the increased likelihood of completion. Two obvious costs are associated with offspring quality and quantity. First, fecundity may be reduced in abbreviated life cycles as egg production is likely to be much lower inside a small, short-lived invertebrate host than in the larger and longer-lived vertebrate definitive host (Poulin, Reference Poulin2001; Poulin and Cribb, Reference Poulin and Cribb2002; Lefebvre and Poulin, Reference Lefebvre and Poulin2005a). Second, trematodes adopting progenesis can only reproduce by selfing within their intermediate host because they are individually enclosed within a cyst, causing inbreeding. Therefore, shorter life cycles could lead to the production of offspring with low genetic heterogeneity, potentially less viable than offspring produced by out-crossing. The deleterious effects of homozygosity induced by self-fertilization are commonly believed to explain why progenesis is not more widespread (Lefebvre and Poulin, Reference Lefebvre and Poulin2005a).
It is also possible that trematode alternative life cycles, and hence reproductive mode, are genetically fixed. The co-existence of both normal and abbreviated cycles in parasite populations could thus reflect an underlying genotypic variation such that different parasite strains, using alternative life cycles, co-exist in the same population (Crossan et al. Reference Crossan, Paterson and Fenton2007). Because the availability of definitive hosts is likely to vary seasonally, each parasite strain's strategy may be periodically advantageous or detrimental but both could be maintained over time by environmental stochasticity. In this case, offspring of parasites reproducing through abbreviated life cycles should be more likely to adopt the shorter route themselves. Trematode species using both the normal three-host cycle and a shorter cycle as alternative strategies are consequently ideal models to determine the heritability of each strategy and to measure the effects of inbreeding depression on important fitness parameters such as infectivity, growth and virulence of the offspring produced by progenetic parasites (Wedekind et al. Reference Wedekind, Strahm and Schärer1998).
The trematode Coitocaecum parvum uses either the typical three-host life cycle or a truncated life cycle in which metacercariae mature precociously (progenesis) inside the amphipod second intermediate host and start producing both eggs and sperm. Progenetic metacercariae from amphipod hosts examined by Holton (Reference Holton1984a), although enclosed within their cyst, had active spermatozoa in the seminal vesicle and seminal receptacle and viable eggs are most likely produced by self-fertilization rather than asexually. Both strategies are observed synchronously in C. parvum populations with up to 60% of the parasites being progenetic (Lefebvre and Poulin, Reference Lefebvre and Poulin2005c) and, although environmental factors can influence the parasite strategy (Poulin, Reference Poulin2003; Lagrue and Poulin, Reference Lagrue and Poulin2007), it is still unclear why not all parasites use progenesis as a reproductive insurance against failed transmission. Because progenetic individuals are enclosed within cysts and can only self-fertilize, it has been suggested that their offspring could be of lower quality (Poulin, Reference Poulin2001). However, the viability and infectivity of C. parvum offspring produced by both progenetic individuals inside the second intermediate host and adult worms in the definitive host are yet to be compared. The adoption of either strategy in C. parvum could also be under genetic influence: offspring from progenetic parents simply adopting progenesis as an inherited feature and therefore reproducing by selfing, and vice-versa for individuals that out-crossed in the definitive host. Once again, this heritability hypothesis remains to be tested.
In this study, we used C. parvum to test for the effects of alternative life strategies on the fitness of offspring produced by selfing via the abbreviated cycle and by adult worms in the definitive host. Fitness parameters such as viability and/or infectivity to the next host, used in previous studies on inbreeding depression (Christen et al. Reference Christen, Kurtz and Milinski2002; Christen and Milinski, Reference Christen and Milinski2003; Milinski, Reference Milinski2006), were measured as an estimate of offspring fitness in C. parvum. We compared the hatching rate of eggs from both origins, infection success of free-living larvae (miracidia and cercariae) and asexual multiplication within the snail first intermediate host. Furthermore, testing whether trematode life cycles, and hence reproductive strategies, are genetically fixed or environmentally adaptable is an important step towards the understanding of the evolution of complex life cycles in parasites. By experimentally infecting crustacean intermediate hosts, we were also able to compare the proportions of progenetic individuals between metacercariae from selfing and out-crossing parents and consequently determine whether progenesis and life-cycle abbreviation are inherited characters in C. parvum.
