Introduction
Hydatidosis or cystic echinococcosis (CE) is an important parasitic zoonotic disease caused by the metacestode (hydatid cyst) of the dog Echinococcus granulosus tapeworm. This disease has a worldwide distribution and is commonly seen in developing and undeveloped countries (Soulsby, Reference Soulsby1982; Taylor et al., Reference Taylor, Coop and Wall2016; Siyadatpanah et al., Reference Siyadatpanah, Anvari, Emami Zeydi, Hosseini, Daryani, Sarvi, Budke, Esmaeelzadeh Dizaji, Mohaghegh, Kohansal, Dodangeh, Saberi and Gholami2019). This infection is of medical, veterinary and economic importance in endemic areas due to its significance in public human health, animal morbidity, and organ and meat condemnation during meat inspection in the abattoir (Schantz, Reference Schantz1991). The transmission of Echinococcus species involves two mammalian hosts, including a carnivore predator (e.g. canid or felid) as a definitive host and its herbivore prey (mostly ungulates and lagomorphs) as the intermediate hosts. However, the infection accidentally occurs in humans via the ingestion of infective eggs through contaminated food/water resources (Budke et al., Reference Budke, Deplazes and Torgerson2006; McManus et al., Reference McManus, Gray, Zhang and Yang2012; Zhang et al., Reference Zhang, Zhang, Wu, Shi, Li, Zhou, Wen and McManus2015).
In CE endemic areas, such as central Asia, South America and the Mediterranean countries, the incidence rate has been estimated to range from <1 to 200 cases per 100 000 individuals; however, the mortality rate is usually low (2–4%) (Craig et al., Reference Craig, McManus, Lightowlers, Chabalgoity, Garcia, Gavidia, Gilman, Gonzalez, Lorca, Naquira, Nieto and Schantz2007, Reference Craig, Li, Qiu, Zhen, Wang, Giraudoux, Ito, Heath, Warnock and Schantz2008; Zhang et al., Reference Zhang, Zhang, Wu, Shi, Li, Zhou, Wen and McManus2015; Galeh et al., Reference Galeh, Spotin, Mahami-Oskouei, Carmena, Rahimi, Barac, Ghoyounchi, Berahmat and Ahmadpour2018; Wen et al., Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019). The average global burden of human CE is between 1 and 3.6 million disability-adjusted life years (Budke et al., Reference Budke, Deplazes and Torgerson2006; Craig et al., Reference Craig, McManus, Lightowlers, Chabalgoity, Garcia, Gavidia, Gilman, Gonzalez, Lorca, Naquira, Nieto and Schantz2007). The development of hydatid cysts is the principal consequence of CE; nonetheless, they occur very slowly, which is mostly lifelong (Possenti et al., Reference Possenti, Manzano-Roman, Sanchez-Ovejero, Boufana, La Torre, Siles-Lucas and Casulli2016). The clinical features of this condition are associated with the damage and/or dysfunction of the target organs, rendering a great deal of health and social consequences for the affected populations (Torgerson and Macpherson, Reference Torgerson and Macpherson2011).
In humans, the diagnosis of CE is established based on clinical findings, imaging results and serology. The clinical manifestations of this disease include cyst rupture, secondary bacterial infection, allergic reactions or anaphylaxis. Ultrasound is a crucially important tool for the diagnosis, staging and follow-up of abdominal CE cysts; however, it has low sensitivity for the detection of small cysts. In addition, the polymerase chain reaction analysis of the biopsy material is another approach for the establishment of a definitive diagnosis. The CE serology is a helpful diagnostic adjunct method that can be used to monitor patients after surgery or pharmacotherapy (McManus et al., Reference McManus, Gray, Zhang and Yang2012). On the other hand, the presence of hydatids as a clinical entity is rarely suspected in domestic animals and never requires a specific diagnosis (Taylor et al., Reference Taylor, Coop and Wall2016). Hydatid cysts are usually identified during the slaughter of domestic animals at slaughterhouses. However, serological methods have been used to diagnose hydatidosis in domestic animals (Dada and Belino, Reference Dada and Belino1978; Dada et al., Reference Dada, Adegboye and Mohammed1981; Haroun et al., Reference Haroun, Omer, Mahmoud and Draz2008; Sazmand et al., Reference Sazmand, Razi Jalali, Hekmatimoghaddam and Asadollahi2013; Igwenagu et al., Reference Igwenagu, Onyiche, Saidu, Chahari, Waziri and Kayeri2018).
Despite the wide use of anthelmintics, improved slaughter hygiene, administration of praziquantel to dogs and health education programmes, CE is still globally prevalent (Budke et al., Reference Budke, Carabin, Ndimubanzi, Nguyen, Rainwater, Dickey, Bhattarai, Zeziulin and Qian2013). Moreover, the long-term under-dosing of antiparasitic drugs leading to drug resistance, as well as the increasing concern regarding the presence of drug residues in the edible animal products, has led researches towards searching for vaccination strategies (Pourseif et al., Reference Pourseif, Moghaddam, Saeedi, Barzegari, Dehghani and Omidi2018).
