INTRODUCTION
One of the most severe pathologies in Mediterranean aquaculture is enteromyxosis caused by the myxozoan Enteromyxum leei (formerly called Myxidium leei Diamant, Lom and Dykova, 1994), which produces serious mortality and economic loss in sparid growing farms (Diamant, 1992; Le Breton and Marques, 1995; Sakiti et al. 1996). Since its first description in cultured gilthead seabream (Sparus aurata L.) in the eastern Mediterranean (Diamant, 1992; Diamant et al. 1994), it has also been detected in many marine fish species belonging to different orders, not only in the Mediterranean (Kent et al. 2001; Padrós et al. 2001) but also in cultured Takifugu rubripes from Japan (Yaganida et al. 2004). Among them, Puntazzo puntazzo is particularly susceptible to this enteromyxosis, which causes up to 80% losses in some stocks (Athanassopoulou, Prapas and Rodger, 1999). Although the life-cycle of this myxozoan is not completely known, fish-to-fish transmission has been achieved by cohabitation, ingestion of developmental stages and by waterborne contamination (Diamant, 1997).
Studies on immune responses to parasites are impaired by the difficulty of adapting fish to laboratory conditions and the lack of continuous in vitro cultures for most of the parasites. Several studies on the innate and adaptive immune response of fish to some parasites have been carried out in recent years (Woo, 1996, 2001; Jones, 2001; Buchmann et al. 2001; Sigh et al. 2004a,b), mainly in those susceptible to culture (at least in the first stages), such as some flagellates (mainly Cryptobia salmositica) and ciliates (especially Ichthyophthirius multifiliis). However, in the case of myxozoans, the lack of in vitro culture techniques and the scarce knowledge of their life-cycle, seriously hamper the use of experimental infection models (Redondo, Palenzuela and Álvarez-Pellitero, 2003; Sitjá-Bobadilla et al. 2004). Therefore, available data are mainly based on natural infections (Foott and Hedrick, 1990; Muñoz, Sitjà-Bobadilla and Álvarez-Pellitero, 1998). Some information has also been obtained for myxozoans susceptible to being experimentally transmitted, such as the turbot parasite Enteromyxum scophthalmi. For this species, the involvement of both innate (Sitjà-Bobadilla et al. 2003) and adaptive (Sitjà-Bobadilla et al. 2004) immune responses has been demonstrated. As regards the fish defence, in studies conducted both in vitro and in vivo, humoral innate parameters (complement, lysozyme, C-reactive protein, lectins or anti-proteases) have been shown to participate in protection against parasitic infections (see Jones, 2001; Buchmann et al. 2001). Complement system and non-specific cytotoxic cell (NCC) activities seem to be the most important innate immune defence mechanisms in the fish response (Graves, Evans and Dawe, 1985; Holland and Lambris, 2002; Nakanishi et al. 2002). On the other hand, whilst some authors have demonstrated the presence of specific circulating antibodies after parasitization periods in several fish species (Furuta, Ogawa and Wakabayashi, 1993; Buchmann et al. 2001; Sitjà-Bobadilla et al. 2004) others have failed to do so (Thoney and Burreson, 1988; Bartholomew et al. 1989). Recent advances in molecular biology have allowed us to study the expression of immune-relevant genes. Thus, mRNA expression of several genes such as cytokines (IL-1β, TNFα, IL-8, TGF-β, etc), complement C3 component, TCR, Ig, iNOS or MHC II has been evaluated by RT-PCR in different fish species after challenge with pathogenic parasites (Lindestrøm, Buchmann and Secombes, 2003; Holland et al. 2003; Saeij et al. 2003; Lindestrøm, Secombes and Buchmann, 2004; Sigh et al. 2004a,b). Deeper knowledge of the immune responses, as well as of the parasite life-cycles and modes of transmission and infection, will jointly lead to future solutions for problems associated with the intensive growth of fish in farms.