MATERIALS AND METHODS
Life cycle of Coitocaecum parvum
Coitocaecum parvum (Trematode, Opecoelidae) is a common parasite of freshwater fish in New Zealand, mostly the common bully (Gobiomorphus cotidianus; Macfarlane, Reference MacFarlane1939; Holton, Reference Holton1984b). Eggs produced by adult worms inside the fish gut are released in host faeces and hatch into free-swimming miracidia. These penetrate mud snails (Hydrobiidae, Potamopyrgus antipodarum) in which they mature as a mother sporocyst before asexually producing daughter sporocysts. Each daughter sporocyst then asexually produces cercariae that actively leave the snail host and enter the amphipod Paracalliope fluviatilis where they encyst as metacercariae in the body cavity. At this stage, metacercariae can either await ingestion by a fish where they will mature and reproduce, or keep growing and reach maturity while still inside the amphipod. Worms that reach maturity in the crustacean intermediate host reproduce by selfing and lay eggs that are contained in their cyst (Holton, Reference Holton1984a; Poulin, Reference Poulin2001); eggs are released after host death and decomposition. Both alternative life cycles occur simultaneously in natural C. parvum populations.
Animal collection
Snails, amphipods and fish were all collected in Lake Waihola, South Island, New Zealand during spring and summer 2006. Amphipods and snails were captured by dragging a dip net (mesh size 500 μm) through macrophytes (Myriophyllum triphyllum). All amphipods and snails were returned alive to the laboratory and kept separated in aerated lake water with strands of M. triphyllum as food source. Fish were captured using a seine net and kept in groups of 10 in 25-litre containers filled with aerated lake water. Fish were fed exclusively with live amphipods from Lake Waihola for several weeks to increase the intensity of infection with C. parvum. Natural prevalence of C. parvum infection in bullies is usually 100% and mating pairs are regularly observed during dissection. However, the average infection intensity is low (between 5 and 10 worms per fish; Poulin, Reference Poulin2001; Lefebvre and Poulin, Reference Lefebvre and Poulin2005b), although some naturally infected fish can contain over 30 and up to 70 C. parvum adults (Lefebvre and Poulin, Reference Lefebvre and Poulin2005b; personal observations). Bullies experimentally fed on amphipods contained between 60 and 100 adult C. parvum with an average of around 75. Adult C. parvum gather in the rectal region of the fish gut and, even in naturally and artificially heavily infected hosts, we did not observe displacement of individuals to other areas of the digestive tract of the host that might be expected with overcrowding (personal observations). Therefore, by increasing the encounter rate between individual parasites, high parasite densities should enhance the opportunities for cross-fertilization without modifying parasite behaviour. In fact, all laboratory fed bullies contained mating pairs of parasites at the time of dissection and, on average, 5 mating pairs were found per fish (personal observations). However, this only represents a snapshot of the situation as these parasites can store sperm after mating. In such high densities, although only a low proportion of worms are actually observed mating, most if not all individuals have had access to a mating partner and possibly stored sperm for cross-fertilization. The number of eggs produced by selfing in these worms should therefore be much lower than in progenetic metacercariae (Lefebvre and Poulin, Reference Lefebvre and Poulin2005b).
Hatching success of eggs
C. parvum eggs were obtained from both progenetic metacercariae and adult worms. Because the prevalence of C. parvum is usually low (<10%) in second intermediate hosts, amphipods were screened under a dissecting microscope and only individuals judged as infected were dissected (Lefebvre and Poulin, Reference Lefebvre and Poulin2005b). In total, 150 amphipods were found to contain a progenetic metacercaria. Approximately 35 eggs per metacercaria were taken and transferred into a Petri dish containing 10 ml of filtrated lake water. Eggs from adult C. parvum in fish were obtained by dissecting the experimentally fed bullies. Adult worms found in fish guts were kept for 24 h in lake water to induce them to release their eggs. After that time, all eggs liberated by the parasites were transferred to another Petri dish filled with water. Typically, only few eggs are released daily by adult parasites (Lefebvre and Poulin, Reference Lefebvre and Poulin2005b) and, in our study, we obtained 400 adult C. parvum that released around 3 eggs each. The large number of parasites used as sources of eggs for both categories should reduce the potential confounding factor of individual variation in egg quality. The 2 pools of eggs (from progenetic metacercariae and from adult worms; i.e. ‘progenetic’ and ‘normal’ eggs) were separated into groups of 20–25 and transferred to 96-well microtitre plates, each well filled with 200 μl of lake water. The number of hatched eggs was counted everyday until all were hatched or no more were seen to be hatching. At the end of the experiment, 450 eggs of each origin (progenetic or normal) were haphazardly chosen and measured, 300 that hatched and 150 that failed to hatch. Assuming a regular ellipsoid shape, individual egg volume was calculated as Vegg=(π∗L∗W2)/6, where L and W are egg length and width, respectively. The proportions of hatched eggs from progenetic metacercariae and adult worms were compared using a Fisher's exact test. A two-way ANOVA with egg type (progenetic or normal) and hatching status (hatched or failed) as independent factors, and egg volume as the dependent variable, was also used to test for any size differences between each type of egg. Egg volume was log-transformed before analysis to normalize the data.