Echinococcus granulosus is characterized by high intraspecific variability (genotypes G1 to G10) (Supplementary Table S1). According to the new molecular phylogeny of genus Echinococcus, E. granulosus is divided into E. granulosus sensu stricto (G1 to G3), E. equinus (G4), E. ortleppi (G5) and E. canadensis (G6 to G10) that has been recently named as E. intermedius (Nakao et al., Reference Nakao, McManus, Schantz, Craig and Ito2006, Reference Nakao, Lavikainen, Yanagida and Ito2013). Camels have an important role in the transmission cycle of the parasite and are usually infected with G6 genotype (Thompson and Lymbery, Reference Thompson and Lymbery1995). Camel strain (G6 genotype) has been detected in humans (Siyadatpanah et al., Reference Siyadatpanah, Anvari, Emami Zeydi, Hosseini, Daryani, Sarvi, Budke, Esmaeelzadeh Dizaji, Mohaghegh, Kohansal, Dodangeh, Saberi and Gholami2019). Camels as important intermediate hosts for hydatidosis, especially in desert areas, and natural intermediate hosts for E. granulosus play an important role in the sustenance of the parasite in the nature, especially in arid regions (Thompson, Reference Thompson2008). Furthermore, the camels scattered in desert or semi-desert areas, where carnivorous animals and other ruminants may live, could be infected with this important genotype (Ebrahimipour et al., Reference Ebrahimipour, Sadjjadi, Darani and Najjari2017).
Various genotypes show differences in morphological characterization, transmission dynamics, life cycle, host range, development levels, pathogenicity and sensitivity to chemotherapeutic drugs (Thompson et al., Reference Thompson, Lymbery and Constantine1995) (Supplementary Table S1). Parasite characterization and the identification of strain differences are particularly important in regions where there are more than one species of intermediate host. These measures can facilitate the identification of the different cycles of transmission and sources of infection for humans. This is the case in the Middle East, Africa and China, where numerous intermediate host species harbour the cysts of E. granulosus (Thompson and Lymbery, Reference Thompson and Lymbery1988; Thompson, Reference Thompson1995). Developmental differences between the strains of Echinococcus, such as the variation in the onset of egg production, will affect transmission and impede the control efforts when regular, adult cestocidal treatment is used to break the cycle (Kumaratilake et al., Reference Kumaratilake, Thompson and Dunsmore1983; Thompson et al., Reference Thompson, Kumaratilake and Eckert1984; Eckert et al., Reference Eckert, Thompson, Michael, Kumaratilake and El-Sawah1989).
The strains of varying pathogenicity will influence the prognosis in patients with hydatid disease. This is of particular significance in cases with alveolar hydatid disease (Kawase and Yagi, Reference Kawase and Yagi1985; Liance et al., Reference Liance, Bresson-Hadni, Vuitton, Bretagne and Houin1990). Nonetheless, it is also important for cystic hydatid disease, caused by E. granulosus. For instance, the sylvatic strain of E. granulosus in North America reportedly causes a benign infection in humans (Wilson et al., Reference Wilson, Diddams and Rausch1968). However, in some parts of Kenya and Libya, there are local virulent strains of E. granulosus (French et al., Reference French, Nelson and Wood1982; Gebreel et al., Reference Gebreel, Gilles and Prescott1983).
Based on the evidence, certain strains of E. granulosus, such as those adapted to horses and pigs, may not be infective to humans (Thompson and Lymbery, Reference Thompson and Lymbery1988, Reference Thompson and Lymbery1991; Shablovskaia et al., Reference Shablovskaia, Bulgakov, Ponomareva, Dan'ko and Voloshchuk1989). It has been suggested that the strains of Echinococcus may differ in their response to particular chemotherapeutic regimens (Saimot et al., Reference Saimot, Meulemans, Hay, Mohler and Manuel1981; Schantz et al., Reference Schantz, Van den Bossche and Eckert1982; Kammerer and Schantz, Reference Kammerer and Schantz1984). These discrepancies have important implications with regard to the epidemiology and control of hydatidosis. Accordingly, it is essential to characterize the causative agent in different endemic regions using molecular techniques in order to identify the transmission patterns, especially where there is more than one genotype in the hydatidosis cycles and the interaction between various cycles is probable (Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Harandi, Rezaeian, Mohebali, Eshraghian, Rahimi and Kia2010).
The mentioned issues highlight the need for performing a study to identify the common genotypes of E. granulosus in cases with hydatid disease, as well as evaluating the overall prevalence of this disease in camels around the world in order to determine the risk factors associated with this zoonotic condition. With this background in mind, the current review was conducted to investigate the genotypic status and worldwide prevalence of hydatid disease in camels.
Methods
Research design
The current review was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). All studies conducted on the prevalence and genotypes of E. granulosus infection in camels up to 1 April 2020 were searched using seven English language databases, including Scopus, PubMed, Science Direct, ProQuest, ISI Web of Science, EMBASE and Google Scholar. The search process was performed using the following MeSH terms (Medical Subject Heading): ‘Echinococcus granulosus’, ‘Prevalence’, ‘Hydatid’, ‘Hydatidosis’, ‘Genotype’, ‘Genetic variation’, ‘Genetic characterization’ and ‘Camel’, alone or in combination with ‘AND’ and/or ‘OR’ in English language.
Inclusion and exclusion criteria
The inclusion criteria were: (A) examination of the prevalence of hydatidosis in camels with detailed results, (B) identification of E. granulosus genotypes (genetic characterization) in camels with hydatidosis, and (C) availability of the full-text version. On the other hand, the review papers and articles irrelevant to the topic under study, as well as the papers presenting no detailed results, were excluded from the review process.
