INTRODUCTION
Parasites can be an important selective force affecting the abundance and distribution of host species (Grenfell and Dobson, Reference Grenfell and Dobson1995), increasing genetic variation within host populations (Anderson and May, Reference Anderson and May1982), favouring the evolution and maintenance of sexual reproduction and certain sexually selected traits (Hamilton, Reference Hamilton1980; Hamilton and Zuk, Reference Hamilton and Zuk1982), and resulting in the development of complex parasite-resistance mechanisms (Anderson and May, Reference Anderson and May1982; Behnke et al. Reference Behnke, Barnard and Wakelin1992). However, to do so, infection with parasites must impose fitness costs on the host. Thus, in order to evaluate the importance of a particular parasite as an evolutionary force on a host species, we must first understand the impact that the parasite has on its host species.
Haemoproteus and Plasmodium species have received a large amount of attention from avian biologists, in part because of the relative ease of detection. For example, substantial effort has gone into determining host specificity (e.g. Waldenström et al. Reference Waldenström, Bensch, Kiboi, Hasselquist and Ottosson2002; Ricklefs et al. Reference Ricklefs, Fallon, Latta, Swanson, Birmingham, Greenberg and Marra2005) and distribution of these vector borne parasites (Valkiūnas, Reference Valkiūnas2005; Garvin et al. Reference Garvin, Szell and Moore2006). The parasites, which infect the red blood cells of vertebrate hosts, cause tissue damage and deplete host resources (Dawson and Bortolotti, Reference Dawson and Bortolotti2000), but do not typically cause direct mortality in birds (Forrester and Spalding, Reference Forrester and Spalding2003), nor are they so virulent as to completely eliminate reproduction (Merino et al. Reference Merino, Moreno, Sanz and Arriero2000; Marzal et al. Reference Marzal, Lope, Navarro and Møller2005). This makes it challenging to assess the impacts of infection on host fitness.
Nevertheless, interactions between haematozoan parasites and their avian hosts are frequently used to model fundamental questions in ecology and evolution (Hamilton and Zuk, Reference Hamilton and Zuk1982; Poulin et al. Reference Poulin, Marshall and Spencer2000); studies testing the assumption that these parasites consistently reduce host fitness in the wild have generally been conducted over relatively short time periods compared to the lifespan of host organisms and they have produced contradictory results. Such studies have adopted a wide variety of proxy measures in order to assess the fitness consequences of these parasites (e.g. body condition, mating success, timing of reproduction, and various measures of annual reproductive success), but there is contradictory evidence as to whether haematozoan infection impacts each of these metrics. Some studies suggest that haematozoan infections decrease host body condition (Dawson and Bortolotti, Reference Dawson and Bortolotti2000; Garvin et al. Reference Garvin, Szell and Moore2006; Groote and Rodewald, Reference Groote and Rodewald2010; Shurulinkov et al. Reference Shurulinkov, Chakarov and Daskalova2012). However, other studies, including one large comparative study of fifteen avian species, have failed to find such a relationship (Ashford, Reference Ashford1971; Smith and Cox, Reference Smith and Cox1972; Bennett et al. Reference Bennett, Caines and Bishop1988; Apanius, Reference Apanius, Nicholls and Clarke1993; Korpimaki et al. Reference Korpimaki, Tolonen and Bennet1995; Wiehn et al. Reference Wiehn, Korpimáki, Bildstein and Sorjonen1997; Garvin et al. Reference Garvin, Szell and Moore2006). Furthermore, contradictory results as to whether haematozoan infection impacts body condition have been reported within the same species, as in the case of American Kestrels (Falco sparverius) (Apanius, Reference Apanius, Nicholls and Clarke1993; Wiehn et al. Reference Wiehn, Korpimáki, Bildstein and Sorjonen1997; Dawson and Bortolotti, Reference Dawson and Bortolotti2000). One reason for this may be that the effects of parasitic infection on host body condition vary with breeding stage, sex, host species, or parasite species (Dawson and Bortolotti, Reference Dawson and Bortolotti2000; Palinauskas et al. Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2011).
Studies investigating the relationship between haematozoan infection and reproductive success or survival have also yielded mixed results. In some cases, infection has been associated with reduced fitness measures such as decreased male mating success (Pruett-Jones et al. Reference Pruett-Jones, Pruett-Jones and Jones1990), delayed arrival at the breeding grounds (Rätti et al. Reference Rätti, Dufva and Alatalo1993) or delayed breeding (Johnson and Boyce, Reference Johnson, Boyce, Loye and Zuk1991; Allander and Bennett, Reference Allander and Bennett1995), potentially resulting in reduced reproductive success (Allander and Bennett, Reference Allander and Bennett1995; Marzal et al. Reference Marzal, Lope, Navarro and Møller2005). Haematozoan infection has also been associated with a decrease in the proportion of eggs fledged (Merino et al. Reference Merino, Moreno, Sanz and Arriero2000), decreased brood size at fledging (Marzal et al. Reference Marzal, Lope, Navarro and Møller2005), or reduced return rates (Dawson and Bortolotti, Reference Dawson and Bortolotti2000). Furthermore, experimental removal of haemosporidian parasites has been shown to increase reproductive success in some host species (e.g. Haemoproteus prognei in the house martin, Delichon urbica (Marzal et al. Reference Marzal, Lope, Navarro and Møller2005)); however, studies such as these do not account for the ecological reality that clearing parasites, and maintaining an immune response capable of doing so, are themselves costly and could diminish investment in reproduction in the absence of medication. Contrary to these results, other studies have failed to find relationships between infection and various measures of fitness or reproduction (Morton, Reference Morton1992; Davidar and Morton, Reference Davidar and Morton1993; MacDougall-Shackleton et al. Reference MacDougall-Shackleton, Derryberry and Hahn2002) and some have found haematozoan infection to be associated with increased reproductive success (Sanz et al. Reference Sanz, Arriero and Moreno2001; Fargallo and Merino, Reference Fargallo and Merino2004; Kilpatrick et al. Reference Kilpatrick, LaPointe, Atkinson, Woodworth, Lease, Reiter and Gross2006; Marzal et al. Reference Marzal, Bensch, Reviriego, Balbontin and De Lope2008; Norte et al. Reference Norte, AraÚJo, Sampaio, Sousa and Ramos2009; Podmokła et al. Reference Podmokła, Dubiec, Drobniak, Arct, Gustafsson and Cichoń2014).