Infectivity of miracidia to the snail first intermediate host
Eggs were again obtained from both progenetic individuals and adults as described above. They were then transferred by groups of 10 to Petri dishes containing 4 ml of lake water and 1 uninfected snail per dish was later added. Eggs were protected from the snail by a wire-netted circular cage (10 mm diameter; mesh size 0·5 mm) glued to the bottom of the container. Uninfected snails used in this experiment were selected by size and shell shape. Altered shell shape being characteristic of infection by C. parvum, any individual displaying unusual shells were discarded (Lagrue et al. Reference Lagrue, McEwan, Poulin and Keeney2007a). Additionally, 100 snails judged as uninfected were dissected to verify the accuracy of our screening technique and none were found to be infected by C. parvum. Only immature snails (2 mm <shell length <3 mm at the time of experimental infections) were used for this experiment as mature snails are less likely to get infected (Zakikhani and Rau, Reference Zakikhani and Rau1999; personal observation). Petri dishes were checked everyday until 1 egg hatched. At that time, the remaining eggs were taken out of the Petri dish; each snail was therefore exposed to only 1 miracidium. Snails were kept individually in lake water and fed with strands of macrophytes for the rest of the experiment. A total of 600 snails were exposed to 1 C. parvum larva each, half of those to miracidia from progenetic eggs and the other half from eggs laid by adult worms. After 10 weeks, half of the snails in each treatment were measured to assess for possible differences in shell growth and their shell volume was calculated using the formula V=1/3(πr2h) where V is the volume, r the radius (half the width of whorl 1) and h the vertical height (total length; see Lagrue et al. Reference Lagrue, McEwan, Poulin and Keeney2007a for details). Snails were then dissected to assess for infection by C. parvum. When infections were found, the number of sporocysts was counted. The remaining snails were used to obtain cercariae for subsequent experimental infections of amphipods. These were also measured to assess for possible differences in shell growth, and dissected 10 months post-infection and again, both the proportion of C. parvum infected snails and the number of sporocysts were recorded. The proportions of snails infected by miracidia from selfed and out-crossed eggs were compared using Fisher's exact tests. The effect of miracidial origin (selfed or out-crossed eggs) on the number of sporocysts inside the snail was tested using a non-parametric test (Mann-Whitney U test).
Infectivity of cercariae to the amphipod second intermediate host and life-history strategy of metacercariae
Uninfected amphipods used for experimental infections were obtained by inspecting each amphipod under a microscope and discarding all amphipods that showed any sign of infection, i.e. an opaque mass in the body cavity corresponding to a metacercaria. This method allows the selection of only uninfected individuals with an accuracy of about 95% (Lefebvre and Poulin, Reference Lefebvre and Poulin2005b). Also, only male amphipods of similar size (mean body length of 3·0±0·0 mm, maximum 3·2 mm and minimum 2·8 mm respectively) were used to reduce the effects of host conditions on the parasite. Snails experimentally infected in the previous experiment were used as sources of cercariae for this part of the study. Cercariae of C. parvum were obtained under controlled conditions to ensure that the cercariae used to experimentally infect amphipods had recently emerged and, therefore, were more likely to penetrate the amphipod. For each round of experimental infections, snails were transferred to large Petri dishes filled with 10 ml of filtrated lake water. Snails were incubated at 25°C for 20 min under constant light, conditions known to induce cercarial release (Hay et al. Reference Hay, Fredensborg and Poulin2005). The Petri dishes were then screened under a microscope and the cercariae transferred to 500 μl Eppendorf tubes using a 20 μl micropipette. One