Study selection and data extraction
In order to select the studies, the papers were subjected to a primary screening of titles or/and abstracts performed independently by three authors. The eligible full-text papers were collected, and the information was recorded. The recorded information included the author's name, publication year, applied diagnostic techniques, geographical area, total sample, number of positive cases, infected organ, fertility rate of the cyst, gender, genotype and gene markers.
Quality assessment
In the current review, the included studies were evaluated based on the Newcastle Ottawa Scale. In this regard, the papers with the scores of <3.5, 3.6–5.25 and 5.26–7 were categorized as low-, moderate- and high-quality papers, respectively (Modesti et al., Reference Modesti, Reboldi, Cappuccio, Agyemang, Remuzzi, Rapi, Perruolo and Parati2016). Therefore, the studies with suitable and acceptable quality were considered for the meta-analysis.
Meta-analysis
Point estimates and 95% confidence intervals (CI) of the pooled prevalence of the included studies were calculated. For the purpose of meta-analysis, a random-effect model was selected to account for the study effect and the expected variation in infection prevalence depending on the studies. In addition, forest plot, Cochrane's Q test and I 2 test were used to determine the heterogeneity among the included studies. The amount of heterogeneity varied from 0 to 100%, indicating the difference in the point estimate that can be attributed to study heterogeneity rather than chance. The I 2 values of <25, 25–50 and >50% were representative of low, moderate and high heterogeneity, respectively. In case of the presence of moderate to high heterogeneity, the datasets were subgrouped to investigate the possible sources of heterogeneity.
In the present review, an I 2 value of >75% was considered to be indicative of significant heterogeneity, warranting the implementation of an analysis with a random-effects model as opposed to the fixed-effects model to adjust for the observed variability. This heterogeneity was further explored through subgroup analyses (Rothstein et al., Reference Rothstein, Sutton and Borenstein2005; Hosseininejad et al., Reference Hosseininejad, Sharif, Sarvi, Amouei, Hosseini, Nayeri Chegeni, Anvari, Saberi, Gohardehi, Mizani, Sadeghi and Daryani2018; Saberi et al., Reference Saberi, Sharif, Sarvi, Aghayan, Hosseini, Anvari, Nayeri Chegeni, Hosseininejad and Daryani2018; Anvari et al., Reference Anvari, Sharif, Sarvi, Aghayan, Gholami, Pagheh, Hosseini, Saberi, Chegeni, Hosseininejad and Daryani2019, Reference Anvari, Saberi, Sharif, Sarvi, Hosseini, Moosazadeh, Hosseininejad, Chegeni and Daryani2020; Chegeni et al., Reference Chegeni, Sharif, Sarvi, Moosazadeh, Montazeri, Aghayan, Balalami, Gholami, Hosseininejad and Saberi2019). The subgroup analyses were performed based on the year, continent, country, gender, infected organ, fertility rate and applied diagnostic test. Fisher's exact test was also used to investigate the interaction (X 2) among the subgroups. Furthermore, based on the Egger's regression test, a funnel plot was designed to check for the existence of publication bias (Rothstein et al., Reference Rothstein, Sutton and Borenstein2005). The data were analysed in StatsDirect software (version 2.7.2). A P value <0.05 was considered statistically significant (Higgins and Thompson, Reference Higgins and Thompson2002).
Results
Qualified studies
Our primary search in seven databases resulted in the identification of a total of 714 articles. After the exclusion of the duplicate papers, 271 articles remained. The screening of the abstracts and titles led to the removal of 138 articles. Out of the remaining 133 studies, 11 papers were eliminated after the investigation of the full-text version of the articles according to the inclusion and exclusion criteria. Accordingly, a total of 70 qualified articles (dataset = 74) were considered for meta-analysis. Figure 1 illustrates a flow chart showing the process of the study evaluation and selection. The characteristics of the qualified studies are presented in Table 1.

Fig. 1. Flow chart of the study process.
Table 1. Characteristics of included studiesa to meta-analysis up to 1 April 2020

Q. A, quality assessment; ME, microscopic examination; HE, histological examination; CCIE, counter current immunoelectrophoresis; DD, Ouchterlony's double diffusion; IH, indirect haemagglutination; CIEP, counter immunoelectrophoresis.
a Type of studies were cross-sectional.
Echinococcus granulosus genotypes in camels with hydatidosis
The E. granulosus genotypes identified in hydatid cysts in camels were G1, G2, G3, G1–G3 complex, G5, G6, G7, G6–G7 complex and G6–G10 complex. The G6 was found to be the most common genotype throughout the world. With regard to the continental distribution of E. granulosus genotypes, G1, G2, G5, G6, G6–G7 and G6–G10 were the common genotypes identified in Africa, while G1, G2, G3, G1–G3, G5, G6, G6–G7, G6–G10 and G7 were the commonly distributed genotypes in Asia. Among the two mentioned continents, G6 genotype had the highest number of reports.
