Introduction
The term ‘haplosporosome’ was coined for cytoplasmic bodies defined as, ‘membrane-bound, osmiophilic structures which are oval, spherical, pyriform or short rods in the haplosporidian Urosporidium crescens’ (Perkins, Reference Perkins1971). This term was then applied to morphologically similar organelles in the paramyxid Marteilia refringens (Perkins, Reference Perkins1976, Reference Perkins1979) because they form in plasmodia, disappear during sporogenesis and reform in spores, as in haplosporidians. Paramyxids were therefore placed with haplosporidians in the class Haplosporea (Perkins, Reference Perkins1976). Similar cytoplasmic organelles in the sporoplasm of a myxosporean myxozoan, Hoferellus gilsoni, that were reminiscent of haplosporosomes, were given the name ‘sporoplasmosomes’ (Lom et al., Reference Lom, Molnár and Dyková1986). Other myxozoan parasites that infect fish and bryozoans, the malacosporeans, contain cytoplasmic electron dense bodies (EDBs) (Seagrave et al., Reference Seagrave, Bucke and Alderman1980; Morris et al., Reference Morris, Adams and Richards2000), which are also called sporoplasmosomes (Morris and Adams, Reference Morris and Adams2008).
Before electron microscopy and molecular phylogenies haplosporidians and paramyxids were classified together in the phylum Ascetosporea, then separated and given phylum status. Subsequently, the Ascetosporea was resurrected as a class in the phylum Cercozoa (Berthe et al., Reference Berthe, Le Roux, Adlard and Figueras2004; Burreson and Ford, Reference Burreson and Ford2004), and currently, they are regarded as endomyxan rhizarians (Cavalier-Smith et al., Reference Cavalier-Smith, Chao and Lewis2018). The class Ascetospora comprises the orders; (1) the Haplosporida (Hartikainen et al., Reference Hartikainen, Ashford, Berney, Okamura, Feist, Baker-Austin, Stentiford and Bass2014a), (2) the Mikrocytida (Hartikainen et al., Reference Hartikainen, Stentiford, Bateman, Berney, Feist, Longshaw, Okamura, Stone, Ward, Wood and Bass2014b), (3) the Paramxyida (Ward et al., Reference Ward, Bennett, Bateman, Stentiford, Kerr, Feist, Williams, Berney and Bass2016), (4) the Claustrosporida, and (5) the Paradinida (Bass et al., Reference Bass, Chao, Nikolaev, Yabuki, Ishida, Berney, Pakzad, Wylezich and Cavalier-Smith2009; Ward et al., Reference Ward, Neuhauser, Groben, Ciaghi, Berney, Romac and Bass2018). The Claustrosporida comprises two species, Claustrosporidium gammari and C. aselli which infect the amphipod Rivulogammarus pulex (see Larsson, Reference Larsson1987). They differ from haplosporidians in having spores that lack an orifice, but molecular data are not available and spore form may not be of taxonomic importance (Burreson and Ford, Reference Burreson and Ford2004). While haplosporidans, paramyxidans and claustrosporidans have haplosporosomes, mikrocytidans appear not to (Hine et al., Reference Hine, Bower, Meyer, Cochennec-Laureau and Berthe2001a ). The spot prawn parasite (Meyers et al., Reference Meyers, Lightner and Redman1994; Bower and Meyer, Reference Bower and Meyer2002) was placed at the base of haplosporidian phylogeny (Reece et al., Reference Reece, Siddall, Stokes and Burreson2004), but appears to be more closely related to Paradinium spp. (Skovgaard and Daugbjerg, Reference Skovgaard and Daugbjerg2008) and is not included here. The phylum Haplosporidia comprises 4 described genera (Urosporidium, Haplosporidium, Bonamia, Minchinia) and several unclassified species (Hartikainen et al., Reference Hartikainen, Ashford, Berney, Okamura, Feist, Baker-Austin, Stentiford and Bass2014a). Forms attributed to possible spore-like stages of B. exitiosa (Fig. 6 in Hine, Reference Hine1991, Figs 12–16 in Hine et al., Reference Hine, Cochennec-Laureau and Berthe2001b) have not been reported from B. exitiosa elsewhere, and are probably a different haplsporidian representing an undescribed genus. The features of most ultrastructurally described haplosporidians were reviewed in 2009 (Hine et al., Reference Hine, Carnegie, Burreson and Engelsma2009), and the reader is referred to that paper for details.
Myxozoans were earlier thought to be related to paramyxids because both have cells contained within other cells and show early development of somatic elements (Desportes and Lom, Reference Desportes and Lom1981; Berthe et al., Reference Berthe, Le Roux, Adlard and Figueras2004). However, electron microscopy showed myxozoan spores contained polar capsules similar to cnidarian nematocysts (Lom and de Puytorac, Reference Lom and de Puytorac1965) and now myxozoans are recognized as cnidarians (Siddall et al., Reference Siddall, Martin, Bridge, Desser and Cone1995; Jiménez-Guri et al., Reference Jiménez-Guri, Philippe, Okamura and Holland2007) that form a sister clade to the cnidarian parasite Polypodium hydriforme (Chang et al., Reference Chang, Neuhof, Rubinstein, Diamant, Philippe, Huchon and Cartwright2015; Foox and Siddall, Reference Foox and Siddall2015). Myxozoans of the class Myxosporea have rich biodiversity and cycle from invertebrate hosts, usually, annelids, in which actinospores are formed, to ectothermic vertebrates, particularly fish, in which myxospores are formed (Lom and Dyková, Reference Lom and Dyková2006; Holzer et al., Reference Holzer, Bartošová-Sojková, Born-Torrijos, Lövy, Hartigan and Fiala2018). Proliferative kidney disease (PKD) in salmonids was found to be caused by a myxozoan (Kent and Hedrick, Reference Kent and Hedrick1985), which based on the stages infecting bryozoan hosts was later named Tetracapsuloides (syn. Tetracapsula) bryosalmonae (Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura1999). This and several related species that use freshwater bryozoans as hosts (Anderson et al., Reference Anderson, Canning and Okamura1999; Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000, Reference Canning, Curry, Hill and Okamura2007, Reference Canning, Curry and Okamura2008), have been placed in the class Malacosporea (Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000). They, and their sister clade, Myxosporea, originally infected invertebrate hosts, and subsequently infected fish with which they co-evolved (Holzer et al., Reference Holzer, Bartošová-Sojková, Born-Torrijos, Lövy, Hartigan and Fiala2018). Because of previous uncertainties regarding the taxonomic placement of T. bryosalmonae, initial descriptions of sporoplasmosomes in this species referred to them variously as haplosporosomes or EDBs (Seagrave et al., Reference Seagrave, Bucke and Alderman1980; Morris et al., Reference Morris, Adams and Richards2000). These bodies are now considered synonymous with the sporoplasmosomes seen in other myxozoan species (Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000). The malacosporean spores formed in the bryozoan are called malacospores, while those found in the fish can be referred to as fish-malacospores (after Lom and Dyková, Reference Lom and Dyková2006).
This paper considers the structure, development, genesis and function of haplosporosomes and sporoplasmosomes of these obligate parasitic groups, how they may inter-relate and the implications for the taxonomy of these disparate protist and metazoan organisms. Ultrastructural studies which contain insufficient information on these bodies are not included.
Haplosporidia
Structure and cytochemistry
Haplosporidian haplosporosomes were originally described in detail from Haplosporidium nelsoni (Perkins, Reference Perkins1968; Fig. 1B) and other species (Perkins, Reference Perkins1979) as having a delimiting unit membrane and an internal membrane separating the medulla from the cortex. While usually spheroid or ovoid, they may also be vermiform or tubular, club-shaped, pyriform, or resembling an axehead (Table 1; Fig. 1C), that in Haplosporidium armoricanum has a hollow core (Hine et al., Reference Hine, Engelsma and Wakefield2007). Formative bodies (FBs) lacking an internal membrane occur in the spherulosomes of spores (Desportes and Nashed, Reference Desportes and Nashed1983; La Haye et al., Reference La Haye, Holland and McLean1984; Ciancio et al., Reference Ciancio, Srippa and Izzo1999; Hine and Thorne, Reference Hine and Thorne2002; Hine et al., Reference Hine, Engelsma and Wakefield2007; Hine et al., Reference Hine, Carnegie, Burreson and Engelsma2009). The internal membrane usually runs parallel to the external membrane, but in U. crescens it is C-shaped, sometimes appearing as 3 concentric rings (Perkins, Reference Perkins1971), in Haplosporidium patagon there is a cup-like indentation of the membrane (Figs 3B, 3C, 4B and 4E in Ituarte et al., Reference Ituarte, Bagnato, Siddall and Cremonte2014), and in H. nelsoni, it is vase-shaped (Perkins, Reference Perkins1968; Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990; Renault et al., Reference Renault, Stokes, Chollet, Cochennec, Berthe, Gérard and Burreson2000). There is a lack of an internal membrane in haplosporosomes of Haplosporidium comatulae in the crinoid Oligometra serripinna (see La Haye et al., Reference La Haye, Holland and McLean1984), and in polymorphic forms in Haplosporidium parisi from the polychaete Serpula vermicularis (see Ormières, Reference Ormières1980). Internal membranes also lack in inclusion bodies in Haplosporidium ascidiarum from the ascidian Ciona intestinalis (Ciancio et al., Reference Ciancio, Srippa and Izzo1999). Some haplosporosomes of a New Zealand abalone parasite (NZAP) (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) have tails (P.M. Hine unpub.obs.).