cercaria was placed in each tube with 2·5 μl of filtrated lake water and an uninfected amphipod was then added. Amphipods were left in the tube along with the cercariae for 5 h, a time after which unsuccessful cercariae stop moving and die. Amphipod survival, at this stage, is usually over 99%. For logistical reasons, amphipods were then haphazardly separated into groups of 5 individuals and placed in plastic Falcon tubes filled with 10 ml of lake water and a strand of macrophyte (Elodea canadensis) was added for food. After 5 weeks, a period sufficient for C. parvum to adopt one or the other strategy (Lagrue and Poulin, Reference Lagrue and Poulin2007), the 412 surviving amphipods were dissected under the microscope to assess their infection status. Metacercariae found in amphipods were recorded as dead (i.e. encapsulated and melanized; Thomas et al. Reference Thomas, Guldner and Renaud2000; Bryan-Walker et al. Reference Bryan-Walker, Leung and Poulin2007) or alive (i.e. moving and within a transparent cyst). Live individuals were measured (length and width) and recorded as ‘normal’ (non egg-producing worm) or ‘progenetic’ (egg-producing worm); in the case of progenetic parasites, eggs were also counted. These included both eggs released by the worm in its thin-walled cyst and eggs still in utero. The body surface of each parasite was then determined and used as a surrogate for body size. This was done using the formula for an ellipsoid, (πLW)/4, where L and W are the length and width of the parasite. The proportion of cercariae, from progenetic and normal descent, successfully penetrating the host and then developing into metacercariae, as well as the proportion of metacercariae adopting progenesis among offspring originating from either reproductive strategy were compared using Fisher's exact tests. The possible effects of parental strategy on metacercarial body size and egg production were assessed using non-parametric tests (Mann-Whitney U test).
RESULTS
Hatching success of eggs
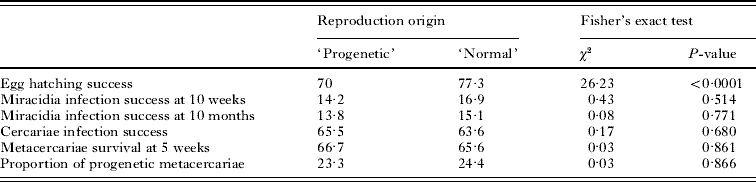
A total of 6415 eggs, 5170 from progenetic metacercariae and 1245 from adult worms, were used in this study. Eggs were observed hatching from day 1 to day 35 and no more eggs hatched after that time. The overall hatching success was 71·4% but there was a significant difference in the hatching success between eggs from progenetic metacercariae (70%) and those from adult worms (77·3%; Table 1). The daily hatching rate of each type of egg was also clearly distinct (Fig. 1): eggs laid by adult worms did not start hatching before day 9 and a large majority (91·7%) hatched between day 12 and day 20. In contrast, eggs from progenetic individuals started hatching from day 1 and a steadier hatching rate was observed during the experiment. Progenetic eggs were also significantly larger (mean±S.E., 112·95±1·1×103 μm3) than normal eggs (108·9±1·1×103 μm3; ANOVA, F1,896=19, P<0·0001; Fig. 2) and, overall, failed eggs were significantly larger than eggs that successfully hatched (128·83±1·5 and 101·97±0·6×103 μm3 respectively; ANOVA, F1,896=361, P<0·0001). However, there was a significant interaction between the two factors (ANOVA, F1,896=14, P=0·0002): while failed progenetic eggs were much larger than failed normal eggs (134·76±2 and 122·89±2·1×103 μm3 respectively; Fisher's LSD, D.F.=896, P<0·0001), there was no significant difference between progenetic and normal eggs that successfully hatched (102·05±0·8 and 101·89±1×103 μm3 respectively; Fisher's LSD, D.F.=896, P=0·625; Fig. 2).

Fig. 1. Daily hatching rate in terms of the proportion (%) of the total number of eggs (‘progenetic’ or ‘normal’) that had hatched at the end of the experiment.

Fig. 2. Mean volumes (±S.E.) of ‘progenetic’ and ‘normal’ eggs, overall and for eggs that successfully hatched into miracidia or failed to hatch and died. Statistically significant differences (P<0·05) are marked by (*).
Table 1. Results of Fisher's exact tests for comparison between offspring from ‘progenetic’ and ‘normal’ eggs
(Note that all values for fitness measurements correspond to proportions (%) of success.)