Further complicating our understanding of the impacts of infection on host fitness, most studies examining the impacts of haematozoan infection on reproductive success and fitness are conducted over just one or two breeding seasons. Extrapolating the results of such experiments to individual fitness consequences requires the assumption that individuals do not alter reproductive investment based on their current resources and condition; this is an untrue assumption that contradicts the basis of life-history theory (see e.g. (Williams, Reference Williams1966; Chastel et al. Reference Chastel, Weimerskirch and Jouventin1995; Love et al. Reference Love, Chin, Wynne-Edwards and Williams2005; Tieleman et al. Reference Tieleman, Dijkstra, Klasing, Visser and Williams2008). Interestingly, one study (Davidar and Morton, Reference Davidar and Morton1993) that attempted to measure the long-term impact of haematozoan infection on reproductive success (a 5-year study of Purple Martins (Progne subis)) found that Haemoproteus infected females had comparable return rates to uninfected females, but arrived earlier on the breeding grounds and fledged significantly more young than uninfected females.
To address this complex issue, we conducted a long-term (11-year) study examining the relationship between naturally occurring infection with haematozoan parasites, and reproductive success and survival of Mountain White-crowned Sparrows (MWCS, Zonotrichia leucophrys oriantha). Specifically, we test the hypothesis that haematozoan infections are costly for infected avian hosts (the Costly Parasite Hypothesis); this hypothesis predicts that birds infected with haematozoan parasites will have reduced survival (as determined by overwinter return rates) and reproductive success. We also test the alternative hypothesis that haematozoan infections exact a relatively low cost from avian hosts such that infection does not compromise host fitness (e.g. because hosts tolerate rather than clearing infections (Sorci, Reference Sorci2013)); this hypothesis predicts either no relationship between parasite infection and fitness, or even a positive relationship between parasite infection and fitness (e.g. if individuals that are more tolerant of infection and, therefore, invest less in controlling parasite infections are able to allocate additional resources to reproduction). Mountain White-crowned Sparrows are ideal for a long-term study of the impact of haematozoan parasites on reproductive success and survival because of their high levels of natal and adult philopatry (Morton, Reference Morton1992), which allow us to collect data on reproductive success and return to the breeding grounds over the course of an individual's lifetime.
METHODS
We investigated the impact of infection with haematozoan parasites on reproductive effort (number of eggs laid and eggs hatched), reproductive success (number of chicks fledged) and long-term survival in a migratory population of MWCS that breed on Tioga Pass Meadow, in the Sierra Nevada of California (37°54′40″N, 119°15′29″W). We captured adults (269 females, 466 males) from May to August of 1996–2006 using a combination of seed-baited Potter traps and mist nets; data on reproductive effort and success were collected in 1996–1999 and 2001–2006. We followed Morton (Reference Morton1992) in considering unbanded adults arriving at the study site to be yearlings, for reasons previously described (MacDougall-Shackleton et al. Reference MacDougall-Shackleton, Derryberry and Hahn2002). Unbanded individuals were marked with a US Fish and Wildlife Service leg band and a unique combination of three colour bands to permit individual identification in the field. Sex was determined based on the presence of an enlarged cloacal protuberance for males and a brood patch for females. In addition, beginning in 1997, a small blood sample was collected by brachial venipuncture to determine both infection status and intensity of infection by haematozoan parasites.
Infection status
For each bird, a drop of whole blood was used to make a blood smear, which was fixed and stained as previously described (Zylberberg et al. Reference Zylberberg, Lee, Klasing and Wikelski2013b ). For each smear, we scanned approximately 10 000 erythrocytes using an oil immersion lens at 1000× magnification and identified haematozoa gametocytes using morphological characteristics (Campbell, Reference Campbell1995); we observed two species of parasites, Haemoproteus beckeri and Plasmodium polare. Fields of view scored for parasites were evenly distributed over the entire surface of the smear to avoid oversampling a single area.
Co-infections were rare (they were only detected in three females and seven males). Due to this small sample size, we chose to include these individuals with individuals exhibiting single parasite infections for the purposes of our analysis, rather than breaking them out into their own group; they were included as infected in both the H. beckeri and P. polare analyses. The results of the H. beckeri analyses remained the same whether or not these doubly-infected individuals were included; however, we did not have a large enough sample size to conduct the P. polare analyses if we excluded doubly-infected individuals (of individuals for which we had data on lifetime reproductive success, 6 females and 10 males were infected with P. polare). For consistency, we included doubly infected individuals in all the analyses reported here.
Individuals were considered ‘infected’ for the purposes of our analysis if they were ever infected over the course of their lifetimes. Blood smears can miss low level chronic or latent Plasmodium spp. or Haemoproteus spp. infections, therefore, we considered this to be the most conservative way to classify individuals for the purposes of the lifetime reproductive effort and success analysis rather than to quantify, for example, the proportion of their lifetime during which an individual was infected based on imperfect data. However, individuals that repeatedly test positive for infection with H. beckeri or P. polare will have higher lifetime average infection intensities. Therefore, to further elucidate the impact of infection on host reproductive effort or success, we examine the relationship between average lifetime infection intensity and reproductive effort and success. We quantified infection intensity as the number of cells infected with H. beckeri or P. polare per 10 000 red blood cells.
Reproduction
Extensive surveys were used to identify all individuals present on Tioga Pass Meadow and particular care was taken to capture and band previously unbanded individuals. Mountain White-crowned Sparrows are socially monogamous and aggressively defend breeding territories. Territories can thus be identified throughout the study site using behavioural observations of adult birds. We made every effort to locate all of the nests initiated on Tioga Pass Meadow and in the surrounding areas, and we checked them every other day until the nestlings fledged or the nest failed. For each nest, we recorded the numbers of eggs laid, eggs hatched, and nestlings fledged. We observed each nest from a distance to identify the colour bands of the attendant male and female. Intraspecific brood parasitism appears not to occur in the study population (MacDougall-Shackleton et al. Reference MacDougall-Shackleton, Derryberry and Hahn2002) thus our measures of female reproductive effort and success are highly accurate. By contrast, extra-pair paternity in this population (MacDougall-Shackleton et al. Reference MacDougall-Shackleton, Derryberry and Hahn2002) likely introduces some error to our estimates of male fitness, in that observed (social) reproductive success may somewhat under- or over-estimate genetic reproductive success.
All birds were processed with the approval of the Institutional Animal Care and Use Committee of the University of California, Davis.
STATISTICAL ANALYSIS
To describe patterns of infection in the study population we first determined if infections varied with age or sex. We used a likelihood ratio chi-square test to determine whether one sex was more likely than the other to be infected with H. beckeri or P. polare, and a two-tailed student's t-test to evaluate sex differences in infection intensity of infected individuals. We used the number of years that an individual bred at the study site as a proxy for age. To determine whether likelihood of infection increased with age, we used a likelihood ratio chi-square test to test for differences in infection probability between first-time breeders at the study site (yearlings), second-year breeders, and those who had bred at the study site for three or more years (for females, N = 142, N = 65, and N = 62, respectively; for males N = 191, N = 131, and N = 144, respectively).