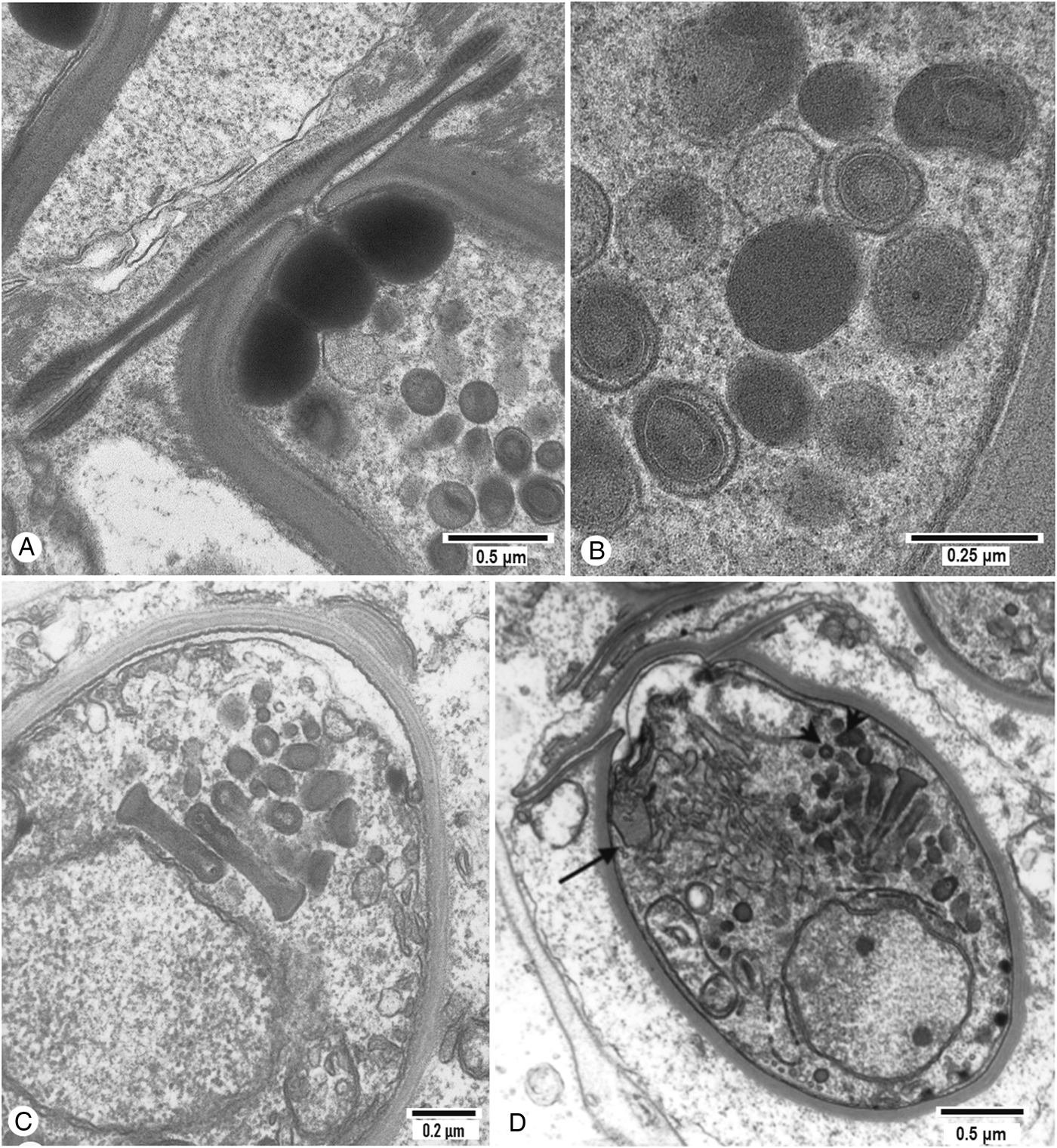
Fig. 1. Ultrastructural aspects of haplosporosomes in haplosplosporidian species. (A) Longitudinal section through the apical portion of a H. nelsoni spore showing spore operculum and internal osmiophilic lipid droplets with numerous cytoplasmic haplosporosomes. (B) Detail of H. nelsoni haplosporosomes showing structural pleomorphism with internal membranes. (C) Spore of H. armoricanum showing detail of the hollow axehead-shaped haplosporosomes. (D) Spore of H. armoricanum showing nuclear membrane-bound Golgi between the nucleus and haplosporosomes, and an MVB (arrowed).
Table 1. The shapes of haplosporidian haplosporosomes in relation to different developmental stages. Numbers in the table refer to the references given below

1. Bower and Meyer, Reference Bower and Meyer2002; 2. Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002; 3. Anderson and Lester, Reference Anderson and Lester1992; 4. Perkins, Reference Perkins1971; 5. Carballal et al., Reference Carballal, Diaz and Villalba2005; 6. Ormières et al., Reference Ormières, Sprague and Bartoli1973; 7. Perkins, Reference Perkins1975; 8. Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004; 9. Hine et al., Reference Hine, Engelsma and Wakefield2007; 10. Perkins, Reference Perkins1969; 11. Bearham et al., Reference Bearham, Spiers, Raidal, Jones and Burreson2008b; 12. Hine and Thorne, Reference Hine and Thorne1998; 13. Comps and Pichot, Reference Comps and Pichot1991; 14. Ituarte et al., Reference Ituarte, Bagnato, Siddall and Cremonte2014; 15. Azevedo, Reference Azevedo1984; 16. Azevedo and Corral, Reference Azevedo and Corral1985; 17. Azevedo and Corral, Reference Azevedo and Corral1987; 18. Perkins, Reference Perkins1968; 19. Renault et al., Reference Renault, Stokes, Chollet, Cochennec, Berthe, Gérard and Burreson2000; 20. Perkins, Reference Perkins1975; 21. Marchand and Sprague, Reference Marchand and Sprague1979; 22. Ormières, Reference Ormières1980; 23. Ciancio et al., Reference Ciancio, Srippa and Izzo1999; 24. Azevedo et al., Reference Azevedo, Balseiro, Casal, Gestal, Aranguren, Stokes, Carnegie, Novoa, Burreson and Figueras2006; 25. Azevedo et al., Reference Azevedo, Casal and Montes2008a; 26. La Haye et al., Reference La Haye, Holland and McLean1984; 27. Catanese et al., Reference Catanese, Grau, Valencia, Garcia-March, Vazquez-Luis, Alvarez, Deudero, Darriba, Carballal and Villalba2018; 28. Hine and Wesney, Reference Hine and Wesney1992; 29. Hine and Wesney, Reference Hine and Wesney1994a; 30. Hine and Wesney, Reference Hine and Wesney1994b; 31. Pichot et al., Reference Pichot, Comps, Tigé, Grizel and Rabouin1979; 32. Hervio et al., Reference Hervio, Chagot, Godin, Grizel and Mialhe1991; 33. Carnegie et al., Reference Carnegie, Burreson, Hine, Stokes, Audemard, Bishop and Peterson2006; 34. Hillman et al., Reference Hillman, Ford and Haskin1990; 35. McGovern and Burreson, Reference McGovern and Burreson1990; 36. Ball, Reference Ball1980; 37. Azevedo, Reference Azevedo2001; 38. Azevedo and Corral, Reference Azevedo and Corral1989; 39. Hine and Thorne, Reference Hine and Thorne2002; 40. Bearham et al., Reference Bearham, Spiers, Raidal, Jones and Nicholls2008a; 41. Desportes and Nashed, Reference Desportes and Nashed1983; 42. Comps and Tigé, Reference Comps and Tigé1997; 43. Dyková et al., Reference Dyková, Lom and Fajer1988; 44. Newman et al., Reference Newman, Johnson and Pauley1976.