In terms of genotype distribution in different countries, G2/G6, G2/G6/G6–G7, G1/G6, G1/G5/G6, G1/G6–G7, G6 and G1/G3/G5/G6/G7/G1–G3/G6–G10 were reported in Algeria, Mauritania, China, Egypt, Ethiopia, India and Iran, respectively. Furthermore, the identified genotypes in Kazakhstan, Kenya, Libya, Mongolia, Nigeria, Oman, Pakistan, Saudi Arabia, Somalia, Sudan, Tunisia and Yemen included G6, G6–G7, G6–G10, G6–G7, G6–G7, G1/G1–G3/G6–G7, G1, G1/G2/G3/G1–G3, G6, G5/G6/G6–G7, G1/G6 and G6, respectively (Table 2, Fig. 2).

Fig. 2. World map showing the genotypes of E. granulosus identified in hydatid cases in camels.
Table 2. Genotypes of Echinococcus granulosus identified in camels hydatidosis cases worldwide up to 1 April 2020
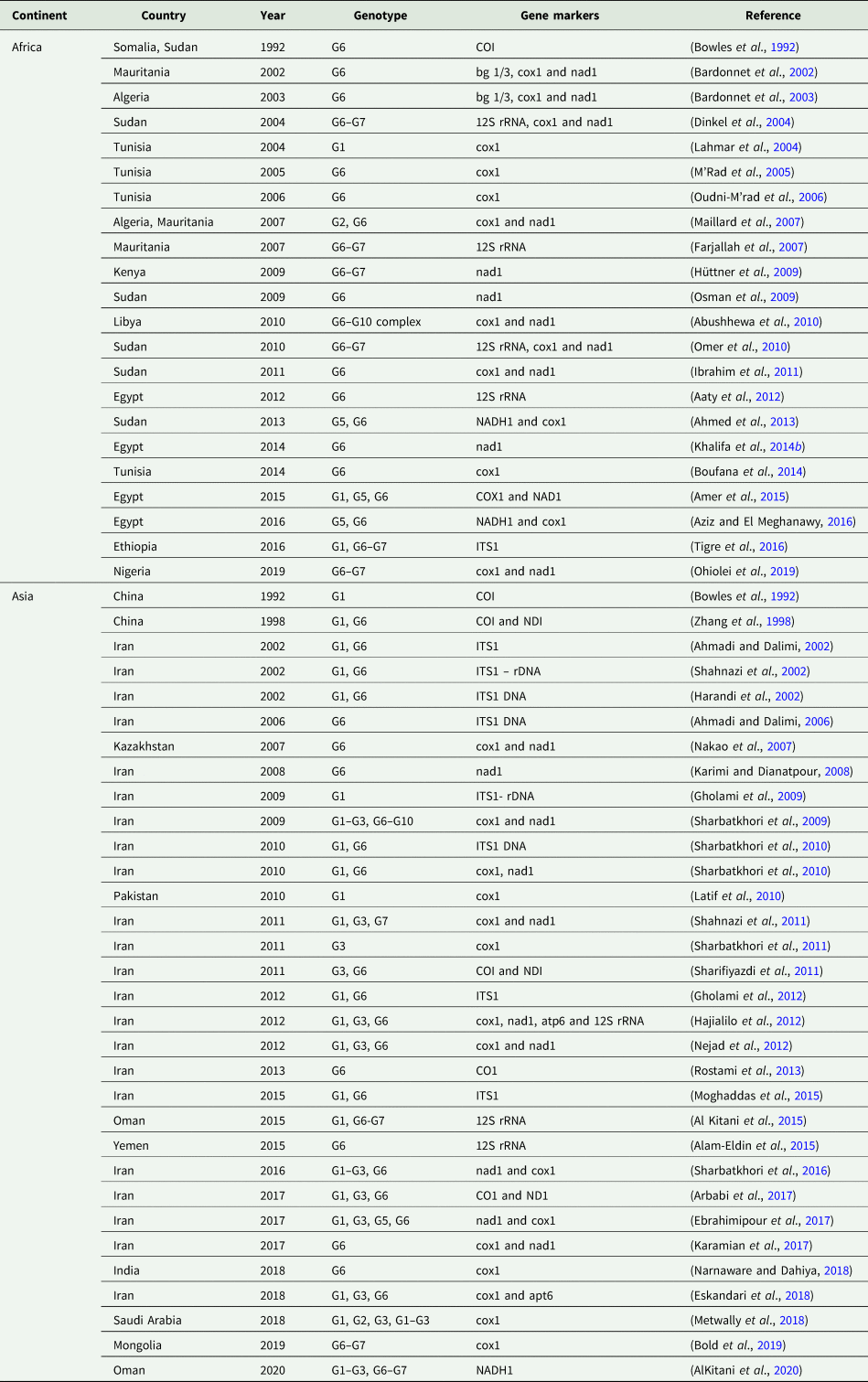
bg 1/3, Echinococcus genus-specific genomic DNA; cox1, mitochondrial cytochrome c oxidase subunit 1; COI, the mitochondrial cytochrome oxidase subunit 1; apt6, mitochondrial ATPase subunit 6; ITS1, ribosomal internal transcribed spacer 1; nad1, mitochondrial NADH dehydrogenase subunit 1; 12S rRNA, mitochondrial 12S small subunit ribosomal RNA.
Results of meta-analysis
Based on the statistical analysis, the weighted and pooled prevalence of hydatidosis in camels around the world was measured at 23.75% (95% CI 20.15–27.55; Fig. 3). The results revealed a strong heterogeneity among the included studies (Q = 10 142.8, I 2 = 99.3%, d.f. = 73, P < 0.001). However, no significant publication bias was found (Egger bias = 1.6, P = 0.382; Fig. 4).