To assess the effect of infection on individual fitness, we examined the relationship between infection and estimated lifetime reproductive success (the total number of chicks fledged) in the subset of the sparrows for which we had this data (83 females and 73 males). To determine if lifetime reproductive effort varied with infection status, we constructed a GLM with a Gaussian link function, which fit our data better than other distributions (e.g. a Poisson distribution) according to residual plots and model AIC values; infection status (H. beckeri infected/uninfected and P. polare infected/uninfected) were fixed effects, and number of nestlings fledged was the outcome variable. This analysis excluded individuals already present on the meadow in the first year of the study (1996) as well as those still present on the meadow in the last year of data collection (2006), as such individuals may have had additional breeding opportunities in the years outside the study period. This and all other models were validated by (1) plotting residuals against fitted values to check for homogeneity; (2) plotting residuals against each explanatory variable; and (3) using histograms of the studentized residuals to check for normality of residuals.
Measures of reproductive effort are often used as proxies for fitness in the literature; therefore, to determine whether using measures of reproductive effort results in similar conclusions compared to using a direct measure of reproductive success we conducted similar, but separate, analyses to determine the impact of haematozoan infection on measures of lifetime reproductive effort (the total number of eggs laid and hatched). The models used in these analyses were the same as that described for the impact of haematozoan parasites on lifetime reproductive success but with the number of eggs laid and eggs hatched as outcome variables.
To determine whether overwinter return rates differed between infected and uninfected individuals, we used a likelihood ratio chi-square test. To determine whether reproductive effort or reproductive success varied with the average intensity of infection over an individual's lifetime, we constructed a linear mixed effects model using only infected individuals with the number of H. beckeri and P. polare parasites per 10 000 red blood cells as the fixed effect, and number of eggs laid, eggs hatched and nestlings fledged as outcome variables. Infection intensity data was normalized via log transformation. Statistical analyses were conducted using JMP 7.0.1 by SAS.
RESULTS
We collected blood smears from 775 individuals over the course of the study (269 females, 466 males, and 40 individuals of undetermined sex). Of these, we obtained lifetime reproductive data for 83 females and 73 males. Many individuals bred at the study site for multiple years, with the longest-lived individuals present on the study site for 9 years.
We detected two species of parasites, H. beckeri and P. polare. There was no significant difference between the sexes in likelihood of infection with H. beckeri (12% of females (33/269) were infected, 16% of males (72/466) were infected; χ 2 733 = 1·44, p = 0·23), or P. polare (5% of females (13/269) were infected, 5% of males (24/466) were infected; χ 2 733 = 0·036, p = 0·85), or in the intensity of infections among those individuals infected with P. polare (t 733 = 1·15, p = 0·25). However, H. beckeri infection intensity was higher in males than in females, when compared across infected individuals (males: 5·4 ± 1·7 infected cells; females: 1·8 ± 0·7 infected cells; t 733 = 1·96, p = 0·05). Because of this observed sex difference in infection intensity, we treat the sexes separately in the following analyses.
Prevalence of H. beckeri and P. polare infection did not vary significantly with age. In females, 14% of yearlings (20/142), 11% of 2-year olds (7/65), and 10% of birds aged three or older (6/62) were infected with H. beckeri (χ 2 269 = 0·62, p = 0·97), whereas 4% of yearlings (6/142), 8% of 2-year olds (5/65), and 3% of birds aged three or older (2/62) were infected with P. polare (χ 2 269 = 1·50, p = 0·47). Similarly, in males 15% of yearlings (28/191), 17% of 2-year olds (22/131), and 15% of birds aged three or older (22/144) were infected with H. beckeri (χ 2 464 = 0·27, p = 0·87), and 5% of yearlings (9/191), 5% of 2-year olds (7/131), and 6% of birds aged three or older (8/144) were infected with P. polare (χ 2 464 = 0·13, p = 0·94).
Of 83 females for which we had data on lifetime reproductive effort and success, 16 (19%) were infected with H. beckeri and 6 (7%) with P. polare. Of the H. beckeri infected females, 10 changed infection status over the course of their lifetimes; 4 became infected, 5 appeared to clear infection (though in these cases it is possible that an undetectably low-level infection remained or that the infection became latent), and 1 became infected and then appeared to clear the infection. Of the P. polare infected females, 2 changed infection status over the course of their lifetimes; 1 became infected, and 1 appeared to clear infection.
Of 73 males for which we had reproductive data, 19 (26%) were infected with H. beckeri and 10 (14%) with P. polare. Of the H. beckeri infected males, 6 changed infection status over the course of their lifetimes; 2 became infected, 3 appeared to clear infection, and 1 became infected and then appeared to clear the infection. Of the P. polare infected males, 7 changed infection status over the course of their lifetimes; 4 became infected, one appeared to clear infection, and 2 became infected and then appeared to clear the infection.
Across all individuals sampled over the course of the study, females infected with H. beckeri in a given breeding season were significantly more likely than uninfected females to return the following year (50% (17/34) vs 29% (78/273) respectively, likelihood ratio chi-square χ 2 307 = 6·07, p = 0·014). We observed no difference in overwinter return rates between P. polare-infected and uninfected females (31% in each case (4/13 and 91/294 returned, respectively), likelihood ratio chi-square χ 2 307=0·0, p = 0·99), H. beckeri-infected and uninfected males (36% in each case (27/74 and 160/450 returned, respectively), likelihood ratio chi-square χ 2 524=0·024, p = 0·88), or P. polare-infected and uninfected males (28% (7/25) and 36% (180/499), respectively, likelihood ratio chi-square χ 2 524 = 0·70, p = 0·40).