The aim of this study was to evaluate changes in the innate immune response (alternative complement activity, peroxidase content and pro-inflammatory cytokines, IL-1β and TNFα, gene expression) provoked by exposure of healthy seabreams to E. leei by cohabitation with infected donor fish. The course of infection was also evaluated by registering mortalities and infection prevalence and intensity in periodical samplings. Implications of the innate immune factors studied in the parasite defence are discussed.
MATERIALS AND METHODS
Fish
Donor gilthead seabream specimens (500–600 g body weight) were obtained from the Instituto de Acuicultura ‘Torre de la Sal’ (IATS), where Enteromyxum leei infections are routinely maintained by cohabitation and effluent transmission. Infected fish were sent to the University of Murcia facilities at the beginning of the experiment.
Recipient and control gilthead seabream (100–150 g body weight) were obtained from CRIMAR S.A. (Burriana, Castellón). Fish were sampled and the intestine processed for histology (see below). Before cohabitation, no signs of E. leei infection were observed in fish chosen as recipients or controls.
Cohabitation protocol and samplings
Recipient fish were acclimated to the University of Murcia facilities for 30 days before cohabitation. Sixty fish were randomly allocated to 2 tanks and used as controls and another group of 60 fish were distributed in 4 tanks and served as recipients. The cohabitation period was started by placing 5 donors in each tank containing recipient fish (ratio of 3 recipients per donor). Fish were fed a commercial pelleted diet (Trouwvit, Spain) at a rate of 1% body weight/day. All fish were kept in 450–500 l of close-recirculating seawater (28‰ salinity) tanks, at 20±2 °C, and 12 h light[ratio ]12 h dark photo-period. Mortalities of donor and recipient fish were registered during the experiment.
Both control and recipient fish were randomly sampled (8 fish per sampling) at 10, 22, 38, 52 and 108 days of cohabitation. Fish were starved 24 h before sampling and anaesthetized with benzocaine (4% in acetone) (Sigma), weighed and measured. Blood, intestine and head-kidney (HK) samples were collected. Blood was allowed to clot at 4 °C for 4 h. After centrifugation, the serum was removed and frozen at −80 °C until use. The intestine from each specimen was excised and cut into pieces, which were processed for histological study. HK portions were stored at −80 °C in TRIzol Reagent (GibcoBRL) for subsequent RNA purification.
Histology
Intestine fragments were fixed in 4% paraformaldehyde solution and embedded in either paraffin or Technovit-7100 resin (Kulzer, Heraeus) for histological processing, following standard histology procedures. Sections were stained with Haematoxylin-Eosin or Giemsa and examined by light microscopy. The prevalence of infection was determined and the infection intensity was also measured using a semi-quantitative scale from 1+ to 6+, according to the number of stages per microscope field at ×250 with the range: 1+=1−5; 2+=6−10; 3+=11−25; 4+=26−50; 5+=51−100; 6+>100.
Alternative complement activity
The activity of the alternative complement pathway was assayed using sheep red blood cells (SRBC, Biomedics) as targets (Ortuño et al. 1998). Equal volumes of SRBC suspension (6%) in phenol red-free Hank's buffer (HBSS) containing Mg2+ and EGTA were mixed with serially diluted serum to give final serum concentrations ranging from 10% to 0·078%. After incubation for 90 min at 22 °C, the samples were centrifuged (400 g, 5 min, 4 °C) to avoid unlysed erythrocytes. The relative haemoglobin content of the supernatants was assessed measuring their optical density at 550 nm in a plate reader (BMG, Fluoro Star Galaxy). The values of maximum (100%) and minimum (spontaneous) haemolysis were obtained by adding 100 μl of distilled water or HBSS to 100 μl samples of SRBC, respectively.
The degree of haemolysis (Y) was estimated and the lysis curve for each specimen was obtained by plotting Y/(1-Y) against the volume of serum added (ml) on a log-log scaled graph. The volume of serum producing 50% haemolysis (ACH50) was determined and the number of ACH50 units/ml was obtained for each experimental group.