The ultrastructure of crustacean haplosporidians has been reported from amphipods (Winters and Faisal, Reference Winters and Faisal2014; Urrutia et al., Reference Urrutia, Bass, Ward, Ross, Bojko, Marigomez and Feist2019), prawns (Dyková et al., Reference Dyková, Lom and Fajer1988; Meyers et al., Reference Meyers, Lightner and Redman1994; Bower and Meyer, Reference Bower and Meyer2002) and crabs (Perkins, Reference Perkins1975; Newman et al., Reference Newman, Johnson and Pauley1976; Marchand and Sprague, Reference Marchand and Sprague1979; Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004, Reference Stentiford, Bateman, Stokes and Carnegie2013). Haplosporidium louisiana (syn. Minchinia cadomensis) has typical haplosporosomes in plasmodia and spores (Perkins, Reference Perkins1975; Marchand and Sprague, Reference Marchand and Sprague1979), and the plasmodia of two unclassified species (Newman et al., Reference Newman, Johnson and Pauley1976; Dyková et al., Reference Dyková, Lom and Fajer1988) also have typical haplosporosomes. Haplosporidium littoralis has haplosporosome-like bodies (H-LBs) which appear to be immature haplosporosomes, and dense vesicles (DVs) with or without an internal membrane, regarded as haplosporosomes. In 3 species (Newman et al., Reference Newman, Johnson and Pauley1976; Dyková et al., Reference Dyková, Lom and Fajer1988; Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004, Reference Stentiford, Bateman, Stokes and Carnegie2013) haplosporosomes cluster around and are in contact with the nucleus. Haplosporidium spp. have DVs without an internal membrane, and some, e.g. H. parisi (Ormières, Reference Ormières1980), appear to lack haplosporosomes and have very different organelles. However, the genus Haplosporidium is paraphyletic, acting as a repository for orphan species, and may contain many genera and families (Hartikainen et al., Reference Hartikainen, Ashford, Berney, Okamura, Feist, Baker-Austin, Stentiford and Bass2014a; Hine, Reference Hine2020).
Homogeneous osmiophilic, probably lipoid, bodies from the spherule in spores of Haplosporidium nelsoni (Fig. 1A), Haplosporidium pickfordi (see Burreson, Reference Burreson2001), Haplosporidium lusitanicum (Azevedo, Reference Azevedo1984; Azevedo and Corral, Reference Azevedo and Corral1985, Reference Azevedo and Corral1987), Haplosporidium armoricanum (see Hine et al., Reference Hine, Engelsma and Wakefield2007), Haplosporidium montforti (see Azevedo et al., Reference Azevedo, Balseiro, Casal, Gestal, Aranguren, Stokes, Carnegie, Novoa, Burreson and Figueras2006), and Haplosporidium sp. (Comps and Tigé, Reference Comps and Tigé1997), which lie peripherally in sporoplasm, are probably not related to haplosporosomes.
Cytochemically, the cortex of H-LBs of the NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002), is labelled by zinc iodide-osmium tetroxide (ZIO) which labels Golgi and tubulovesicular systems. ZIO also labels the haplosporosomes and multivesicular bodies (MVBs) of Bonamia exitiosa (Fig. 2), but imidazole-buffered OsO4 showed lipid is weak on the haplosporosome surface (Hine and Wesney, Reference Hine and Wesney1994a). The haplosporosomes of the NZAP, Bonamia ostreae and B. exitiosa lack acid phosphatases (Hervio et al., Reference Hervio, Chagot, Godin, Grizel and Mialhe1991; Hine and Wesney, Reference Hine and Wesney1994a; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). Haplosporosomes of H. lusitanicum react weakly for glycoproteins, the external unit membrane reacting more strongly than the more lipoidal inner membrane (Azevedo and Corral, Reference Azevedo and Corral1985, Reference Azevedo and Corral1987). Haplosporidium lusitanicum spores have haplosporosomes containing polysaccharides and basal and apical peripheral membrane-bound DVs lying just under the plasma membrane which stain intensely for polysaccharides (Azevedo, Reference Azevedo1984) and may be involved in the formation of the spherule (Azevedo and Corral, Reference Azevedo and Corral1985). FBs and haplosporosomes in H. nelsoni are positive with the Feulgen stain for DNA (Perkins Reference Perkins1968, Reference Perkins1979).

Fig. 2. Zinc iodide-osmium tetroxide positive MVBs in the cytoplasm of Bonamia exitiosa.
Development and haplosporogenesis
Haplosporidian haplosporosomes occur in presporogonic plasmodial, stages, disappear at the onset of sporogony and are then formed in the sporoplasm. The methods of formation in relation to species are given in Table 2. Haplosporogenesis in some haplosporidian plasmodia and spores begins when granular material, apparently of nuclear origin occurs in an indentation in the nuclear surface (INS) (Fig. 3A). Budding of the nuclear membrane occurs near nuclear membrane-bound Golgi (NM-BG) along which balls of the putative nuclear material are processed. H-LBs bud from a trans-Golgi network (TGN) and condense to form haplosporosomes (Perkins, Reference Perkins1979; Hine and Wesney, Reference Hine and Wesney1992; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). NM-BG may also occur in spores (Fig. 1D) (Hine et al., Reference Hine, Engelsma and Wakefield2007). In Haplosporidium pinnae H-LBs appear to bud from the nuclear membrane (Catanese et al., Reference Catanese, Grau, Valencia, Garcia-March, Vazquez-Luis, Alvarez, Deudero, Darriba, Carballal and Villalba2018). The H-LBs mature into haplosporosomes in the TGN (Perkins, Reference Perkins1979; Hine and Wesney, Reference Hine and Wesney1992; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). The cytoplasmic distribution of haplosporosomes in the NZAP may be related to the occurrence of endoplasmic reticulum (Fig. 3B). In H. nelsoni plasmodia, haplosporosomes are formed when membranous vesicles in MVBs bud off to acquire an outer membrane (Perkins, Reference Perkins1968, Reference Perkins1979; Renault et al., Reference Renault, Stokes, Chollet, Cochennec, Berthe, Gérard and Burreson2000). In spores, the putative homolog of Golgi, the spherulosome or spherule, either produces haplosporosomes directly (Urosporidium spp.) or produces membrane-bound dense FBs which may be striated. They may remain as FBs (Perkins, Reference Perkins1968; Azevedo, Reference Azevedo1984; La Haye et al., Reference La Haye, Holland and McLean1984) or haplosporosomes may develop from them (Perkins, Reference Perkins1979; Desportes and Nashed, Reference Desportes and Nashed1983; Ciancio et al., Reference Ciancio, Srippa and Izzo1999). Haplosporosomes may also form by budding of membranous vesicles from FBs or MVBs in the plasmodia of H. nelsoni (Perkins, Reference Perkins1975) and in spores (Table 2). The spore of H. armoricanum has both a spherule and NM-BG (Hine et al., Reference Hine, Engelsma and Wakefield2007) (Fig. 1D). MVBs reported from the NZAP (Fig. 9 in Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) and B. exitiosa (Fig. 22 in Hine and Wesney, Reference Hine and Wesney1992), from which tubules extend contain putative haplosporosome cores They may be autophagic or crinophagic lysosome-like bodies. They are not similar to the MVBs associated with haplosporosomes in paramyxids.

Fig. 3. Haplosporosomes of a New Zealand abalone parasite. (A) Putative nuclear material in a nuclear membrane indentation in the NZAP. (B) Haplosporosomes arrayed around endoplasmic reticulum in the NZAP.
Table 2. The process of haplosporosome and sporoplasmosome/electron dense body (EDB) formation in different taxa. Numbers in the table refer to the references given below

EDBs, electron dense bodies; FBs, formative bodies; H-LBs, haplosporosome-like bodies; INS, indentations in the nuclear surface; MVBs, multivesicular bodies; NM-BG, nuclear membrane-bound bodies.
Superscript numbers indicate variations in structure. 1Some DVs with membrane, many without. 2No internal membrane. 3Also in C2, but of unknown origin. 4In outer sporoplasm. 5Elongated vesicle-like structures.