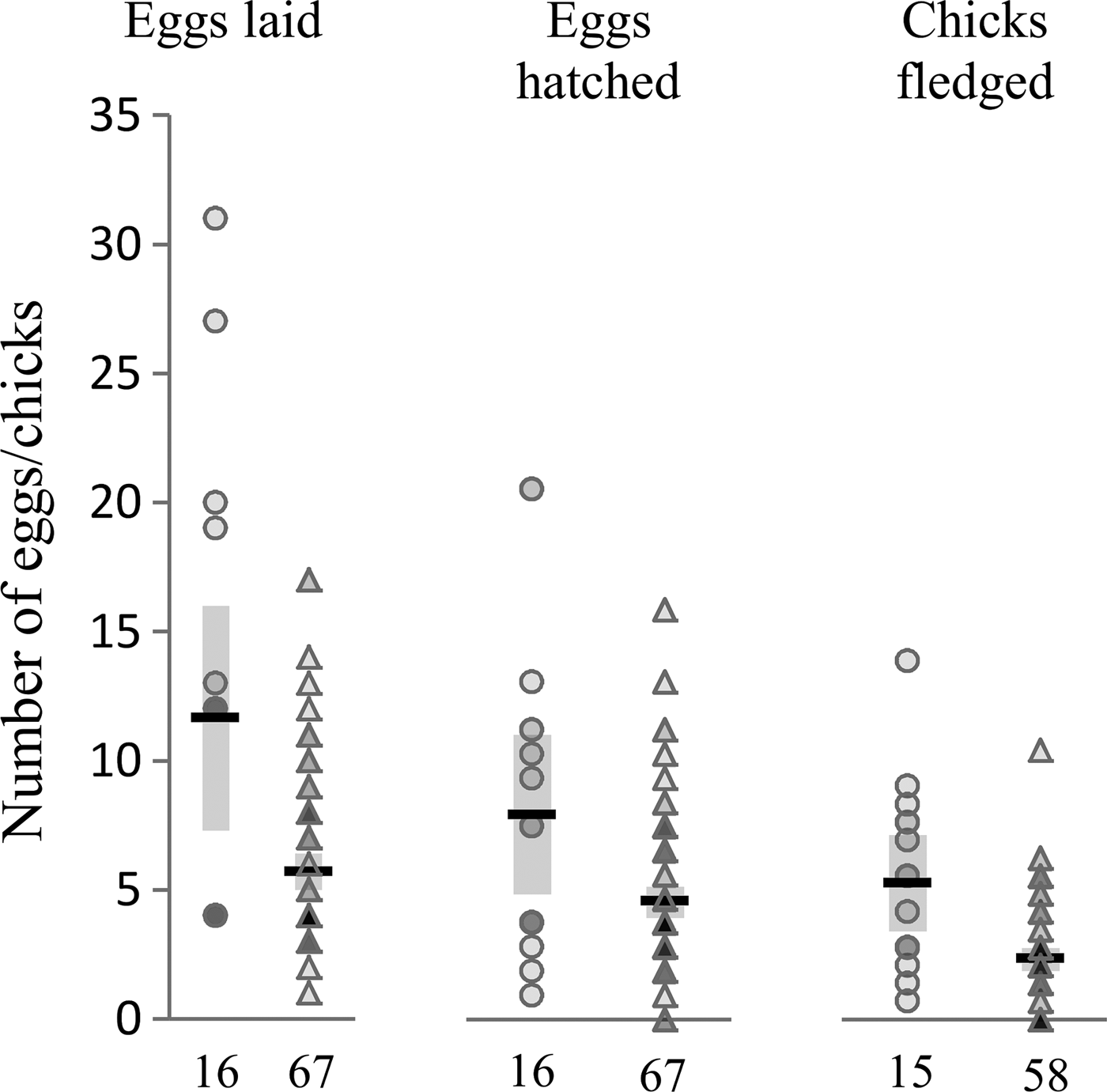
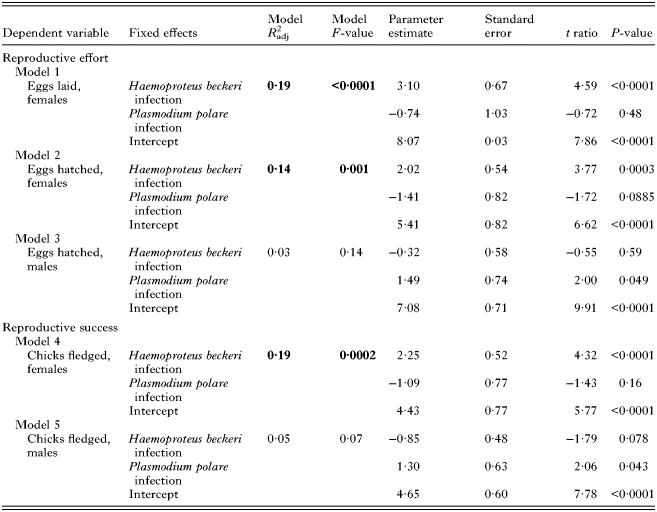
Contrary to our expectations, females infected with H. beckeri did not exhibit reduced reproductive effort and success compared to uninfected females. Instead, females that were infected with H. beckeri at some point in their lives exhibited substantial, and statistically significant, increases in lifetime reproductive success compared with uninfected individuals. Ultimately, H. beckeri-infected females laid and hatched about twice as many eggs and fledged about twice as many chicks as did uninfected females (Fig. 1, Table 1). By contrast, H. beckeri infection was not associated with lifetime reproductive effort or success of males, nor was P. polare infection associated with lifetime reproductive effort or success in either females or males (Table 1).
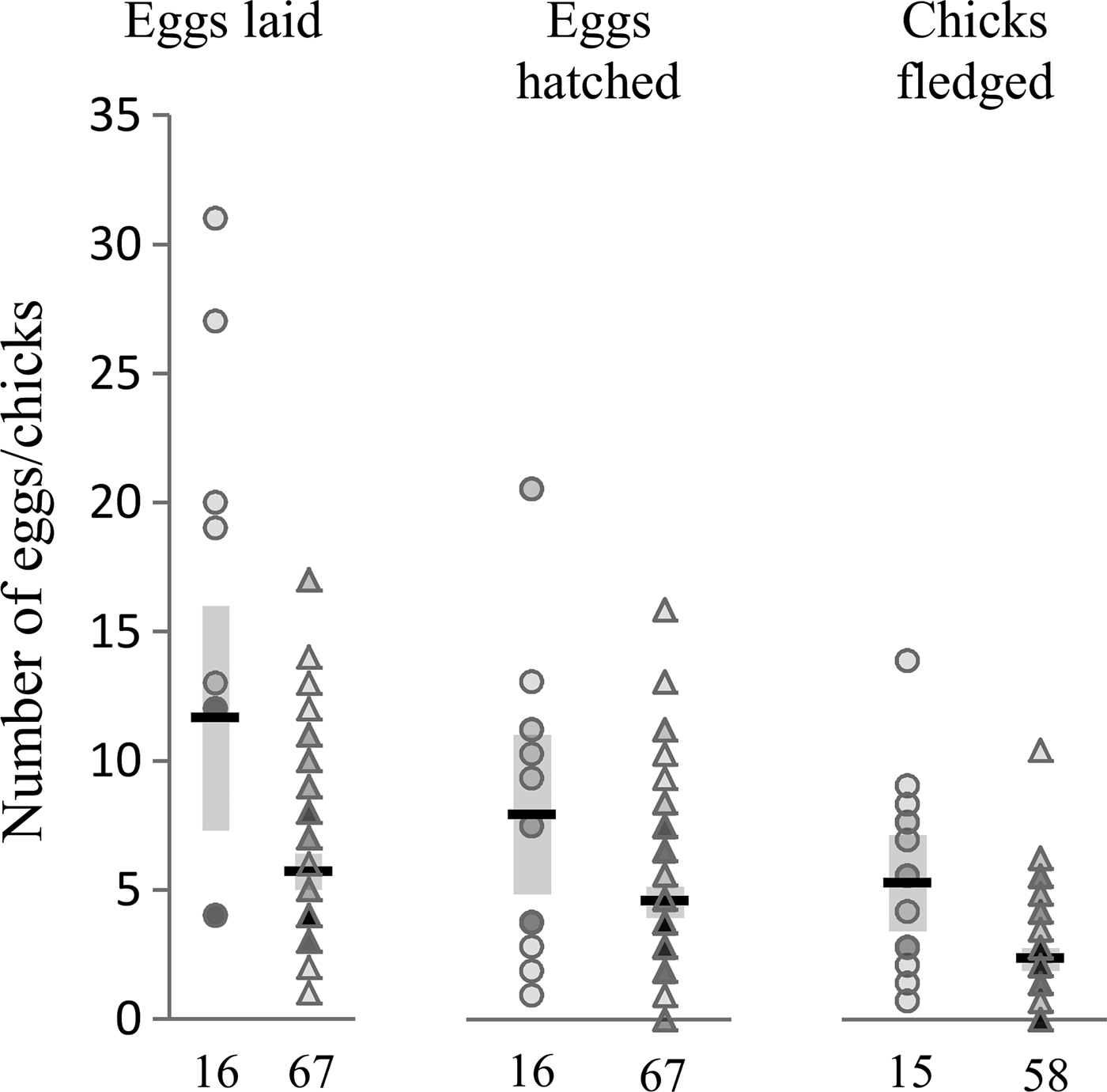
Fig. 1. Lifetime reproductive effort and success in H. beckeri infected and uninfected females. Figure shows number of eggs laid, eggs hatched and chicks fledged in infected females (circles) and uninfected females (triangles), with group means indicated by horizontal black lines, 95% confidence intervals around the means indicated by grey boxes, and sample sizes shown at bottom. Data points are transparent, with darker markers indicating multiple overlapping data points. Infected females laid on average 11·6 eggs, hatched 8·3 eggs and fledged 7·3 chicks; uninfected females laid on average 5·6 eggs, hatched 4·7 eggs and fledged 3·2 chicks.
Table 1. Relationship between haematozoan infection and lifetime reproductive effort and success
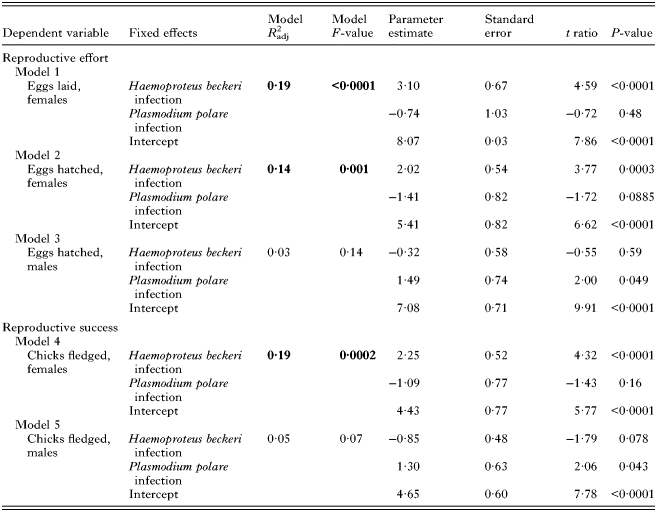
Statistically significant models are highlighted in bold font.
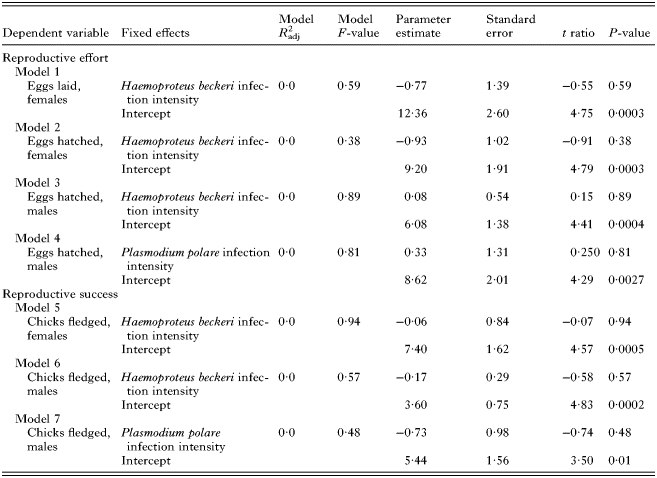