Peroxidase content
The total peroxidase content present in serum was measured according to the method described by Quade and Roth (1997). Briefly, 15 μl of serum were diluted with 135 μl of HBSS without Ca2+ or Mg2+ in flat-bottomed 96-well plates. Then, 50 μl of 20 mM 3,3′,5,5′-tetramethylbenzidine hydrochloride (TMB) (Sigma) and 5 mM H2O2 were added. The colour-change reaction was stopped after 2 min by adding 50 μl of 2 M sulphuric acid and the optical density was read at 450 nm in a plate reader. The wells without serum were used as blanks.
Cytokine gene expression
HK fragments stored at −80 °C in TRIzol Reagent were used for RNA purification following the manufacturer's instructions. Pro-inflammatory cytokine (IL-1β and TNFα) expression was evaluated using RT-PCR (Pelegrín et al. 2001; García-Castillo et al. 2002). The first strand of cDNA was synthesized by reverse transcription of 1 μg of total RNA using the ThermoScriptTM RNase H− Reverse Transcriptase (Invitrogen) with an oligo-dT12–18 primer (Invitrogen). PCR reactions were carried out in a volume of 20 μl containing 2 μl of 10× reaction buffer, 1 μl of forward and reverse primers (10 μM each; Invitrogen), 0·5 μl of dNTP mix (2·5 mM each), 0·6 μl of MgCl2 (50 mM), 0·1 μl of Taq polymerase (5 units/μl; Roche Applied Science), 13·8 μl of Dnase/Rnase-free distilled water and 1 μl of ester cDNA. The cycling reaction was performed in a MasterCycler Gradient PCR (Eppendorf) for 1 cycle of 95 °C for 5 min, 30 cycles of 95 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s, followed by 1 cycle of 72 °C for 10 min. The IL-1β primers (5′-ATGCCCGAGGGGCTGGGC-3′ and 5′-CAGTTGCTGAAGGGAACAGAC-3′) and TNFα primers (5′-TCGTTCAGAGTCTCCTGCAG-3′ and 5′-AAGAATTCTTAAAGTGCAAACACACCAAA-3′) used gave fragments of 593 and 309 bp, respectively. As a control, the constitutively expressed β-actin gene (primers 5′-ATCGTGGGGCGCCCCAGGCACC-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′) was also amplified (543 bp). PCR products were separated on a 1% agarose (Sigma) gel containing 0·5 μg/ml ethidium bromide (Sigma), visualized under UV light and photographed. Band intensity was calculated by 1D Image Analysis Software v3.6 (Kodak) and normalized to that of β-actin.
Statistical analysis
For both fish groups (recipient and control) mean absorbance values were calculated at each sampling time. Graphs show the stimulation index obtained dividing recipient fish values by the mean control fish value at the corresponding sampling time. Thus, values higher than 1 indicate activation while lower values reflects inhibition. Data are represented as means+S.E. A Student's t-test was applied to analyse differences between recipient and control values (significant levels at P[les ]0·1 or P[les ]0·05). Differences within the recipient group along the sampling points were analysed using one way-ANOVA, followed by the Tukey's method (P[les ]0·05). A two way-ANOVA, followed by a Student-Knewman-Keuls test (P[les ]0·05), was used to study the effect of the sampling time and the fish parasitic status (parasitized vs non-parasitized) on the humoral parameters of recipient fish.
RESULTS
Course of Enteromyxum leei infections
Histological examination of recipient fish allowed the identification of different parasitic stages in the gut mucosa (Table 1). The first 2 positive fish were detected at the first sampling time (day 10). Prevalence rose from the third sampling onwards and reached a maximum of 62·5% at the last sampling time (108 days of cohabitation). In the first 2 samplings, infection intensity was low, and only early trophozoites were found. The intensity increased in subsequent samplings and more advanced developmental stages were observed. Sporoblasts and spores were scarce at day 38, whereas they predominated in the last two samplings. In some fish, parasite stages appeared degraded, and sometimes engulfed by macrophages.

Mortalities were observed throughout the cohabitation experiment in both donor and recipient groups. Donor fish died from day 4 onwards, and only 6% survived at the end of the experiment. Mortality in recipient fish was recorded at days 71 (4 fish) and 77 (5 fish). The total infection prevalence in sampled fish was 45%, although including the dead fish, prevalence reached 67·5% of recipient fish.