Fig. 3. Forest plot of the global prevalence of hydatid disease infection in camels up to 1 April 2020. A square is appointed to each individual study with a horizontal line as confidence intervals and the area of each square is proportional to the study's weight in the meta-analysis. Moreover, a diamond is assigned to the meta-analysed measure of effect. A vertical line representing no effect is also plotted. If the confidence intervals for individual studies overlap with this line, it indicates that at the given level of confidence their effect sizes do not differ from no effect for the individual study.

Fig. 4. Funnel plot from Egger for the prevalence of hydatid disease in camels across the world up to 1 April 2020. In the absence of publication bias, it assumes that studies with high accuracy will be plotted near the average, and studies with low accuracy will be spread evenly on both sides of the average, creating a roughly funnel-shaped distribution. Deviation from this shape can indicate publication bias.
Table 3 shows the results of subgroup analysis. The overall prevalence rates of hydatidosis in camels were obtained as 37.13, 21.11, 17.44, 16.05 and 11.94% in years ⩽2000, 2011–2015, 2006–2010, 2001–2005 and >2015, respectively. Accordingly, the results of subanalysis indicated a decrease in the worldwide prevalence of hydatidosis in camels in recent years (>2015). In the same vein, the data were indicative of a decrease in the prevalence of hydatidosis in camels in both Africa and Asia in recent years (>2015) (Supplementary Table S2). Furthermore, African camels had the highest prevalence of hydatidosis (26.17%). With regard to the pooled prevalence of hydatidosis in camels in different countries, Iraq, Pakistan, Ethiopia, Sudan and Nigeria had the prevalence rates of 36.27% (95% CI 7.75–71.77), 35.94% (95% CI 4.34–77.44), 33.73% (95% CI 20.46–48.45), 30.93% (95% CI 15.71–0.48.65) and 28.69% (95% CI 11.85–49.38), respectively. In addition, these rates were reported as 25.95% (95% CI 9.88–46.39), 25.74% (95% CI 19.11–32.97), 22.40% (95% CI 10.93–36.52), 12.94% (95% CI 4.17–25.57), 12.18% (95% CI 6.25–19.72) and 3.15% (95% CI 0.44–8.21) in Jordan, Iran, Libya, Saudi Arabia, Egypt and Oman, respectively. Moreover, based on the results of the meta-analysis, Iraq and Oman were found to have the highest (36.27%) and lowest (3.15%) prevalence of hydatidosis in camels, respectively. Furthermore, the overall prevalence rate of hydatidosis in camels was higher in females (37.00%) as compared to that in males (29.06%).
Table 3. Subgroup analysis of hydatidosis in camels according to year, continent, country, mean temperature, precipitation, camel population, gender, infected organ, fertility rate and diagnostic method up to 1 April 2020
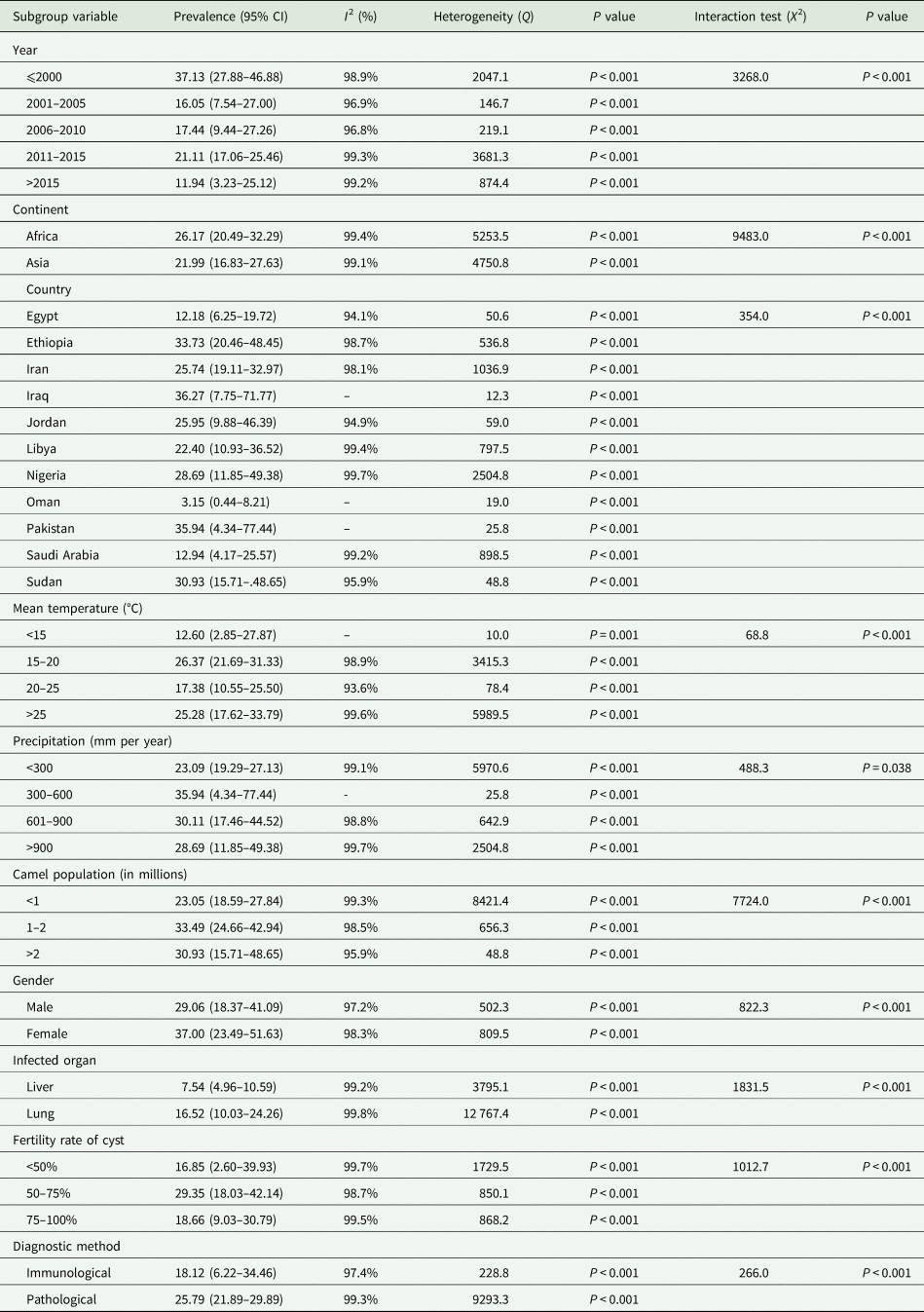
Moreover, the highest prevalence of hydatidosis was observed in regions with a camel population of 1–2 million (33.49%; 95% CI 24.66–42.94), mean temperature of 15–20°C (26.37%; 95% CI 21.69–31.33) and precipitation rate of 300–600 mm (35.94%; 95% CI 4.34–77.44; Table 3). In addition, a comparison was made based on the infected organ, fertility rate of the cyst and applied diagnostic method. With regard to the infected organ, the lung (16.52%) and liver (7.54%) were the most frequently infected organs in camels with hydatidosis. Furthermore, the highest prevalence of infection was found in the group that had cysts with 50–75% fertility (29.35%). In terms of the diagnostic technique, the prevalence rates of hydatidosis in camels were estimated at 25.79% and 18.12% based on pathological and immunological tests, respectively.