To further explore the impact of parasite infection on reproductive effort and success, we examined whether reproductive effort or reproductive success varied with the average intensity of infection over an individual's lifetime; this analysis included only infected individuals. We found no relationship between infection intensity and reproductive effort or success. The average intensity of H. beckeri infection over an individual's lifetime was not correlated with any measure of female reproductive effort or success (Table 2); sample size of P. polare-infected females was not sufficiently large (N = 6) to confidently ascertain whether intensity of P. polare infection predicts female reproductive effort or success. In males, neither H. beckeri nor P. polare infection intensity were correlated with reproductive effort or success. It is important to note that results regarding the impact of infection intensity on reproductive effort and success over an individual's lifetime are based on small sample sizes, and should be interpreted with caution.
Table 2. Relationship between haematozoan infection intensity and lifetime reproductive effort and success

DISCUSSION
The impact of haematozoan infection on host fitness has received substantial attention since Hamilton and Zuk posited that parasites are important drivers of sexual selection (1982). However, this is the first study, to our knowledge, that measures the impact of haematozoan infection on lifetime reproductive effort and success. We found evidence of sex differences in susceptibility to haematozoan infection, as well as an association between parasite infection status and reproductive effort and success in females. Interestingly, however, the relationship we found was contrary to the predictions of the Costly Parasite Hypothesis – the hypothesis that is most intuitive: specifically, H. beckeri-infected females exhibited increased overwinter return rates (interpreted as survival) and fledged more than twice as many chicks during their lifetimes as did uninfected females. Based on our results we argue that, in the case of less virulent pathogens, investment in excessive immune defence may decrease lifetime reproduction.