Infectivity of miracidia to the snail first intermediate host
After 10 weeks, none of the 600 experimentally infected snails were producing cercariae. Among the 300 snails dissected at that time, the mortality was very low (1%) and snail growth was minimal. The overall prevalence of C. parvum was 15·5% and no difference was found in the proportion of infected snails between individuals exposed to miracidia from progenetic (14·2%) and normal eggs (16·9%; Table 1). After 10 months, the mortality among the 300 remaining snails was 18·4% and C. parvum prevalence was 14·5%. The mortality was similar between the two treatment groups (19·4% and 17·4% respectively, Fisher's exact test, χ2=0·21, P=0·648) and again, no difference was found in the proportions of infected snails between individuals exposed to miracidia from progenetic (13·8%) and normal eggs (15·1%; Table 1). Although there was a slight difference in size (shell volume) between snails exposed to progenetic and normal miracidia (3·77±0·14 and 3·30±0·12 mm3 respectively; Mann-Whitney U test, Z=2·406, P=0·016), we found no size difference among snails actually infected by C. parvum (2·29±0·23 and 2·03±0·14 mm3 respectively; Mann-Whitney U test, Z=0·466, P=0·641). Consequently, any difference in the number of sporocysts between snails infected by progenetic or normal miracidia would not be influenced by snail size. The reproductive origin of miracidia (progenetic or normal eggs) had no effect on the mean number of sporocysts inside snail hosts, either after 10 weeks (6·7±0·4 and 6·8±0·4 respectively; Mann-Whitney U test, Z=−0·073, P=0·941) or 10 months (44·1±3·6 and 41·6±3·4 respectively; Mann-Whitney U test, Z=0·449, P=0·653).
Infectivity of cercariae to the amphipod second intermediate host and life-history strategy of metacercariae
A total of 1030 amphipods were experimentally exposed to C. parvum; 511 to cercariae descending from selfed (progenetic) eggs and 519 from eggs obtained from adult worms in fish. After 5 weeks, 412 of the treated amphipods (40%) were still alive (206 in each group); high mortality is commonly observed in laboratory maintained P. fluviatilis (Lagrue and Poulin, Reference Lagrue and Poulin2007). Of these 412 individuals, 266 (64·6%) had been successfully infected by C. parvum although 33·8% of the cercariae (90 out of 266) that penetrated the amphipod did not survive and were encapsulated by the host immune system. We found no difference in the proportion of cercariae, produced by either progenetic or normal parents, successfully penetrating the host (65·5% and 63·6% respectively; Table1) and subsequently surviving to develop into metacercariae (66·7% and 65·6% respectively of cercariae that penetrated the host; Table1). Thus, there was no difference in the probability of a cercaria being encapsulated depending on its parental origin (normal or progenetic: 34·4 and 33·3% respectively; Fisher's exact test, χ2=0·03, P=0·86).
There was no difference in the proportion of metacercariae adopting the progenetic developmental strategy between offspring of progenetic and normal parents (23·3% and 24·4% respectively; Table1). Furthermore, the parental reproductive strategy (selfing or out-crossing) did not seem to influence the fitness of the offspring, either in terms of metacercariae body size (0·054±0·005 and 0·057±0·005 mm2; respectively; Mann-Whitney U test, Z=0·786, P=0·432) or egg production of progenetic individuals (5·3±1·3 and 7·5±1·5 respectively; Mann-Whitney U test, Z=−0·926, P=0·354).
DISCUSSION
Inbreeding depression has long been thought to reduce the genetic value of inbred progeny, with strong deleterious effects on the fitness of plants and animals having been documented (see Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987 for review). This includes parasitic organisms: for example, the effects of inbreeding depression on the fitness of the cestode Schistocephalus solidus include reduced growth, lower infection and transmission success as well as limited intraspecific competitive ability (Christen et al. Reference Christen, Kurtz and Milinski2002; Christen and Milinski, Reference Christen and Milinski2003; Milinski, Reference Milinski2006). Whatever induces these deleterious effects, inbreeding depression is often strong and should drive the evolution of breeding systems and life-history strategies that avoid inbreeding (Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987). However, in this study, we found no evidence of such depression on any fitness parameter of C. parvum offspring. Although the expression of inbreeding depression depends largely on the environment in which its effects are tested, our experimental setups should have allowed us to detect some of these potential effects. Still, the progeny of progenetic metacercariae and adult worms were equally successful in terms of infectivity to snail and amphipod intermediate hosts, asexual multiplication inside snails, and survival and growth inside the amphipod host.