1. Perkins, Reference Perkins1969; 2. Carnegie et al., Reference Carnegie, Burreson, Hine, Stokes, Audemard, Bishop and Peterson2006; 3. Comps and Pichot, Reference Comps and Pichot1991; 4. Hine et al., Reference Hine, Engelsma and Wakefield2007; 5. Hine and Thorne, Reference Hine and Thorne2002; 6. Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002; 7. Hine and Wesney, Reference Hine and Wesney1992; 8. Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004; 9. Hine et al., Reference Hine, Carnegie, Burreson and Engelsma2009; 10. La Haye et al., Reference La Haye, Holland and McLean1984; 11. Perkins, Reference Perkins1968; 12. Ormières et al., Reference Ormières, Sprague and Bartoli1973; 13. Anderson et al., Reference Anderson, Newman and Lester1993; 14. Azevedo, Reference Azevedo1984; 15. Azevedo and Corral, Reference Azevedo and Corral1989; 16. Ituarte et al., Reference Ituarte, Bagnato, Siddall and Cremonte2014; 17. Azevedo et al., Reference Azevedo, Balseiro, Casal, Gestal, Aranguren, Stokes, Carnegie, Novoa, Burreson and Figueras2006; 18. Azevedo et al., Reference Azevedo, Casal and Montes2008a; 19. Bearham et al., Reference Bearham, Spiers, Raidal, Jones and Nicholls2008a; 20. Bearham et al., Reference Bearham, Spiers, Raidal, Jones and Burreson2008b; 21. Comps and Tigé, Reference Comps and Tigé1997; 22. Hine and Thorne, Reference Hine and Thorne1998; 23. Renault et al., Reference Renault, Stokes, Chollet, Cochennec, Berthe, Gérard and Burreson2000; 24. Ball, Reference Ball1980; 25. Hillman et al., Reference Hillman, Ford and Haskin1990; 26. Marchand and Sprague 1979; 27. Perkins, Reference Perkins1975; 28. Desportes, Reference Desportes1981; 29. Perkins and Wolf, Reference Perkins and Wolf1976; 30. Carrasco et al., Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013; 31. Comps, Reference Comps1983, [1985]; 32. Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009; 33. Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a; 34. Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979b; 35. Ruiz et al., Reference Ruiz, López, Lee, Rodríguez and Darriba2016; 36. Villalba et al., Reference Villalba, Iglesias, Ramilo, Darriba, Parada, No, Abollo, Molares and Carballal2014; 37. Itoh et al., Reference Itoh, Yamamoto, Kang, Choi, Green, Carrasco, Awaji and Chow2014; 38. Perkins, Reference Perkins1979; 39. Desportes and Lom, Reference Desportes and Lom1981; 40. Perkins, Reference Perkins1976; 41. Canning et al., Reference Canning, Curry, Hill and Okamura2007; 42. Canning et al., Reference Canning, Curry and Okamura2008; 43. Morris and Adams, Reference Morris and Adams2006; 44. Morris and Adams, Reference Morris and Adams2007; 45. Morris and Adams, Reference Morris and Adams2008; 46. Morris, Reference Morris2012.
Putative functions of haplosporidian haplosporosomes
The large plasmodia of the NZAP and H. nelsoni are extracellular and release their contents to destroy surrounding cells, but intracellular Bonamia spp. have small plasmodia and retain their haplosporosomes (Hine, Reference Hine2020). It cannot be assumed that haplosporosomes have the same functions in plasmodia and spores, or that within those stages they have a uniform function. For example, Minchinia occulta spores (Bearham et al., Reference Bearham, Spiers, Raidal, Jones and Nicholls2008a) have concurrent ultrastructurally different haplosporosome populations (Hine and Thorne, Reference Hine and Thorne2002). Also, differences in the density of the matrix in compartments such as the cortex and medulla (Figs 2 and 9 in Azevedo and Corral, Reference Azevedo and Corral1985, Fig. 11 in Hine and Thorne, Reference Hine and Thorne2002, Figs 2 and 10 in Perkins, Reference Perkins1979, Fig. 12 in Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004), suggest differences in content. The internal membrane, when present, may have the dual functions of separating the content of different compartments and the external membrane of fusion with the plasma membrane before exocytosis of the internal matrix. It has been proposed that plasmodial haplosporosomes participate in thickening the plasma membrane in the early stages of sporogony (Perkins, Reference Perkins1971, Reference Perkins1979), while spore haplosporosomes facilitate the release of the sporoplasm from the spore (Azevedo and Corral, Reference Azevedo and Corral1989). This suggests that haplosporosomes in plasmodial and spore stages have unique and distinct functions.
Plasmodia of 3 early-diverging haplosporidians (Reece and Stokes, Reference Reece and Stokes2003; Reece et al., Reference Reece, Siddall, Stokes and Burreson2004; Ward et al., Reference Ward, Neuhauser, Groben, Ciaghi, Berney, Romac and Bass2018), the NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002), U. crescens (Perkins, Reference Perkins1971) and H. nelsoni (Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990), release haplosporosomes. While those of the NZAP cause massive damage to surrounding cells (Diggles et al., Reference Diggles, Nichol, Hine, Wakefield, Cochennec-Laureau, Roberts and Freidman2002; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002), those of H. nelsoni and U. crescens plasmodia appear not to (Perkins, Reference Perkins1971, Reference Perkins1979). However, at sporulation in U. crescens, the release of haplosporosomes causes extensive damage to surrounding cells resulting in the host being ‘bags’ of spores (Perkins, Reference Perkins1971). Histologically, NZAP plasmodia are surrounded by a halo, due to destruction of surrounding host cells, leaving the host as bags of plasmodia (Diggles et al., Reference Diggles, Nichol, Hine, Wakefield, Cochennec-Laureau, Roberts and Freidman2002; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). Many NZAP haplosporosomes appear to accumulate in autophagic or crinophagic vacuoles.
The cores of some NZAP haplosporosomes occur in coated pits or thickenings of the plasma membrane and some lie beneath the plasma membrane. They become elongated and orientate at right angles to the plasma membrane, sometimes piercing it (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). Similarly, plasmodia of H. nelsoni are closely juxtaposed to host cells, align haplosporosomes (>65 nm) beneath the plasma membrane with the narrow end of the flask-shaped internal membrane orientated toward the exterior (Fig. 5 and 6 in Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990). In vitro, when oyster (Crassostrea virginica) granular haemocytes come in contact with H. nelsoni plasmodia they quickly retreat and agranular haemocytes become inactive, suggesting the plasmodia produce a substance inhibitory to haemocytes (Ford et al., Reference Ford, Ashton-Alcox and Kanaley1993). Such a substance may derive from the haplosporosomes aligned beneath the plasmodial membrane. Treatment of H. nelsoni plasmodia with enzymes and metabolic inhibitors increase phagocytosis by haemocytes, particularly the glycolysis inhibitor iodoacetate (Ford and Ashton-Alcox, Reference Ford and Ashton-Alcox1998).
The function or functions of haplosporosomes in spores is/are uncertain. In U. crescens, the disappearance of haplosporosomes at the onset of sporulation coincides with damage to surrounding cells which intensifies as sporulation progresses (Perkins, Reference Perkins1971). This loss of haplosporosomes also occurs widely in spore-forming species, but damage at sporulation has seldom been reported (Hine et al., Reference Hine, Carnegie, Burreson and Engelsma2009). Other membrane-bound dense bodies at the periphery of spores of H. lusitanicum (Azevedo and Corral, Reference Azevedo and Corral1985, Reference Azevedo and Corral1987), H. armoricanum (Hine et al., Reference Hine, Engelsma and Wakefield2007), H. patagon (Ituarte et al., Reference Ituarte, Bagnato, Siddall and Cremonte2014), and a haplosporidian resembling Haplosporidium costale (Comps and Pichot, Reference Comps and Pichot1991) may not be related to haplosporosomes, They are formed by the spherule and are released from the sporoplasm to lie between it and the spore wall. Their function is unknown.