The results of subgroup analysis demonstrated significant differences between the prevalence of hydatid disease in camels based on year, geographic area (e.g. continent and country), climate parameters (e.g. mean temperature and precipitation), camel population, gender, infected organ, fertility rate of the cyst and laboratory diagnostic technique (Table 3).
Discussion
Based on the results of the included studies, the most common genotype of E. granulosus in camels with hydatid disease was G6. This genotype has been also reported in other intermediate hosts (Khademvatan et al., Reference Khademvatan, Majidiani, Foroutan, Tappeh, Aryamand and Khalkhali2019), especially humans (Siyadatpanah et al., Reference Siyadatpanah, Anvari, Emami Zeydi, Hosseini, Daryani, Sarvi, Budke, Esmaeelzadeh Dizaji, Mohaghegh, Kohansal, Dodangeh, Saberi and Gholami2019). In addition, after G6, the G6–G7 genotype in Africa and G1 genotype in Asia were the most commonly reported strains (Table 2, Fig. 2). There is now a widespread agreement based on morphological, molecular and ecological criteria that E. granulosus s.l. should be split into E. granulosus sensu stricto (s.s.) (including the genotypes G1, G2, G3; sheep and buffalo strains), E. equinus (G4; horse strain), E. ortleppi (G5; cattle strain) and E. canadensis (G6–10). Recently, the results of a study (Lymbery et al., Reference Lymbery, Jenkins, Schurer and Thompson2015) using similar criteria demonstrated that G6 (camel strain) and G7 (pig strain) genotypes represent a single species that is different from both G8 and G10 genotypes (cervid strains). They suggested the names E. intermedius (G6, G7), E. borealis (G8) and E. canadensis (G10). In addition, E. felidis (lion strain) has been described in Africa (Nakao et al., Reference Nakao, McManus, Schantz, Craig and Ito2007; Saarma et al., Reference Saarma, Jõgisalu, Moks, Varcasia, Lavikainen, Oksanen, Simsek, Andresiuk, Denegri and González2009). Given the identification of other genotypes, in addition to G6, in camels, this animal can be a highly important intermediate host for rotating different strains between the definitive host and other intermediate hosts in the world.
An important point in studying hydatid disease is the consideration of the nature and extent of genetic variation in Echinococcus species. The design and implementation of control measures are dependent upon such data, especially those related to the transmission cycle patterns of parasites as a risk to human health (Thompson, Reference Thompson2008). As indicated in Table 2, almost all important genotypes of E. granulosus have been reported in camels around the world, especially in Asia. Therefore, it seems that camels play an important role in the persistence of hydatid disease, as well as the continuity of E. granulosus transmission among other intermediate and final hosts.
Based on the results of meta-analysis, the global prevalence of hydatidosis in camels was 23.75% (95% CI 20.15–27.55) within 1969–2019. In addition, the results were indicative of a significant association between the pooled prevalence of hydatidosis in camels and year, geographic area (e.g. continent and country), climatic parameters (e.g. mean temperature and precipitation), camel population, gender, infected organ, fertility rate of the cysts and applied diagnostic technique.