SEX DIFFERENCES
It remains an open question whether there are consistent sex differences in pathogen susceptibility. Although some meta-analyses have found that males are more frequently and more heavily infected with parasites (Poulin, Reference Poulin1996; Schalk and Forbes, Reference Schalk and Forbes1997), others have shown females to have higher parasite prevalence than males (McCurdy et al. Reference McCurdy, Shutler, Mullie and Forbes1998). However, this latter result appears confined to polygynous species whereas the same study found that monogamous species showed little difference among the sexes in parasite prevalence (McCurdy et al. Reference McCurdy, Shutler, Mullie and Forbes1998). Consistent with this, we did not observe sex differences in the prevalence of H. beckeri or P. polare in socially monogamous Mountain White-crowned Sparrows. However, males in this species had higher intensity H. beckeri infections than did females. With no difference in prevalence, it appears that exposure does not differ between the sexes; instead, males and females may differ in their ability to successfully control haematozoan infections following exposure, perhaps due to differences in immune function or hormone profiles (Sheridan et al. Reference Sheridan, Poulin, Ward and Zuk2000; Cornelius et al. Reference Cornelius, Zylberberg, Breuner, Gleiss and Hahn2014).
REPRODUCTIVE SUCCESS AND INFECTION
Contrary to the Costly Parasite Hypothesis, we found no evidence of a correlation between haematozoan infection and reduced reproductive success or survival in either males or females. Instead, a major and unexpected finding of this study is that H. beckeri infection was associated with increased female reproductive effort and success. Infected females laid and hatched approximately 2-fold more eggs and fledged more than twice as many chicks as uninfected females over the course of their lifetimes, supporting the hypothesis that haematozoan infections do not compromise host fitness. It is noteworthy that we found a similar pattern in how H. beckeri infection impacted measures of both lifetime reproductive effort and success. This suggests that measures of lifetime reproductive effort may be adequate proxies for lifetime reproductive success, if success is not measurable.
The observed relationship between H. beckeri infection and female reproductive success could be confounded by age as older individuals have more opportunities to breed and also more opportunities to become infected with haematozoa. However, if increased reproductive success and infection were correlated solely as a result of lifespan, we would expect (1) a positive relationship between reproduction and infection status in both males and females; and (2) a positive relationship between reproduction and infection for both H. beckeri and P. polare. Instead, we see differences in reproductive success based on both sex and haematozoan species, suggesting that the observed relationship is not simply an artefact of longer-lived birds having more opportunity to become infected. Nonetheless, the timing and duration of infection and the health status of an individual's mate are both likely to alter the impact of infection on individual reproductive success; future studies would do well to examine these topics.
It is possible our results reflect a sampling bias towards high quality infected individuals (those able to survive and breed despite infection). However, this is unlikely as the prevalence of haematozoan infections among the overall population was comparable to, and even slightly lower than, prevalence for the subset of individuals included in our analysis of lifetime reproduction. This suggests that infected individuals are no less likely to breed than uninfected individuals and, therefore, that the observed relationship between parasitism and female reproduction cannot be attributed to sampling bias.
It remains possible that a third, unmeasured, factor (e.g. territory quality or migration route) results in both increased lifetime reproduction and increased infection risk, or that these ‘parasites’ are in fact beneficial to the host in some way. It also remains possible that infection did impact male reproduction as we did not measure extra pair, but only social, paternity. In our study, a large portion of P. polare infected individuals were also infected with H. beckeri; therefore, the impacts of double infection could be a confounding factor in our understanding of the impacts of P. polare infection on reproductive success and survival. In addition, despite collecting infection and lifetime reproductive data on over 200 individuals, we nonetheless have a relatively small sample size of infected individuals (35 in total); future studies would do well to examine the impact of infection on lifetime reproductive success using larger samples sizes. Finally, it is worth noting that we estimated prevalence based on analysis of blood smears. It has been suggested that this method underestimates parasite prevalence; however, Valkiūnas et al. (Reference Zylberberg, Klasing and Hahn2006) showed that microscopy results in estimates of haematozoan prevalence comparable to PCR, indicating that this is unlikely to be an important source of bias in our study.
ARE HAEMATOZOAN INFECTIONS COSTLY?
There have been two main approaches to addressing the relationship between reproduction and infection with a parasite or pathogen (Siikamaki et al. Reference Siikamaki, Ratti, Hovi and Bennett1997), each assuming that infection decreases current or future reproductive success. The first approach is to examine the impact of parasitism on host reproduction, and posits that infection decreases reproductive success (e.g. Korpimaki et al. Reference Korpimaki, Hakkarainen and Bennett1993; Hurd, Reference Hurd2001). The second approach examines the impact of current reproductive effort on current or future susceptibility to parasitic infection; this approach posits that reproductive effort increases susceptibility to infection and predicts that, within any given breeding attempt, parasitism should be positively related to reproduction. Within this second framework, findings of a positive relationship between parasitism and host reproductive output (e.g. Festa-Bianchet, Reference Festa-Bianchet1989; Apanius et al. Reference Apanius, Deerenberg, Visser and Daan1994; Richner et al. Reference Richner, Christe and Oppliger1995) have generally been interpreted as evidence that individuals allocate resources to reproduction at the expense of parasite and pathogen defence mechanisms and, ultimately, at the expense of future reproductive success (Sheldon and Verhulst, Reference Sheldon and Verhulst1996). Such conclusions are based on the assumption that increased investment in reproduction in a given season decreases future reproductive potential as the result of trade-offs between current and future reproductive effort or between reproduction and self-maintenance (Tuomi et al. Reference Tuomi, Hakala and Haukioja1983).
The long-term nature of our study allows us to challenge this longstanding assumption. Females infected with H. beckeri were actually more likely to return overwinter than their unparasitized counterparts and ultimately enjoyed substantially (approximately 2-fold) higher lifetime reproductive success than uninfected females. This counterintuitive finding mirrors that reported for Purple Martins (Progne subis) in which Haemoproteus-infected females also had higher reproductive success over the course of 5 years than their uninfected counterparts (Davidar and Morton, Reference Davidar and Morton1993) (our study extends this finding to lifetime reproductive success).
It is possible that the MWCS studied here, and the Purple Martins studied by Davidar and Morton (Reference Davidar and Morton1993) were simply infected with parasites that were not particularly virulent to those host species or populations (Palinauskas et al. Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2008, Reference Palinauskas, Iezhova, Križanauskienė, Markovets, Bensch and Valkiūnas2013); indeed, little is known about the pathogenicity of either H. beckeri or P. polare (Valkiūnas Reference Valkiūnas2005). Nonetheless, the stark contrast in results between the findings of these two long-term studies (our study and that of Davidar and Morton (Reference Davidar and Morton1993)), and short-term studies of the impacts of haematozoan infection on reproductive effort and success, indicate that more long-term studies of the impacts of haematozoan infection on lifetime reproductive success are needed.