There is some evidence regarding the haplosporosomes of some haplosporidians as viruses or virus-like. In the NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) and haplosporidians infecting ostreid oysters (Hine and Wesney, Reference Hine and Wesney1992; Hine and Thorne, Reference Hine and Thorne2002; Hine et al., Reference Hine, Engelsma and Wakefield2007) balls of putative nuclear material pass from indentations in the nuclear membrane (Fig. 3A) to the NM-BG where, at the TGN, H-LBs are formed (Table 2). The haplosporosomes of H. nelsoni are Feulgen +ve indicating the presence of DNA (Perkins, Reference Perkins1968, Reference Perkins1979). Occasionally H-LBs of B. exitiosa (Hine: unpub. obs.) and H. littoralis (Stentiford et al., Reference Stentiford, Bateman, Stokes and Carnegie2013) are hexagonal in cross-section (Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004). These features resemble the ultrastructure of nucleocapsids of some DNA viruses (Hine, Reference Hine1992). Furthermore, B. exitiosa may contain multiple cylindrical confronting cisternae which are associated with underlying viral infection in mammalian cells (Hine and Wesney, Reference Hine and Wesney1992). Against a viral interpretation is that haplosporosomes do not invade other cells, do not appear to hijack the nucleus to replicate and do not then exit the cell to infect other cells. Also, incorporation of nuclear material has not been reported from other haplosporidians and paramyxids.
Paramyxida
Haplosporosome structure in relation to developmental stages
In the absence of a uniform nomenclature, the developmental stages of paramyxids will be designated C1–C6, C1 being the plasmodial stage, C2 being equivalent to a sporont, C3 being equivalent to the outer cell of the spore and C4–6 being equivalent to the cells within the spore. Haplosporosomes occur in C1, C3, C4 and C5 (Table 3), but it is unclear whether the dense inclusions in C1 of Paramyxa paradoxa (see Desportes, Reference Desportes1981), in C1 and C3 of Paramarteilia orchestiae (Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a, Reference Ginsburger-Vogel and Desportes1979b) and in flattened elongated ‘vesicles’ of Marteilia octospora (Ruiz et al., Reference Ruiz, López, Lee, Rodríguez and Darriba2016) are haplosporosomes. The C4 cells of Marteilia cochillia (Fig. 6D in Carrasco et al., Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013) and Marteilia sydneyi (Fig. 10 in Perkins and Wolf, Reference Perkins and Wolf1976) have tubular haplosporosomes aligned at ~90° to the plasma membrane. The C4 of M. refringens (Fig. 9 in Perkins, Reference Perkins1979) and Marteilia granula (Fig. 6E in Itoh et al., Reference Itoh, Yamamoto, Kang, Choi, Green, Carrasco, Awaji and Chow2014) have vesicles in the same position, that resemble the vesicles in MVBs of M. sydneyi (Fig. 9, stage 2, in Perkins and Wolf, Reference Perkins and Wolf1976). They are observed during haplosporogenesis and are therefore identified as haplosporosomes. In Paramarteilia canceri, C3 contains two haplosporosome populations. One population is comprised of cylindrical and bacilliform forms as in C1. The second is comprised of large (>3.5 μm long) forms with one end bulbous to give a tadpole-like body. They have complex membranes giving the appearance of two eyes and an apical sheath behind the ‘head’ running nearly half-way towards the tail (Fig. 4A and B) (Fig. 23 in Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009). However, in the congeneric P. orchestiae (Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a), the dense bodies in C3, while being similar in shape and size to those of P. canceri, appear to lack internal membranes. They have a heterogeneous glycogen-like content, migrate to the surface of the endospore and eject their filamentous content into the space between endospore and epispore.

Fig. 4. Details of haplosporosomes in paramyxids species. (A) Tertiary cell of Paramarteilia canceri showing the presence of numerous small haplosporosome stages ranging from bacilliform to curved and more rounded containing linked electron-dense bodies (arrow). Larger bulb-shaped haplosporosomes with internal membranous internal structure and striations are prominent. (B) Detail of bacilliform haplosporosome also present in the tertiary cell of P. canceri. (C) Section through spores of Paramyxoides nephtys showing the presence of numerous spherical haplosporosomes. (D) Detail of P. nephthys haplosporosomes showing pleomorphism with internal electron-dense structure at one end and elongation of the haplosporosomes.
Table 3. The shapes of paramyxid haplosporosomes in relation to different developmental stages. C1–C5 indicate developmental stages (see text). Numbers in the table refer to the references given below
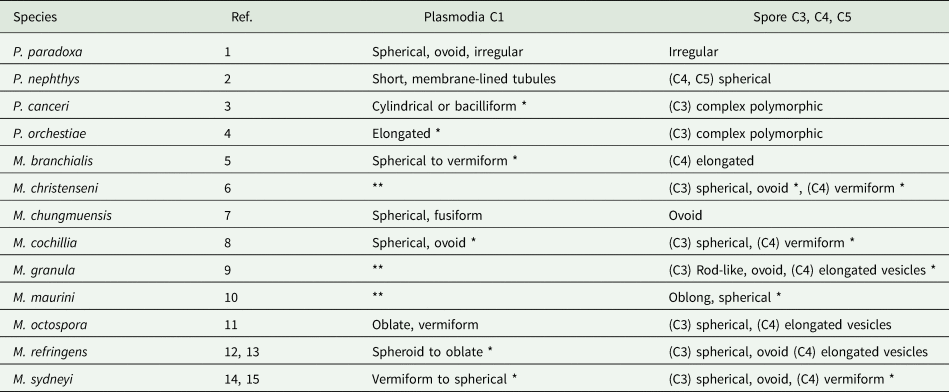
* typical haplosporosomes as defined by Perkins (Reference Perkins1971).
** not reported.
1. Desportes (Reference Desportes1981); 2. Larsson and Koie (Reference Larsson and Køie2005); 3. Feist et al. (Reference Feist, Hine, Bateman, Stentiford and Longshaw2009); 4. Ginsburger-Vogel and Desportes (Reference Ginsburger-Vogel and Desportes1979a, ); 5. Anderson and Lester (Reference Anderson and Lester1992); 6. Comps Reference Comps(1983 [1985]); 7. Comps et al. (Reference Comps, Park and Desportes1986); 8. Carrasco et al. (Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013); 9. Itoh et al. (Reference Itoh, Yamamoto, Kang, Choi, Green, Carrasco, Awaji and Chow2014); 10. Auffret and Poder Reference Auffret and Poder(1983 [1985]); 11. Ruiz et al. (Reference Ruiz, López, Lee, Rodríguez and Darriba2016); 12. Longshaw et al. (Reference Longshaw, Feist, Matthews and Figueras2001); 13. Perkins (Reference Perkins1979); 14. Kleeman et al. (Reference Kleeman, Adlard and Lester2002); 15. Perkins and Wolf (Reference Perkins and Wolf1976).
Haplosporogenesis
In C1, haplosporosomes in some species are formed in MVBs (Table 2) as bilaminar vesicles that probably obtain their outer membrane by budding from the vesicles, as in some haplosporidians (Perkins, Reference Perkins1968). Golgi has not been reported from C1. The haplosporosomes of C3 of M. sydneyi also arise from vesicles (Perkins and Wolf, Reference Perkins and Wolf1976), but their genesis in other species is unknown, their appearance seeming to be rapid. In the C4 of P. paradoxa, haplosporosomes derive from Golgi (Figs 9A and 9B in Desportes, Reference Desportes1981), and in the C4 and C5 of Paramyxoides nephthys, haplosporosome like bodies arise from vacuoles (Fig. 4C and D) (Fig. 19 in Larsson and Koie, Reference Larsson and Køie2005). In P. orchestiae it is claimed that the large dense inclusions of C3 arise from Golgi (Figs 15 and 16 in Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a), but an alternative interpretation is that they derive from circular profiles of sER, as would be expected if they contain mucopolysaccharides.
Putative function
There are no clues to the function of C1 haplosporosomes, and although they underlie the fibrous ectoplasm in the periphery of C1 in P. orchestiae (Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a, Reference Ginsburger-Vogel and Desportes1979b), they do not orientate toward the exterior as in the haplosporidians NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) and H. nelsoni (Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990). However, in the intermediate spore cell (C4) of Marteilia christenseni (Figs 5 and 6 in Comps, Reference Comps1983 [1985]), M. sydneyi (Figs 9–11 in Perkins and Wolf, Reference Perkins and Wolf1976), and M. cochillia (Fig. 6A in Carrasco et al., Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013), the thin vermiform haplosporosomes are orientated at right angles to the C4 plasma membrane. In M. sydneyi the distal end appears to contain osmiophilic matter, but it is unlikely to be involved in spore wall formation as this occurs around the previous C3 cell (Fig. 11 in Perkins and Wolf, Reference Perkins and Wolf1976). In P. orchestiae the heterogeneous content of dense bodies in C3, probably haplosporosome homologs, contain glycogen and are thought to be involved in spore wall formation (Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a).