The only difference found in our study was in the hatching success of eggs: a significantly higher proportion of eggs produced by adult worms in fish hatched into miracidia. However, this is unlikely to be the effect of inbreeding depression but rather due to the different mechanisms of egg release. Adult C. parvum lay their eggs into the gut lumen of their fish definitive host; the eggs are rapidly released to the environment along with host faeces (Poulin and Cribb, Reference Poulin and Cribb2002). These eggs are then free to hatch when the miracidium has developed. Because progenetic metacercariae are contained inside the body cavity of their amphipod host, eggs can be released only after the host has died and decomposed; waiting for host death is the most common way by which progenetic parasites release their eggs (Lefebvre and Poulin, Reference Lefebvre and Poulin2005a). Some trematode species show high levels of virulence to accelerate host death and therefore the liberation of their eggs (Pampoulie et al. Reference Pampoulie, Lambert, Rosecchi, Crivelli, Bouchereau and Morand2000), although Coitocaecum parvum seems to lower its virulence to extend host life and thus to maximize its egg output (Poulin, Reference Poulin2001). While this might be advantageous in terms of total egg production, it also means that eggs are trapped within the host for prolonged periods of time, waiting to reach the external environment to hatch. This could explain both the observed volume difference between progenetic and normal eggs and the peak of progenetic egg hatching on day 1 post-release. Eggs do not hatch within the cysts and those that are retained for longer than the optimal incubation period inside the amphipod should hatch immediately or might not be viable anymore when they eventually reach the external environment, hence the lower hatching success. At the same time, it is possible that eggs of progenetic metacercariae have a slower development when contained within the cyst, waiting to be released from the host to achieve maturation and hatch, hence the longer hatching time observed in this study and the bimodal shape of the daily hatching rate distribution for progenetic eggs. On the other hand, eggs contained within the amphipod host are protected against potential pathogens (fungi or bacteria) and predation; for instance, snails actively graze on the substrate and consume the eggs (personal observations). Eggs produced by adult worms in the fish host are quickly released in the external environment and remain unprotected during the incubation period, on average 15 days and virtually no eggs hatched before 12 days. Progenetic eggs that hatch during that time after host death, almost a quarter (22·6%) in our study, should be less likely to be predated upon or infected by pathogens, therefore compensating for the lower overall hatching success of eggs produced in the amphipod host.
It has been suggested that high levels of inbreeding should purge the population of deleterious alleles and mutations and therefore quickly reduce or eliminate the effects of inbreeding depression (Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987). Previous studies on trematodes have shown that some species can survive using self-fertilization without noticeable deleterious effects, at least for a few generations (see Lefebvre and Poulin, Reference Lefebvre and Poulin2005a for review). Producing eggs by selfing inside the second intermediate host can be seen as a cheap reproductive insurance against failed transmission (Wang and Thomas, Reference Wang and Thomas2002), particularly if there is no cost associated with inbreeding. It is then possible that progenesis in the second intermediate host is actually not an alternative life-history strategy, when transmission to the definitive host fails, but the preferred reproductive strategy used by C. parvum; the high rate of selfing in C. parvum populations could cause a constant purging of strongly deleterious alleles, reducing or even eliminating the short-term effects of inbreeding depression (Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987).