No control fish died during the experiment nor showed signs of disease, and all of them were negative for E. leei in the histological study.
Alternative complement activity
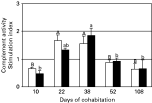
The complement activity was lower in recipient fish than in controls at day 10, whereas an increase over control fish was observed after 22 and 38 days of cohabitation (stimulation index up 1·66-fold compared with the control fish group) (Fig. 1). Differences were statistically significant at these sampling times. At day 52, the complement activity of recipient fish was slightly lower than that of the control group (though not significant). However, a statistically significant reduction of this activity in recipient fish was found after 108 days of cohabitation (P<0·1), when levels similar to those found at day 10 were observed. Within the recipient group, the complement activity at days 10, 52 and 108 was significantly lower than that observed after 22 and 38 days of cohabitation (P<0·05), and the same pattern was observed when analysing separately the data from parasitized and non-parasitized fish, although values peaked earlier in the latter (Fig. 2). Furthermore, the two way-ANOVA revealed a statistically significant effect of sampling time on the complement values (F=11·636, P<0·001), but not of the parasitic status, and no interaction between the two factors (F=0·664), P=0·622).

Fig. 1. Serum alternative complement activity of recipient gilthead seabream in cohabitation with Enteromyxum leei donor fish. Data are presented as the stimulation index (mean value+S.E.; n=8) obtained by dividing each recipient fish value by the mean value of the control group. Thus, values > or <1 mean an increase or decrease with respect to the controls, respectively. Symbols * and+denote statistically significant differences (P[les ]0·05 and P[les ]0·1, respectively) between recipient and control fish. Different letters stand for statistically significant differences (P[les ]0·05) for recipient group between sampling times.

Fig. 2. Serum alternative complement activity of parasitized (□) and non-parasitized ([squf ]) recipient gilthead seabream in cohabitation with Enteromyxum leei donor fish. Data are presented as the stimulation index (mean value+S.E.) obtained by dividing each recipient fish value by the mean value of the control group. Thus, values > or <1 mean an increase or decrease with respect to the controls, respectively. Different letters stand for statistically significant differences (P[les ]0·05) within each group of parasitized (low case letters) and non-parasitized (capital letters) fish during the sampling points.
Serum peroxidase levels
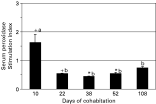
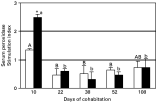
Serum peroxidase, which is released by activated circulating leucocytes, significantly increased in recipient fish with respect to control fish at day 10 (P=0·062) (Fig. 3). However, peroxidase levels of recipient fish fell in subsequent sampling times, although the difference with respect to control fish was statistically significant at the second to fourth samplings but not at the last sampling (108 days of cohabitation). The peroxidase level of the recipient fish at day 10 was significantly higher (P<0·05) than that in the remaining sampling times. A similar pattern was observed when analysing separately the data from parasitized and non-parasitized recipient fish, though in the latter no statistically significant differences were detected at the last sampling point (Fig. 4). Thus, two way-ANOVA revealed a statistically significant effect of the sampling time on the peroxidase level (F=13·09, P<0·001) but not of the parasitic status. In addition, an interaction between the two factors was detected (though at the significant limit: F=2·659, P=0·052), probably related to the significant difference between parasitized and non-parasitized recipient fish observed at the first sampling (P<0·05).

Fig. 3. Serum peroxidase contents in recipient gilthead seabream. Data are presented as the stimulation index (mean value+S.E.; n=8) obtained by dividing each recipient fish value by the mean value of the control group. Thus, values > or <1 mean an increase or decrease, respectively, with respect to controls. Symbols * and+denote statistically significant differences (P[les ]0·05 and P[les ]0·1, respectively) between recipient and control fish. Different letters stand for statistically significant differences (P[les ]0·05) for recipient group between sampling times.