The results of sub-analysis also indicated a decrease in the worldwide prevalence of hydatidosis in camels, as well as in both African and Asian camels separately in recent years (>2015; Table 3 and Supplementary Table S2). This finding may be due to the use of improved maintenance systems to better raise and keep domestic animals, such as cattle, camels and sheep (Jasra and Mirza, Reference Jasra and Mirza2004). Furthermore, in recent years, the improvement of the quality of breeding and keeping camels, controlling and treating echinococcosis in dogs, and preventing the entry of infected dogs into the breeding or living areas of camels have significantly reduced the prevalence of this disease among camels (Craig et al., Reference Craig, Hegglin, Lightowlers, Torgerson and Wang2017; Umhang et al., Reference Umhang, Possenti, Colamesta, d'Aguanno, La Torre, Boué and Casulli2019).
The results of the present meta-analysis highlighted the higher prevalence of camel hydatid disease in Africa than in Asia. This finding may be due to the high popularity of camel breeding and its products in Africa, leading to the implementation of more studies to monitor the health of this beneficial animal in the mentioned continent (Jasra and Mirza, Reference Jasra and Mirza2004; Ali et al., Reference Ali, Baby and Vijayan2019a, Reference Alİ, Akyol, Ceyhan, Dilawar, Firdous, Zia-Ul-Qasim and Ahmad2019b). There have been many studies confirming the benefits of the meat and especially milk of camels in the treatment of diseases. Camel milk has been indicated as a highly valuable substance, which contains many antibodies and protective enzymes against diseases (El Sayed et al., Reference El Sayed, Ruppanner, Ismail, Champagne and Assaf1992; Dubey et al., Reference Dubey, Lal, Mittal and Kapur2016; Ali et al., Reference Alİ, Akyol, Ceyhan, Dilawar, Firdous, Zia-Ul-Qasim and Ahmad2019b). In addition, due to the better climatic conditions of some regions of Africa, the survival of E. granulosus eggs and chance of completing the parasite cycle and its transmission to camels are greater in Africa than in Asia (Laux et al., Reference Laux, Kunstmann and Bárdossy2008; Rauch et al., Reference Rauch, Bliefernicht, Laux, Salack, Waongo and Kunstmann2019).
Based on geographic regions, the subgroup analysis revealed that the prevalence of hydatid disease in camels varies widely across the globe. This variation may be related to some important factors, such as proper environmental and ecological conditions, non-industrial abattoir, home slaughtering, immigrant population and large number of infected stray dogs (Fallah et al., Reference Fallah, Taherkhani and Sadjjadi1995; Mehrabani et al., Reference Mehrabani, Oryan and Sadjjadi1999; Seimenis, Reference Seimenis2003). In addition, prevalence variations observed among different geographical zones in this meta-analysis may have resulted from the environmental and/or meteorological parameters, extent of contact with dogs, sampling design, sample size and serological tests and their varied cut-off points (Zhang et al., Reference Zhang, Zhang, Wu, Shi, Li, Zhou, Wen and McManus2015; Anvari et al., Reference Anvari, Saberi, Sharif, Sarvi, Hosseini, Moosazadeh, Hosseininejad, Chegeni and Daryani2020).
Our results showed heterogeneity in the prevalence of hydatidosis based on the geographic and climate parameters (e.g. mean temperature and precipitation). In this regard, the highest prevalence was observed at the mean temperature of 15–20°C (26.37%; 95% CI 21.69–31.33) and precipitation rate of 300–600 mm (35.94%; 95% CI 4.34–77.44). Moreover, the climatic conditions and geographical location reportedly have a major effect on the survival of E. granulosus eggs (Thevenet et al., Reference Thevenet, Jensen, Mellado, Torrecillas, Raso, Flores, Minvielle and Basualdo2003). It is well known that the survival rate of E. granulosus eggs is higher in areas with higher humidity (75 ± 15%) and at an average temperature of 20°C (eggs stay alive within the temperature range of 0–30°C) in soil (Wachira et al., Reference Wachira, Macpherson and Gathuma1991; Thevenet et al., Reference Thevenet, Jensen, Mellado, Torrecillas, Raso, Flores, Minvielle and Basualdo2003). In general, according to the life-cycle pattern of each helminth species, climate variables are able to affect the prevalence, intensity and geographical distribution of helminths. Nevertheless, the comparison of the prevalence rates among regions based on climatic conditions should be made with caution since there are several confounding factors, such as different management practices (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2008).
As the current review demonstrated, the highest prevalence of hydatid disease was observed in regions with a camel population of 1–2 million (33.49%; 95% CI 24.66–42.94). The camel population and distribution vary from region to region. While the human population living in deserts has substantially decreased, the trend of nurturing camels in the desert is increasing at the global level. The camel population was estimated to be 27 million in 2014 according to the Food and Agriculture Organization (Ali et al., Reference Ali, Baby and Vijayan2019a). More than 80% of the world's camel population is estimated to be in Africa. Clearly, areas with a high number of camels need to adopt more care, control and monitoring measures to ensure the health of this valuable animal.