Myxozoa
The Myxozoa is divided into two main clades; the Malacosporea and the Myxosporea. The Myxosporea is further sub-divided based on phylogeny, development, host and habitat into four further clades; Sphaerospora, marine species, Kudoa and freshwater species (Morris and Adams, Reference Morris and Adams2008; Holzer et al., Reference Holzer, Bartošová-Sojková, Born-Torrijos, Lövy, Hartigan and Fiala2018). All myxozoan spores are multicellular and usually contain a single sporoplasm which infects the subsequent host in the life cycle. Exceptions are malacospores which contain two sporoplasms and Sphaerospora myxospores which can contain two or more sporoplasms. For freshwater clade actinospores, malacospores and Kudoa myxospores, the sporoplasms are multicellular composed of a sporoplasm primary cell within which one or more secondary cells reside. For marine clade actinospores a single sporoplasm is usually described with no secondary cells present, however, in one study secondary cells have been reported (Rangel et al., Reference Rangel, Azevedo, Casal and Santos2012).
Malacosporea
Sporoplasmosome structure in relation to developmental stages
H-LBs (not structurally like haplosporidian H-LBs) of malacosporeans were called EDBs (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Morris et al., Reference Morris, Adams and Richards2000) but are now recognized as a type of sporoplasmosome (Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000; Morris and Adams, Reference Morris and Adams2006; Canning et al., Reference Canning, Curry, Hill and Okamura2007; Morris and Adams, Reference Morris and Adams2007, Reference Morris and Adams2008). They occur in primary (1°) cells, pre-saccular cells and mural cells of T. bryosalmonae (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Morris et al., Reference Morris, Adams and Richards2000; Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2008), Buddenbrockia plumatellae (Canning et al., Reference Canning, Curry, Hill and Okamura2007; Morris and Adams, Reference Morris and Adams2007) and Buddenbrockia allmani (Canning et al., Reference Canning, Curry, Hill and Okamura2007), although they are relatively sparse in mural cells (Canning et al., Reference Canning, Curry and Okamura2008).
To date, two morphologically distinct types of sporoplasmosomes have been described in malacosporeans. These can exist within the life-cycle of a single species and it is possible that this is common within the group. The only malacosporean for which the life cycle has been examined ultrastructurally in both hosts is T. bryosalmonae (Ferguson and Needham, Reference Ferguson and Needham1978; Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000; Morris and Adams Reference Morris and Adams2006, Reference Morris and Adams2007, Reference Morris and Adams2008). For this species, the sporoplasmosomes observed in the sporoplasm of the fish malacospore and the presaccular cells observed in the bryozoan host appear identical. However, they are distinct from the sporoplasmosomes observed in the 1° cell of the intra-piscine extrasporogonic stage of T. bryosalmonae and sporoplasm of the malacospore released from the bryozoan.
Structurally the malacosporean sporoplasmosomes are membrane-bound dense spherical bodies around 200 nm in diameter. Those that exist in the 1° cells of intra-piscine extrasporogonic stages and the malacospore sporoplasm contain a lucent bar extending into a lipid-rich core (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Morris et al., Reference Morris, Adams and Richards2000; Morris and Adams, Reference Morris and Adams2007, Reference Morris and Adams2008). The top of the lucent bar is capped by an additional membrane (Morris et al., Reference Morris, Adams and Richards2000). While observed in the 1° cell they may be endocytosed by secondary (2°) cells (Morris et al., Reference Morris, Adams and Richards2000). In the intra-bryozoan presaccular cells and the sporoplasm of the fish malacospore there is no bar, but an elliptical lucent region at one pole of the dense core (Canning et al., Reference Canning, Curry, Hill and Okamura2007, Reference Canning, Curry and Okamura2008, Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2008). Interestingly T. bryosalmonae also has a labyrinthine structure resembling the spherule in haplosporidian spores (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984), although this appears to have been a unique observation and may represent an unusual plane of section through the TGN (Morris et al., Reference Morris, Adams and Richards2000).
Intra-piscine presporogonic stages of other malacosporeans have yet to be described, however, parasites comparable to T. bryosalmonae have been noted in carp (Voronin, Reference Voronin1993; Voronin and Chernysheva, Reference Voronin and Chernysheva1993; Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádkova, Jurajda, Tyml and Holzer2014). These parasites may represent presporogonic stages of B. plumatellae, or related Malacosporea (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2010a, Reference Grabner and El-Matbouli2010b).
Sporoplasmogenesis
Malacosporean sporoplasmosomes form at Golgi, on the trans-face (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984, Fig. 1D in Feist and Bucke, Reference Feist and Bucke1987; Morris et al., Reference Morris, Adams and Richards2000; Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2007). This occurs in the presaccular proliferating stages within the invertebrate host and the proliferating extrasporogonic stage of T. bryosalmonae in the vertebrate host. The sporoplasmosomes of T. bryosalmonae also array along confronting cisternae (CC) of ER (Fig. 14 in Morris et al., Reference Morris, Adams and Richards2000) and are also found in cup-like bodies (Fig. 13 in Morris et al., Reference Morris, Adams and Richards2000) that lack a thick double membrane (unlike paramyxid MVBs). There is no evidence that either of these represents a site of formation and conversely, the latter is considered evidence of autophagocytosis (Morris et al., Reference Morris, Adams and Richards2000).
Putative function
Sporoplasmosomes array under the plasma membrane (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Morris et al., Reference Morris, Adams and Richards2000; Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2007; Canning et al., Reference Canning, Curry and Okamura2008). Within the intra-piscine extrasporogonic stages of T. bryosalmonae they orientate the lucent bar at right angles to the plasma membrane (Ferguson and Needham, Reference Ferguson and Needham1978; Morris et al., Reference Morris, Adams and Richards2000), For the sporoplasmosomes observed in the proliferative stages in bryozoans hosts the lucent area orients parallel to the plasma membrane (Morris and Adams, Reference Morris and Adams2006, Reference Morris and Adams2007).
The contents of the lucent inclusion may be secreted externally by T. bryosalmonae (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984) or beneath the plasma membrane by B. plumatellae (Morris and Adams, Reference Morris and Adams2007). However, it remains unclear whether the sporoplasmosomes (Morris et al., Reference Morris, Adams and Richards2000) or their lucent inclusions fuse with the plasma membrane (Ferguson and Needham, Reference Ferguson and Needham1978; Morris et al., Reference Morris, Adams and Richards2000; Morris and Adams Reference Morris and Adams2006, Reference Morris and Adams2007). The sporoplasmosomes of T. bryosalmonae may be exocytosed or released when the 1° cell of the extrasporogonic stage disintegrates, but there is no evidence that they are expelled (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984; Morris et al., Reference Morris, Adams and Richards1997, Reference Morris, Adams and Richards2000; Morris and Adams, Reference Morris and Adams2006).
There is a change in the distribution and orientation of sporoplasmosomes of intra-piscine T. bryosalmonae depending on site within the host. When the parasite is interdigitated with macrophages in the kidney interstitium they lie beneath and orientate to the plasma membrane. However, when the parasite is released into the kidney tubule lumen and not associated with macrophages the sporoplasmosomes have random cytoplasmic distribution. It has been suggested that sporoplasmosomes may be associated with the formation of the plasma membrane (Morris et al., Reference Morris, Adams and Richards2000), membrane recycling (Morris et al., Reference Morris, Adams and Richards2000), or aid in recognition and adherence/fusion of certain cell types (Morris and Adams, Reference Morris and Adams2006).
Myxosporea
Sporoplasmosome structure in relation to developmental stages
While myxosporeans are considered to have indirect life cycles, these have only been completed for a relatively few species in two of the four main myxosporean clades. However, given available knowledge, it is considered that freshwater species usually cycle between fish and oligochaetes and marine species usually cycle between fish and polychaetes. The life cycles of any Kudoa and Sphaerospora species have yet to be elucidated (Holzer et al., Reference Holzer, Bartošová-Sojková, Born-Torrijos, Lövy, Hartigan and Fiala2018). Sporoplasmosomes occur in actinospore developmental stages within invertebrate hosts of invertebrate hosts and in the myxospores of vertebrate hosts (Lom and Dyková, Reference Lom and Dyková1997). In actinospores, they are reported from binucleate pre-pansporocyst stages (Morris and Freeman, Reference Morris and Freeman2010; Morris, Reference Morris2012), which contain Golgi (Lom et al., Reference Lom, Feist, Dyková and Kepr1989; Morris and Freeman, Reference Morris and Freeman2010) and the actinospore cytoplasm. One study observed the sporoplasmosomes orientating to the plasma membrane within the binucleate stages in a similar manner to the sporoplasmosomes observed in the Malacosporea (Morris and Freeman, Reference Morris and Freeman2010). The sporoplasmosomes of Sphaeractinomyxon, Aurantiactinomyxan, and Triactinomyxon observed in the actinospore sporoplasm are diverse in structure (Lom and Dyková, Reference Lom and Dyková1997) and do not resemble typical sporoplasmosomes (Fig. 5D). The sporoplasmosomes in the actinospore sporoplasm appear to be a different structure from those observed in the pre-pansporocyst binucleate cells (Morris and Freeman, Reference Morris and Freeman2010; Morris, Reference Morris2012).