Fig. 4. Serum peroxidase contents in parasitized (□) and non-parasitized ([squf ]) recipient gilthead seabream in cohabitation with Enteromyxum leei donor fish. Data are presented as the stimulation index (mean value+S.E.) obtained by dividing each recipient fish value by the mean value of the control group. Thus, values > or <1 mean an increase or decrease with respect to the controls, respectively. Different letters stand for statistically significant differences (P[les ]0·05) within each group of parasitized (lower case letters) and non-parasitized (capital letters) fish along the sampling points. Symbol * denote statistically significant differences (P[les ]0·05) between parasitized and non-parasitized fish.
Cytokine gene expression
We also evaluated mRNA expression of the cytokines, IL-1β and TNFα, in some specimens. Although great variability was found, both cytokines were constitutively expressed in all the control fish (Fig. 5). In recipient fish, the expression of IL-1β mRNA was up-regulated at days 10 (6-fold), 22 and 52 but decreased after 38 and 108 days of cohabitation. On the other hand, TNFα expression was similar in recipient and control fish at days 10 and 22, although it was significantly lower in recipient than in control fish in later samplings.

Fig. 5. Cytokines, IL-1β and TNFα, gene expression in head-kidney from gilthead seabream unexposed (control) or exposed (recipient) to Enteromyxum leei by cohabitation with donor fish. (A) RT-PCR was performed using the gene specific primers for IL-1β and TNFα. The expression of β-actin was used as internal control. C, control fish; R, recipient fish. D.c., days of cohabitation. (B) Histogram showing IL-1β (□) and TNFα([squf ]) mRNA expression. Data are presented as the stimulation index (mean value+S.E.) obtained by dividing each recipient fish value by the mean value of the control group. Thus, values > or <1 mean an increase or decrease, respectively, with respect to controls. Symbols * and + denote statistically significant differences (P[les ]0·05 and P[les ]0·1, respectively) between recipient and control fish.
DISCUSSION
Gilthead seabream is one of the most important fish species in Mediterranean marine culture. Production reached more than 100000 tons in 2003, of which 12% were obtained in Spain (FAO; www.fao.org). While production losses due to handling have been almost completely overcome, the yield of seabream is still seriously impaired by pathogens, one of the most important parasitosis being the enteromyxosis produced by Enteromyxum leei. This emerging disease causes important financial losses in culture, since mortalities can be high and chronically infected fish show a caquectic appearance, which renders them unmarketable. To date, no prophylaxis or successful treatment has been available for myxosporean parasites. The difficulty of establishing in vitro cultures of E. leei seriously hampers the necessary studies of different aspects of the host-parasite relationship. However, the availability of an experimental transmission model in vivo has provided the opportunity to evaluate some innate immune mechanisms involved in the seabream defence against E. leei.
In the present work, the parasitosis caused by E. leei in gilthead seabream has been effectively transmitted from fish-to-fish by cohabitation, as demonstrated previously by Diamant (1997). Fish-to-fish transmission has also been reported for the related myxosporean E. scophthalmi in turbot (Redondo et al. 2002). Histological examination revealed a relatively high prevalence (45%) for the total of fish examined in our experiments. Both prevalence and mean intensity increased progressively during the cohabitation period. In the two first samplings, only initial developmental stages were found, sometimes degraded. Parasite maturation occurred in subsequent samplings, and spores were observed in parasitized intestines from the third sampling (day 38) onwards. At the same time, the severity of lesions was higher in some fish, and mortalities occurred at days 74 and 77 (cumulative mortality 7·7%).