TOLERANCE AS A PARASITE COPING METHOD
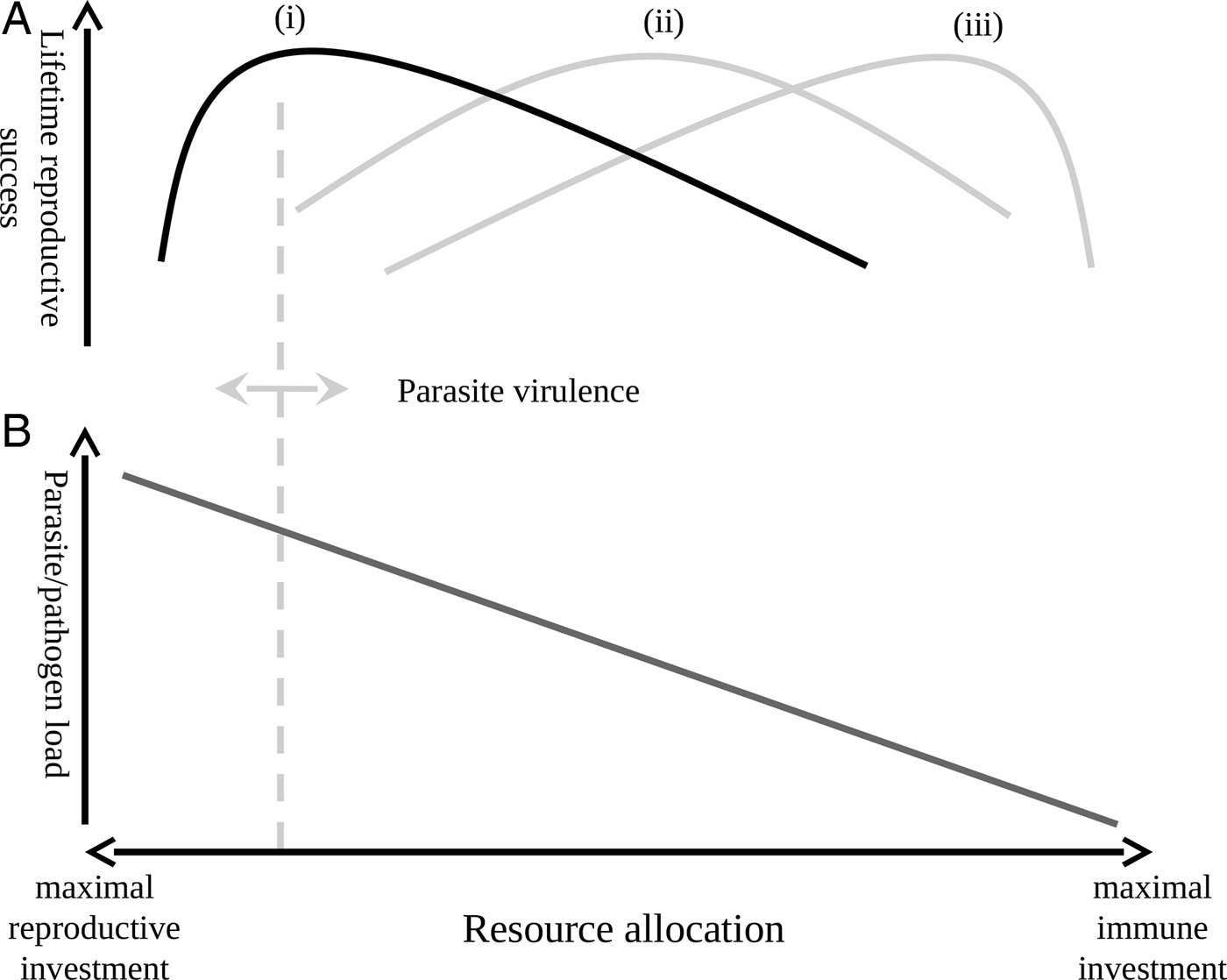
One interesting possibility suggested by our findings is that, contrary to prevailing dogma, parasitic infection may not always decrease host fitness. That is not to say that parasites do not exact a cost from their host in terms of lost resources; experimental treatment of malarial parasites has been shown to increase reproductive success (e.g. (Marzal et al. Reference Marzal, Lope, Navarro and Møller2005; Knowles et al. Reference Knowles, Palinauskas and Sheldon2010a )). However, when treatment studies show that removing parasites in the absence of a cost associated with that removal increases reproductive success, they do not take into account that the clearing of parasites, and the maintenance of an immune response capable of doing so, are themselves costly (Klasing, Reference Klasing1998; Martin et al. Reference Martin, Scheuerlein and Wikelski2003; Lee et al. Reference Lee, Cory, Wilson, Raubenheimer and Simpson2006; Owen-Ashley and Wingfield, Reference Owen-Ashley and Wingfield2007; Zylberberg et al. Reference Zylberberg, Klasing and Hahn2013a , Reference Zylberberg, Klasing and Hahn2014; Zylberberg, Reference Zylberberg2014). There is now a burgeoning literature on parasite resistance and tolerance which suggests that, under certain circumstances, tolerating a parasitic infection may be the least costly strategy (as reviewed in Ayres and Schneider, Reference Ayres and Schneider2012; Medzhitov et al. Reference Medzhitov, Schneider and Soares2012; Sorci, Reference Sorci2013). This suggests an optimal level of investment in immune function whereby less virulent parasites are tolerated below a threshold infection intensity; above this threshold host individuals might benefit from investing in parasite control mechanisms, but below the threshold further reduction of the parasite population becomes a waste of resources that could be better invested in reproduction (Fig. 2).