The present meta-analysis revealed a higher prevalence of hydatid disease in female camels than in their male counterparts. Since most of the female camels subjected to routine slaughtering are culled from herds due to their age and completion of maximum production potential, old female camels have a higher chance of being positive for hydatid cyst. In contrast, males are usually slaughtered early in their life; therefore, they have a lower chance of acquiring hydatid infection (Ibrahim, Reference Ibrahim2010; Al Kitani et al., Reference Al Kitani, Al Riyami, Al Yahyai and Hussain2015).
The overall prevalence of hydatid disease in camels was higher in the lung than in the liver. Based on the evidence, camels do not have bile ducts; as a result, the oncosphere passes through the blood, flows to the lungs and stays there. Furthermore, the solid and tough tissues of the camel liver make it difficult for the oncosphere to grow easily. On the other hand, the lung tissue is softer and smoother; therefore, it is easier for the oncosphere to grow faster in this organ (Elmajdoub and Rahman, Reference Elmajdoub and Rahman2015). Moreover, camels are slaughtered at an older age during which the liver capillaries are dilated, and a great number of oncospheres pass directly to the lungs. Additionally, Echinococcus oncospheres may enter the lymphatic circulation and be carried via the thoracic duct to the heart and lung in such a way that the lung may be infected before or instead of the liver as observed (Ismail and Al-Thebaiti, Reference Ismail and Al-Thebaiti2019). Furthermore, based on some evidence, the G6 genotype of E. granulosus has a specific affinity, for example, to the brain, in human hosts (Sadjjadi et al., Reference Sadjjadi, Mikaeili, Karamian, Maraghi, Sadjjadi, Shariat-Torbaghan and Kia2013). There is still no definitive reason for the higher prevalence of hydatid cysts in the lungs. However, it seems that there are a number of factors related to the anatomical structure of the camel's circulatory system, tendency and efficacy of antigenic contaminants to infect and grow in the camel's lungs, and the involved immunological interactions. Therefore, it is required to perform further studies in this domain.
The highest prevalence of hydatidosis was observed in camels that had cysts with 50–75% fertility rate (29.35%). Hydatid cyst fertility means the presence of live protoscoleces inside the cyst. The cysts that have live protoscoleces is called fertile, these types of cysts are infective for the definitive hosts. The purpose of the fertility rate is to determine the fertility degree in the hydatid cysts found in camels and the risk of infection transmission from camels to the definitive host (Yildiz and Gurcan, Reference Yildiz and Gurcan2003; Tappe et al., Reference Tappe, Mousavi and Barazesh2011). Data on the fertility and viability of hydatid cysts in different livestock animals play an important role in providing credible indicators regarding the importance of each livestock as a possible source of infection for the definitive hosts, especially dogs. Hydatid cysts usually have different rates of fertility, depending on the host and the location and size of the cysts (Tashani et al., Reference Tashani, Zhang, Boufana, Jegi and McManus2002; Daryani et al., Reference Daryani, Alaei, Arab, Sharif, Dehghan and Ziaei2007; Elmajdoub et al., Reference Elmajdoub, Elhoti and Haded2007). According to a number of studies, the availability of nourishment is probably the most important factor that is influenced by the location of the parasite and condition of the adventitious layer. Moreover, the development of sterile hydatid cysts may be due to infection by unspecific strains (Dew, Reference Dew1928; Elmajdoub and Rahman, Reference Elmajdoub and Rahman2015).
The current meta-analysis demonstrated the higher efficacy of pathological methods in identifying hydatid cysts in camels as compared to that of the immunological methods. The most reliable diagnostic technique is cyst detection during meat inspection or at post-mortem examination via visualization and palpation for dead animals. However, with regard to the living animals, serology and ultrasonography are the diagnostic methods of choice (Dada and Belino, Reference Dada and Belino1978; Igwenagu et al., Reference Igwenagu, Onyiche, Saidu, Chahari, Waziri and Kayeri2018). Therefore, serological methods are highly valuable and useful for monitoring and controlling hydatid disease in camels. In this regard, the use of appropriate antigens for the diagnosis and vaccination of animals against hydatid disease is a matter of fundamental importance.
The limitations of the current review included: (a) incomplete study on the effects of some important factors, such as type breeding, contact with dogs and age upon hydatidosis incidence, (b) inadequate data concerning the severity or condition of hydatidosis, and (c) use of various diagnostic tests without equal specificities and sensitivities.
Concluding remarks
According to the results of the reviewed articles, the most common genotypes in camels across the world were G6 and G1. Therefore, diagnostic and control measures, including vaccine design, should be based on these genotypes. In addition, the relatively high prevalence of hydatid cysts in camels worldwide can cause fatal and severe damage to the camel breeding industry and contribute to the sustainability and circulation of E. granulosus among other intermediate and final hosts. Therefore, it is required to pay attention to hydatidosis and consider prevention and control strategies for this disease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001705.
Acknowledgements
The authors thank all collaborators who have contributed to this study in the Mazandaran University of Medical Sciences, Sari, Iran.
Author contributions
DA, SG, AD, AS and SS designed the study. DA, NP, SR, AF, MH, MRN, MK and FP were involved in searching the databases. DA and SAH analysed the data. DA, NP and SG performed the screen of the papers and data extraction. DA wrote the manuscript and finally revised by SG. All authors read and approved the final manuscript.
Financial support
This research received no grant.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical standards
Not applicable.