Information on the fish immune defence against parasites is scarce. Available information mainly deals with some flagellates (Cryptobia salmositica and Trypanoplasma sp.), for which parasitic material for experiments is available from in vitro cultures (Woo, 2001). Some ciliates, mainly Ichthyophthirius multifiliis, have also been studied, since early stages can be cultured in vitro (Nielsen and Buchmann, 2000; Xu et al. 2000) and infections can be maintained in vivo through passage from infected to healthy fish (Buchmann et al. 2001; Xu, Klesius and Shelby, 2004). Knowledge regarding other fish parasites is mostly based on in vitro anti-parasitic actions of fish serum/leucocytes or in parasite-immunized fish. Teleost fish have complement system constituents and activation pathways similar to those of mammals. The involvement of fish complement in the response to infections, including parasitosis, has been demonstrated (see Buchmann, 1998; Jones, 2001; Holland and Lambris, 2003). Thus, the in vitro ability of fish serum to lyse different parasites via the alterative pathway of complement is well documented for Gyrodactylus spp. (Buchmann, 1998; Harris, Soleng and Bakke, 1998), Cryptobia spp. (Bower and Woo, 1977; Wehnert and Woo, 1980; Ardelli and Woo, 1997), Trypanoplasma spp. (Plouffe and Belosevic, 2004; Scharsack et al. 2004), Cryptocotyle lingua (Wood and Matthews, 1987), Discocotyle sagittata (Rubio-Godoy, Porter and Tinsley, 2004), Ichthyophthirius multifiliis (Buchmann and Nielsen, 1999), and Tylodelphys sp. (Olabuenaga, 2000). Additionally, C3 factor gene expression appears to be activated in carp parasitized by Trypanoplasma borreli (Saeij, de Vries and Wiegertjes, 2003) and in rainbow trout infected by Ichthyophthirius multifiliis (Sigh et al. 2004a), leading to greater complement activity against the parasites. However, few articles have paid attention to the involvement of the complement system, especially the classical or antibody-dependent pathway, in natural or provoked parasitosis in vivo (Bower and Evelyn, 1988; Li and Woo, 1995; Mehta and Woo, 2002). Information on the fish immune response to myxosporean parasites is very scarce, and on some occasions has only been obtained from immunized fish (Muñoz, Sitjà- Bobadilla and Álvarez- Pellitero, 2000). The involvement of different factors of the innate response, complement included, has been demonstrated in turbot infected by Enteromyxum scophthalmi (Sitjà-Bobadilla et al. 2003), but no data are available for E. leei. Bearing this in mind, we performed an experiment to elucidate the humoral innate immune responses in a group of gilthead seabream exposed to E. leei infection by cohabitation with parasitized fish. Of note is the fact that the alternative pathway of the complement system of recipient fish varied during the experiment. After a statistically significant decrease in exposed fish with respect to control fish at the first sampling time (10 days), the complement activity increased in recipient fish (at days 22 and 38) compared with the unexposed group, but a fall occurred at the two last sampling days. No differences in complement activity were observed when comparing parasitized and non-parasitized recipient fish. However, it is noteworthy that the values peaked earlier in the former ones (day 22). In turbot exposed to E. scophthalmi, complement levels also increased after 20 days but the subsequent pattern was different from the one described in the present study for E. leei since a decrease occurred at 40 days followed by an increase at 43 days (in severely affected fish) (Sitjà-Bobadilla et al. 2003).
Apart from complement activity, lysozyme and C-reactive protein seem to take part in fish defence against parasites (Buchmann et al. 2001; Jones, 2001). However, the serum content in peroxidases has never been studied after any kind of fish infection. Myeloperoxidase and eosinophil peroxidase are found in the granules of phagocytic cells (Meseguer, López-Ruiz and Esteban, 1994; Rodríguez, Esteban and Meseguer, 2003) which, together with H2O2 and halide ions, form chlorides and chloramines that are highly toxic for pathogens (Quade and Roth, 1997). Moreover, leucocytes can release their granule contents (including peroxidases) when activated (Klebanoff, 1998; Rodríguez et al. 2003). In the current study, serum peroxidase content was raised in seabream exposed to E. leei at day 10, and this increase was mainly due to the high values of the few parasitized fish, significantly higher than those of non-parasitized fish, even with their low parasitization intensity. Nevertheless, in subsequent samplings serum peroxidase levels were clearly lower in recipient fish than in control fish, and no differences were detected between parasitized and non-parasitized fish. These changes could reflect variations in peroxidase-producing cell numbers in blood, probably due to an exhaustion of active cells after the initial stimulation by the first parasite contact.