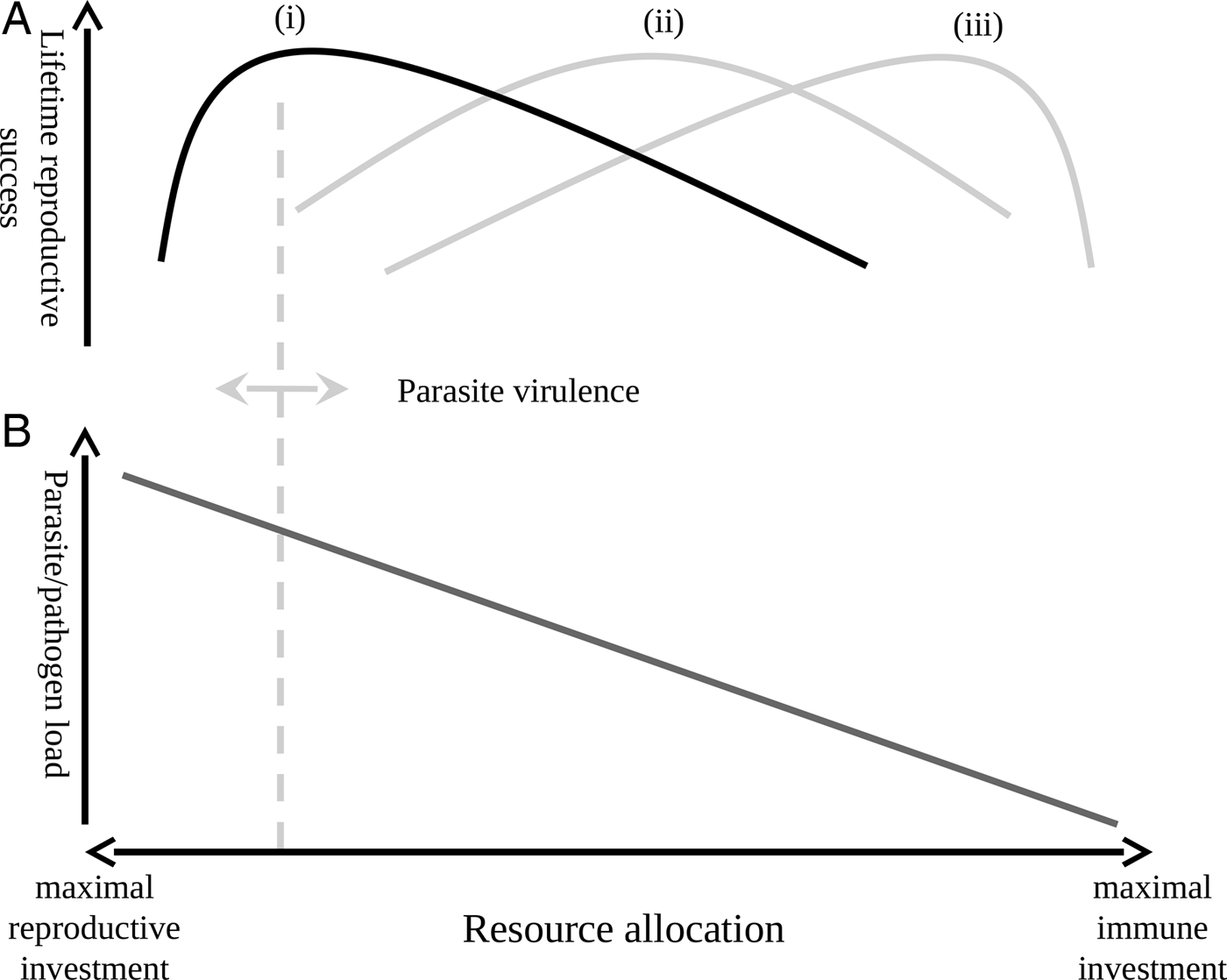
Fig. 2. Lifetime reproductive success as a function of immune investment, reproductive effort and pathogen virulence. The upper panel (A) shows how lifetime reproductive success is expected to vary with shifts in resource allocation in the case of a low-virulence (i), medium virulence (ii) and high-virulence (iii) pathogen. The lower panel (B) shows how shifts in resource allocation are expected to impact the parasite/pathogen load of an infected individual. The vertical dashed line is the threshold above which increased investment in immune function decreases reproductive success; this threshold shifts, as indicated by the double-headed arrow, with changes in pathogen virulence. In the case of a low-virulence pathogen (i, highlighted in black), the point at which increasing immune investment to control parasite/pathogen load is quickly reached and individuals would then do better to tolerate a low-level infection and invest in reproduction.
The hypothesis that over-investment in excessive immune defence decreases lifetime reproduction under certain circumstances may explain why we see differences in the impacts of infection on MWCS reproduction by parasite species and with host sex. In the case of parasite species, Haemoproteus spp. parasites are generally considered to be less virulent than Plasmodium spp. parasites (Van Riper et al. Reference Van Riper, Van Riper, Goff and Laird1986; Atkinson and van Riper, Reference Atkinson, van Riper, Loye and Zuk1991; Bennett et al. Reference Bennett, Peirce and Ashford1993); therefore, investing a given amount in defences when infected with Haemoproteus spp. parasites may be a waste of resources better spent on reproduction, while an equal investment in defences during Plasmodium spp. infection may be beneficial to the host (Fig. 2). In the case of sex differences, males exhibited higher intensity H. beckeri infections than females, suggesting a decreased ability to control H. beckeri infections and, therefore, less investment in immune function compared to females. If breeding males do indeed invest less in immune function than females, then they would be less likely to cross the threshold at which excessive investment in immune function results in a decrease in reproductive success, and less likely to exhibit a relationship between H. beckeri infection and reproduction than females. In considering these possibilities, it should be noted that we have a small sample size of individuals infected with P. polare, and that an impact of H. beckeri infection on male reproductive success could be obscured by our measure of social (as opposed to genetic) male reproductive success. Furthermore, while our data suggest some intriguing possibilities, we did not collect data on immune function and so are unable to test whether or not variation in immune investment underlies the observed results. Ultimately, an experimental study is required to further test this hypothesis; this could be accomplished by causing infected individuals to mount an immune response themselves that would clear haematozoan infections (e.g. by exposing individuals to an appropriate immune challenge) and then comparing their reproductive success to control individuals that remain infected.
Concluding remarks
Our results suggest that caution must be used in deciding whether specific blood parasites are suitable for testing ecological and evolutionary hypotheses, and that the details, and especially costs, of these and other parasite systems must be understood before undertaking such studies. Although haematozoan parasites are easily detected, they make up only a small portion of the many infectious pathogens (including fungal, bacterial and viral pathogens) to which birds are exposed, some of which likely have much more ecologically and evolutionarily important impacts on their host species. In order to test hypotheses regarding the ecology, evolution and physiology of hosts in relation to their pathogens, we need to focus on the pathogens that have the greatest impact on their hosts. This goal requires that we first develop a much more complete picture of the entire community of pathogens to which a host is exposed, not just those that we are already aware of, and that we develop a better understanding of how exactly these pathogens affect their hosts. Recent technological advances have made broad-scale pathogen surveys feasible (Wang et al. Reference Wang, Coscoy, Zylberberg, Avila, Boushey, Ganem and DeRisi2002), and researchers have begun to conduct species-based pathogen surveys (Li et al. Reference Li, Victoria, Wang, Jones, Fellers, Kunz and Delwart2010). It is time to begin to address the relationship between avian hosts and their pathogens in a thorough and methodical way, and to focus these efforts on pathogens with important ecological impacts on their host species.
ACKNOWLEDGEMENTS
We express our appreciation to numerous field assistants and undergraduate researchers who assisted with data collection.
FINANCIAL SUPPORT
Funding was provided by the National Science Foundation (grants to T.P.H (IBN-0235911) and C.W.B. (PSI0747361 and IBN-0236536)).