Indeed, quantitative models have shown that the balance between the advantages of self-fertilization and costs of inbreeding depression should result in populations using either selfing or out-crossing only (Charlesworth et al. Reference Charlesworth, Morgan and Charlesworth1990). In any given population, C. parvum should use preferentially one or the other reproductive strategy. However, it has also been predicted that environmental stochasticity could produce evolutionarily stable mixed mating systems in which both strategies co-occur (Cheptou and Dieckmann, Reference Cheptou and Dieckmann2002). In the case of C. parvum, environmental stochasticity in the second intermediate host is likely to be high due to the host itself (host sex, size, age, body condition and stress levels) and/or competition (intra or interspecific) between co-infecting parasites, and could either prevent or induce metacercariae to adopt progenesis (Lagrue and Poulin, Reference Lagrue and Poulin2007). C. parvum may use progenesis whenever possible but still have to use the fish definitive host when necessary: limited space and resources in the amphipod host, intra and or interspecific competition is known to limit parasite growth and prevent any egg production (Lagrue and Poulin, Reference Lagrue and Poulin2008b). In this situation, C. parvum populations may be mainly inbred with only a few individuals descending from outcrossing parents. Previous genetic studies have detected very low levels of heterozygosity and high linkage disequilibrium in C. parvum populations (Lagrue et al. Reference Lagrue, McEwan, Poulin and Keeney2007a, Reference Lagrue, Waters, Poulin and Keeneyb; unpublished data); both are characteristics of predominantly self-fertilizing populations (Lymbery et al. Reference Lymbery, Constantine and Thompson1997; Annan et al. Reference Annan, Durand, Ayala, Arnathau, Awono-Ambene, Simard, Razakandrainibe, Koella, Fontenille and Renaud2007). However, the presence of heterozygous genotypes in populations of C. parvum shows that some outcrossing does occur (Lagrue et al. Reference Lagrue, McEwan, Poulin and Keeney2007a, Reference Lagrue, Waters, Poulin and Keeneyb; unpublished data). In fact, within fish dissected for this study, some mating pairs were observed, providing direct evidence for sexual reproduction. Still, even though the relative importance of each reproductive strategy is unknown, progenesis could easily become the main way to produce offspring, especially in situations where fish are scarce or absent, as there are no obvious costs linked to inbreeding depression. It is also possible that adult worms in fish use selfing as well as outcrossing when sexual partners are scarce, thus increasing even further the inbreeding coefficient of the population (Lefebvre and Poulin, Reference Lefebvre and Poulin2005a). However, in our study, a substantial number of offspring from adult C. parvum should be produced by cross-fertilization as access to mating partners was not limited and mating was indeed seen to happen; a comparison with the purely selfed offspring of progenetic worms should thus detect fitness differences, if there are any.
Finally, the proportion of progenesis observed in the next generation was comparable in offspring from progenetic metacercariae and those from adults. This shows that the reproductive strategy adopted by parents is not strictly heritable, i.e. it has no effect on the strategy adopted by their offspring, at least in the first generation. It suggests that life-cycle truncation in C. parvum is a conditional strategy rather than genetically predetermined (Poulin and Cribb, Reference Poulin and Cribb2002), therefore corroborating our previous hypothesis that the adoption of progenesis by metacercariae depends on the environment provided by the amphipod host in which metacercariae develop (Lagrue and Poulin, Reference Lagrue and Poulin2007, Reference Lagrue and Poulin2008a, Reference Lagrue and Poulinb).
In conclusion, inbreeding is unlikely to have any effect on the quality of offspring produced by metacercariae selfing in their second intermediate host. Furthermore, it seems that the progenetic strategy is not inherited from parents. Nevertheless, if there are no genetic costs of producing offspring by selfing and no genetic basis of progenesis, then why don't all metacercariae adopt the shorter life cycle and reproduce by selfing? First, it is possible that progenetic metacercariae in their second intermediate host cannot produce as many eggs as adult worms in the fish (Lefebvre and Poulin, Reference Lefebvre and Poulin2005a) and the two strategies may have on average equal offspring output: progenesis insuring the production of at least some eggs whereas reaching the definitive host provides a low probability of higher fecundity (Poulin, Reference Poulin2001). Second, it is also possible that not all C. parvum individuals can achieve progenesis in the amphipod intermediate host. The amphipod host might be ingested by a fish definitive host before any egg production occurs, in which case the parasite would have no other solution but to reproduce in the fish. Also, progenesis is characterized by a drastic increase in the size of metacercariae, egg production being positively correlated with parasite size (Lagrue and Poulin, Reference Lagrue and Poulin2007). Therefore, intrinsic host resources and/or intra-host competition between individual parasites (hetero or conspecific) can restrict the growth of metacercariae and prevent the production of eggs, leaving no choice to the parasite but to wait for transmission to the definitive host (Lagrue and Poulin, Reference Lagrue and Poulin2008b). Overall, a multitude of interconnected factors have been documented to influence the life-history strategy of Coitocaecum parvum (Poulin, Reference Poulin2001, Reference Poulin2003; Lagrue and Poulin, Reference Lagrue and Poulin2007,Reference Lagrue and Poulin2008a, Reference Lagrue and Poulinb). Whatever the reason for the co-occurrence of the two strategies, from our results, it is likely that inbreeding depression has little if any short-term effects on the maintenance of the normal three-host cycle in Coitocaecum parvum populations.
This research was supported by a grant from the Marsden Fund (The Royal Society of New Zealand) to R. P.