Our results clearly demonstrate that exposure to parasites is sufficient to trigger the immune system response. This triggering was detected earlier in peroxidase levels than in complement ones, and the subsequent fall also occurred earlier (day 22 vs day 52). Nevertheless, it remains unclear whether the activation of these innate humoral factors is responsible for the existence of non-parasitized recipient fish, which could have a higher capability to react to E. leei than the parasitized fish. However, the absence of parasites in some exposed fish could be just a consequence of a low exposure levels to the parasite, as there is no way to secure the infective dose to which each individual fish is exposed. These interesting data point to the need for further studies of the mechanisms involved in possible resistance against E. leei. For example, using both selected gynogenetic clones (with increased resistance to the parasitosis via the complement system) and transgenic fish have already been used as effective control measures against cryptobiosis in salmonids (Forward and Woo, 1996; Woo, 2001).
Cytokines are soluble glycoproteins mainly produced and released by activated leucocytes (Secombes et al. 2001). They exert autocrine and paracrine actions playing a role as immune mediators and exerting pleiotropic functions in other body tissues. Thus, cytokine production is vital in the immune response at both local and systemic levels. Recent studies have described the expression of some other immune-related genes (C3, CXC, A2M, SAA, MHC-II, COX-2, TGF-β, iNOS or IgM), apart from cytokines (IL-1β, IL-1RII, IL-8 or TNFα), in several tissues (skin, head-kidney, liver or spleen) of fish parasitized by Tetracapsuloides bryosalmonae (the myxozoan causing proliferative kidney disease, PKD) (Holland et al. 2003), I. multifiliis (Sigh et al. 2004a,b), Gyrodactylus derjavini (Lindestrøm et al. 2003; 2004) or Trypanoplasma borreli (Saeij et al. 2003). Although an up-regulation of the main immune-related genes in parasite-exposed fish has been demonstrated in these studies, their specific role remains partially unknown. We have studied the expression of two gilthead seabream pro-inflammatory cytokines, IL-1β and TNFα, after exposure to E. leei in order to gain insight into the possible correlation between gene expression and fish disease. Although a high variability among specimens was detected, some valuable information can be drawn from the obtained data. An up-regulation in the head-kidney expression of IL-1β in exposed fish occurred at day 10, whereas no clear difference was detected in subsequent samplings with respect to the control. These data agree with those of other authors describing the up-regulation of IL-1β and its recipient in fish exposed to parasites for a short period, whereas the expression levels returned to values similar to the control fish when exposed for long periods (Lindenstrøm et al. 2003; Saeij et al. 2003). On the other hand, no clear differences in TNFα expression were observed in seabream exposed to E. leei with respect to control fish at the first sampling day, although some down-regulation occurred from day 38 onwards. However, up-regulation of TNFα expression has been described in parasitized fish (Saeij et al. 2003; Lindenstrøm et al. 2004). Gilthead seabream infected by Vibrio anguillarum also showed up-regulation of IL-1β (Pelegrin et al. 2001) but no regulation of TNFα (García-Castillo et al. 2002). Summarizing, both cytokines seem to have a certain involvement in the immune response of gilthead seabream to E. leei, although further studies are necessary to elucidate their precise role. Our knowledge on the immune response against fish parasites at the molecular level is in its infancy, partly due to the lack of suitable tools for evaluating the expression of the involved immune factors.
To conclude, we have evaluated for the first time the in vivo immune response of gilthead seabream exposed to E. leei. Serum complement activity and peroxidase content, as well as the head-kidney expression of pro-inflammatory cytokines, were affected by exposure to the parasite. These results clearly demonstrate that the fish immune response can be triggered by exposure to the parasite but no differences were detected between E. leei parasitized and non-parasitized seabreams. However, other immune factors, innate or adaptive, must be also involved in the response to the parasite. More studies are needed to elucidate these mechanisms. Such information would contribute to the development of control measures for this important disease, which affects cultured fish.
This work has been funded by an EU Project (QLRT-2001-00722 MyxFishControl). A. C. and I. S. are beneficiaries of Fundación CajaMurcia and Fundación Séneca fellowships, respectively. Primers for cytokines were designed by Dr V. Mulero of the Fish Innate Immune System Group of the University of Murcia.