Fig. 5. Ultrastructural details of sporoplasmosomes in malacosporeans and actinospores. (A) Sporoplasmosomes of Tetracapsuloides bryosalmonae with internal membranes containing electron-dense material and with an invagination aligned perpendicularly along the plasma membrane of the primary cell of the parasite. (B) Formation of T. bryosalmonae sporoplasmosomes via the Golgi in close association with the endoplasmic reticulum. (C) Sporoplasmosomes of presaccular Buddenbrockia plumatellae stages forming on Golgi apparatus. Inset: B. plumatellae sporoplasmosome within the sporoplasm of a malacospore. There they resemble those of T. bryosalmonae. In the presaccular stages they have a different organization. (D) Sporoplasmosomes of Triactinomyxon A showing complex structure.
Despite the description of >2596 myxosporean species by 2018 (Okamura et al., Reference Okamura, Hartigan and Naldoni2018), the sporoplasmosomes of myxospores are seldom illustrated or described (but see Azevedo et al., Reference Azevedo, Lom and Corral1989, Casal et al., Reference Casal, Matos and Azevedo2003, Vita et al., Reference Vita, Corral, Matos and Azevedo2003, Casal et al., Reference Casal, Matos and Azevedo2006, Reference Casal, Costa and Azevedo2007, Azevedo et al., Reference Azevedo, Casal, Matos and Matos2008b, Reference Azevedo, Casal, Matos, Alves and Matos2011), those that have, show great diversity in form (Fig. 6A–H). All reports have been from the sporoplasm contained within the binucleate sporoplasm of the maturing spore. In one study (Lom et al., Reference Lom, Feist, Dyková and Kepr1989), the polymorphic sporoplasmosomes of 14 myxosporean spores are illustrated. Kudoa lunata sporoplasmosomes were described as occurring in a variety of forms (linear, groove-like and ring-like) that were interpreted as cross-sections through dense tubes that were cut at different angles (Lom and Dyková, Reference Lom and Dyková1988). No myxosporean sporoplasmosomes have an internal membrane as in the original descriptions of haplosporosomes (Perkins, Reference Perkins1971, Reference Perkins1979). However, nearly all the sporoplasmosomes have a complex substructure and some appear to have compartments delimited by membranes (Figs 6C and 6H) (Vita et al., Reference Vita, Corral, Matos and Azevedo2003; Azevedo et al., Reference Azevedo, Casal, Matos, Alves and Matos2011).

Fig. 6. Ultrastructural details of sporoplasmosomes in myxospores. (A) Sporoplasmosomes (Ss) and some cisternae of endoplasmic reticulum (white arrowheads) of a Ceratomyxa tenuispora myxospore. (B) Detail of some sporoplasmosomes (Ss) and glycogen particles (arrows) of a myxospore of Henneguya amazonica. (C) Sporoplasmosomes (arrows) of a myxospore of Myxobolus braziliensis. (D) Sporoplasmosomes (arrows) resembling the teardrop with an unusual electron density externally obtained from myxospore of Henneguya friderici. (E) Detailed aspect of some sporoplasmosomes shown in figure D. (F) Sporoplasmosomes, each with an eccentric, dense structure with a half crescent section located in a matrix formed by granular masses obtained from myxospore of Myxobolus metynnis. (G) Sporoplasmosomes of Sphaeromyxa balbiani showing a variety of profiles from apparent tubes to circular structures indicating a cup-shaped structure of the mature sporoplasmosome. (H) Internal structure of Myxobolus cotti sporoplasmosome with an internal membrane containing electron-dense material.
Sporoplasmogenesis and function
The method of sporoplasmosome formation is unknown in myxosporeans. While attributed to Golgi in one study (Morrison et al., Reference Morrison, Martell, Leggiadro and O'Neil1996), no evidence is presented and it appears to be based on the origin of sporoplasmosomes in malacosporeans. The functions of sporoplasmosomes are also unknown. The structure of sporoplasmosomes in the binucleate stages observed in annelids is proposed to resemble that observed in the corresponding myxospore sporoplasm released from the fish host (Morris and Freeman, Reference Morris and Freeman2010). The alignment along the plasma membrane further suggests a functional relationship with sporoplasmosomes observed in malacosporeans.
Overview
Haplosporidians and paramyxids are linked by haplosporosome morphology, haplosporidians and malacosporeans by the array of haplosporosomes under the plasma membrane and alignment of their internal structures, and paramyxids and myxozoans by cell within cell development. In the 3 groups, haplosporosomes and sporoplasmosomes occur in feeding plasmodial stages, they disappear afterwards and reappear during sporogony. In all 3 groups (Haplosporidia, Paramyxa and Myxozoa) the plasmodia are trophic, but methods of feeding are different. Haplosporidian (except Bonamia spp.) and paramyxid plasmodia are extracellular, presumably feeding on nutrients gained from lysing surrounding host cells. Myxozoan plasmodia lie in close inter-digitating contact with host cells and feed by pinocytosis (Azevedo et al., Reference Azevedo, Carmona De São Clemente, Casal, Matos, Oliveira, Al-Quraishy and Matos2013).
Comparative morphology of haplosporosomes and sporoplasmosomes
Comparison of haplosporosomes and sporoplasmosomes is constrained by the vagueness of their definitions. Haplosporosomes of U. crescens were defined as ‘membrane-bound, osmiophilic structures which are oval, spherical, pyriform or short rods’ (Perkins, Reference Perkins1971). Similarities between haplosporosomes of haplosporidians and paramyxids were recognized by Perkins (Reference Perkins1979). Subsequently, similar bodies in the sporoplasm of the myxozoan Hoferellus gilsoni were described as having ‘an electron dense core and a thin lucent envelope’ that ‘to a slight extent remind of haplosporosomes’, and were named sporoplasmosomes (Lom et al., Reference Lom, Molnár and Dyková1986). However, these bodies differ greatly in form and in different developmental stages of haplosporidians (Table 1), as do haplosporosomes in paramyxids (Table 3) and sporoplasmosomes in myxozoans (Lom et al., Reference Lom, Feist, Dyková and Kepr1989). The situation is complicated by the involvement of FBs, DVs and MVBs in haplosporogenesis in haplosporidians and paramyxids (Table 2). It is therefore difficult to identify which of the many forms in the 3 groups are haplosporosomes, which are sporoplasmosomes and which are unrelated.
Understanding patterns in the occurrence and morphology of haplosporosomes in haplosporidians is limited by taxonomic constraints and sampling. The NZAP, Urosporidium spp., Bonamia spp., Minchinia spp. and some Haplosporidian spp. (Perkins, Reference Perkins1968, Reference Perkins1969, Reference Perkins1971, Reference Perkins1975, Reference Perkins1979; Marchand and Sprague, Reference Marchand and Sprague1979; Hine et al., Reference Hine, Engelsma and Wakefield2007, Reference Hine, Carnegie, Burreson and Engelsma2009) have haplosporosomes sensu Perkins (Reference Perkins1971). However, studies have been mainly on infections in molluscs and crustaceans, and the genus Haplosporidium is a paraphyletic repository for orphan species (Hine et al., Reference Hine, Carnegie, Burreson and Engelsma2009). It may therefore comprise several genera or even families that vary greatly in haplosporosome morphology. This is supported by the diverse forms of cytoplasmic bodies, but not haplosporosomes sensu Perkins (Reference Perkins1971), observed in H. parisi from a polychaete (Ormières, Reference Ormières1980), H. ascidiarum infecting an ascidian (Ciancio et al., Reference Ciancio, Srippa and Izzo1999) and H. comatulae reported from a crinoid (La Haye et al., Reference La Haye, Holland and McLean1984).
The haplosporosomes of paramyxids are polymorphic, may show the classic structure of haplosporidian haplosporosomes (Perkins and Wolf, Reference Perkins and Wolf1976; Desportes and Lom, Reference Desportes and Lom1981; Comps et al., Reference Comps, Park and Desportes1986; Anderson and Lester, Reference Anderson and Lester1992; Longshaw et al., Reference Longshaw, Feist, Matthews and Figueras2001; Larsson and Koie, Reference Larsson and Køie2005; Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009; Carrasco et al., Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013) and in some cases (C1 in Desportes and Lom, Reference Desportes and Lom1981, C3 in Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009, C3 in Perkins and Wolf, Reference Perkins and Wolf1976) an internal membrane is not distinct. The haplosporosomes of the C3s of the two Paramarteilia spp. are different from those of other genera, but they also differ markedly. Those of P. canceri have a complex substructure (Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009) with membranes dividing the haplosporosome into compartments (Fig. 4A).
Sporoplasmosomes of myxozoans show great diversity in size and shape (Lom et al., Reference Lom, Feist, Dyková and Kepr1989). The internal membranes/inclusions of haplosporosomes/sporoplasmosomes are also very varied from apparently absent (Desportes and Nashed, Reference Desportes and Nashed1983; Lom et al., Reference Lom, Feist, Dyková and Kepr1989; Bower and Meyer, Reference Bower and Meyer2002; Vita et al., Reference Vita, Corral, Matos and Azevedo2003; Stentiford et al., Reference Stentiford, Feist, Bateman and Hine2004; Casal et al., Reference Casal, Costa and Azevedo2007) to complex (Auffret and Poder, Reference Auffret and Poder1983 [1985]; Lom et al., Reference Lom, Feist, Dyková and Kepr1989; Morris et al., Reference Morris, Adams and Richards2000; Azevedo et al., Reference Azevedo, Casal, Matos and Matos2008b; Feist et al., Reference Feist, Hine, Bateman, Stentiford and Longshaw2009; Azevedo et al., Reference Azevedo, Casal, Matos, Alves and Matos2011). Presumably, when present, they separate functional compartments and therefore have different content/functions.
Myxosporean sporoplasmosomes do not resemble haplosporosomes, except for the sporoplasmosomes (EDBs) of malacosporean 1° cells, presaccular and mural cells of Tetracapsuloides bryosalmonae (Feist and Bucke, Reference Feist and Bucke1987; Morris et al., Reference Morris, Adams and Richards2000). Like the haplosporosomes of H. nelsoni plasmodia they are round, electron-dense and arrayed along the plasma membrane with the internal membrane orientated toward the exterior. The internal membrane is like a squat vase in H. nelsoni (Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990) and like a T-bar in non-osmicated T. bryosalmonae (Morris et al., Reference Morris, Adams and Richards2000). However, the T. bryosalmonae bars have a separate and distinct membrane that caps the bar (Morris et al., Reference Morris, Adams and Richards2000). In the C3 and C4 of some paramyxids tubular haplosporosomes are orientated toward the plasma membrane (Perkins and Wolf, Reference Perkins and Wolf1976; Comps, Reference Comps1983 [1985]; Carrasco et al., Reference Carrasco, Hine, Durfort, Andree, Malchus, Lacuesta, González, Roque, Rodgers and Furones2013), before spores occur in the C5 and C6 stages.
Function of haplosporosomes and sporoplasmosomes
Little is known of the functions of haplosporosomes and sporoplasmosomes. In haplosporidians they appear to act extracellularly in the NZAP and H. nelsoni, but remain intracellular in derived groups (Hine, Reference Hine2020). Their occurrence in plasmodia, disappearance during sporogony and reappearance in spores suggests they may be involved in spore wall formation in haplosporidians (Perkins, Reference Perkins1971, Reference Perkins1979) and paramyxids (Ginsburger-Vogel and Desportes, Reference Ginsburger-Vogel and Desportes1979a). However, there is no evidence for this in myxozoans.
The haplosporosomes of the NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) and H. nelsoni (Scro and Ford, Reference Scro, Ford, Perkins and Cheng1990), and the sporoplasmosomes of T. bryosalmonae (Morris et al., Reference Morris, Adams and Richards2000) resemble each other in their positioning beneath the plasma membrane (Fig. 5A). However, they differ in details of their interaction with the plasma membrane. The outer membrane of the NZAP haplosporosomes appears to fuse with the plasma membrane, releasing the content between the 2 haplosporosome membranes and the intact cores. These intact cores are then endocytosed into lysosomes. The outer membrane of H. nelsoni haplosporosomes appears to fuse with the plasma membrane. Presumably, its contents are discharged, but there is no subsequent evidence of intact cores being endocytosed. The sporoplasmosomes of T. bryosalmonae may also fuse with the plasma membrane (Ferguson and Needham, Reference Ferguson and Needham1978) and may (Smith et al., Reference Smith, Morrison, Ramsey and Ferguson1984) or may not (Morris et al., Reference Morris, Adams and Richards2000) release their contents, or their lipid-rich content may interact with the plasma membrane (Morris et al., Reference Morris, Adams and Richards2000). Similarly, to the endocytosed cores of the NZAP, T. bryosalmonae may sometimes be engulfed by MVBs and enter the lysosomal pathway (Morris et al., Reference Morris, Adams and Richards2000). The discharge of haplosporosome contents from the NZAP and from plasmodia of H. nelsoni is associated with the destruction of surrounding cells (Hine, Reference Hine2020) which then nourish the parasites. The haplosporosomes sensu Perkins (Reference Perkins1971) of haplosporidians and paramyxids are probably homologous.
Concluding remarks
Haplosporosomes and sporoplasmosomes may have a common origin or similarities between them may be due to convergent evolution. Eukaryotic protists and cnidarians have deep evolutionary roots, both occurring in the Neoproterozoic (Van Iten et al., Reference Van Iten, Marques, de Moraes Leme, Forancelli Pacheco and Guimaraes Simões2014), with myxozoans occurring at the same time as basal bilaterians (Evans et al., Reference Evans, Holder, Barbeitos, Okamura and Cartwright2010), and the Malacosporea and the Myxosporea separating in the Cambrian (Kodádková et al., Reference Kodádková, Bartošová-Sojková, Holzer and Fiala2015). If derived from a common origin the diversity of structure and evolution of a variety of functions may be expected, given the long evolutionary history of these organisms.
There is ultrastructural proof that haplosporosome exocytosis to destroying surrounding cells in the NZAP (Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002) and of haplosporosomes containing nuclear material (Hine, Reference Hine1992; Hine and Wesney, Reference Hine and Wesney1992; Hine et al., Reference Hine, Wakefield, Diggles, Webb and Maas2002). However, there is no good evidence of the involvement of haplosporosomes or sporoplasmosomes in spore wall formation. Haplosporosomes containing nuclear material may indicate the presence of endogenous viral elements. Otherwise, the 3 groups form haplosporosomes/sporoplasmosomes for no apparent reason, despite their utilization of cellular resources. Our review highlights critical areas for further research. These include:
• examining the relationship between H. nelsoni haplosporosomes and malacosporean sporoplasmosomes,
• determining the identity of the nuclear content in haplosporosomes of B. exitiosa and whether they may represent viral elements,
• the role of haplosporosomes in sporogony and spore wall formation,
• the structure and behaviour of sporoplasmosomes within proliferative, pre-sporogonic stages,
• determining any morphological similarities between sporoplasmosomes in myxospores, corresponding binucleate stages within annelid hosts and phylogenies,
• and the role of haplosporosomes and sporoplasmosomes in a wider range of species of each group.
Functional and genomic studies would clarify the role of these bodies in these groups, which include serious pathogens of fish and shellfish.
Acknowledgements
All these reported figures are reprinted with permission of: Diseases of Aquatic Organisms, Figs 1C and 1D, (2007) 77, 225–233; Fig. 2, (1994) 20, 207–217; Bulletin of the European Association of Fish Pathologists, Figs 5A and 5B, (1997) 17, 209–214; International Journal for Parasitology, Fig. 5C (inset) (2007) 37, 1163–71; Folia Parasitologica, Fig. 6A, (2007) 54, 165–171; Parasitology Research, Fig. 6B, (1989) 76, 131–134; European Journal of Protistology, Fig. 6C, (1996) 32, 123–127; Parasitology, Figs 6D and 6E, (2003) 126, 313–319; Journal of Parasitology Fig. 6F, (2006) 92, 817–821.
Financial support
The support of the Department for Environment, Fisheries and Rural Affairs (contracts FB002 and FX001) to SWF is gratefully acknowledged.
Ethical standards
Not applicable.
Conflict of interest
None.












