Introduction
Pneumocystis organisms are opportunistic and airborne-transmitted fungal parasites that infect the lungs of humans and other mammalian species (Aliouat-Denis et al. Reference Aliouat-Denis, Chabé, Demanche, Aliouat, Viscogliosi, Guillot, Delhaes and Dei-Cas2008; Chabé et al. Reference Chabé, Aliouat-Denis, Delhaes, Aliouat, Viscogliosi and Dei-Cas2011). They may induce severe Pneumocystis pneumonia in immunocompromised individuals but are also able to colonize asymptomatically the lungs of immunocompetent and healthy hosts, which constitute potential infection sources (Chabé et al. Reference Chabé, Dei-Cas, Creusy, Fleurisse, Respaldiza, Camus and Durand-Joly2004). Due to the inability to reproducibly culture these microorganisms in vitro, Pneumocystis life-cycle and basic biology are still poorly understood (Martinez et al. Reference Martinez, Halliez, Moukhtar Aliouat, Chabé, Standaert-Vitse, Fréalle, Gantois, Pottier, Pinon, Dei-Cas and Aliouat-Denis2013). Most of the available data on these parasites have been obtained using immunosuppressed laboratory animals, but studies focused on wild mammals have also led to important advancements regarding Pneumocystis diversity, evolution and life cycle (Aliouat-Denis et al. Reference Aliouat-Denis, Chabé, Demanche, Aliouat, Viscogliosi, Guillot, Delhaes and Dei-Cas2008; Chabé et al. Reference Chabé, Aliouat-Denis, Delhaes, Aliouat, Viscogliosi and Dei-Cas2011).
Once considered as a unique taxonomic entity named ‘Pneumocystis carinii’, molecular genetic studies have revealed that the Pneumocystis genus is highly diversified and includes numerous divergent taxonomic entities characterized by strong host specificity [see Aliouat-Denis et al. (Reference Aliouat-Denis, Chabé, Demanche, Aliouat, Viscogliosi, Guillot, Delhaes and Dei-Cas2008) for review], as confirmed by the failure of cross-infection experiments (Aliouat et al. Reference Aliouat, Mazars, Dei-Cas, Cesbron and Camus1993, Reference Aliouat, Mazars, Dei-Cas, Delcourt, Billaut and Camus1994; Gigliotti et al. Reference Gigliotti, Harmsen, Haidaris and Haidaris1993). Marked host species-related genetic divergence among Pneumocystis species has been observed and specific gene sequences could be attributed to parasites from different host species (Wakefield et al. Reference Wakefield, Stringer, Tamburrini and Dei-Cas1998; Demanche et al. Reference Demanche, Berthelemy, Petit, Polack, Wakefield, Dei-Cas and Guillot2001; Guillot et al. Reference Guillot, Demanche, Hugot, Berthelemy, Wakefield, Dei-Cas and Chermette2001; Ma et al. Reference Ma, Imamichi, Sukura and Kovacs2001; Hugot et al. Reference Hugot, Demanche, Barriel, Dei-Cas and Guillot2003; Akbar et al. Reference Akbar, Pinçon, Aliouat-Denis, Derouiche, Taylor, Pottier, Carreto-Binaghi, González-González, Courpon, Barriel, Guillot, Chabé, Suarez-Alvarez, Aliouat, Dei-Cas and Demanche2012). The strong host specificity of these fungal parasites suggests that they probably resulted from a long host–parasite co-evolution process that led to co-speciation (Demanche et al. Reference Demanche, Berthelemy, Petit, Polack, Wakefield, Dei-Cas and Guillot2001; Guillot et al. Reference Guillot, Demanche, Hugot, Berthelemy, Wakefield, Dei-Cas and Chermette2001; Derouiche et al. Reference Derouiche, Deville, Taylor, Akbar, Guillot, Carreto-Binaghi, Pottier, Aliouat, Aliouat-Denis, Dei-Cas and Demanche2009). The comparison of the respective phylogenies of Pneumocystis and their primate hosts revealed a high number of homologous nodes that may result from co-divergence events between the parasites and their hosts (Guillot et al. Reference Guillot, Demanche, Hugot, Berthelemy, Wakefield, Dei-Cas and Chermette2001; Hugot et al. Reference Hugot, Demanche, Barriel, Dei-Cas and Guillot2003).
Despite this large diversity, only five Pneumocystis species have been formally described and accepted so far. Pneumocystis carinii (Frenkel, Reference Frenkel1999) is the type species of the genus and has been identified in the lungs of laboratory rats (Rattus norvegicus). Pneumocystis wakefieldiae (Cushion et al. Reference Cushion, Keely and Stringer2004) is the second species described in laboratory rats while P. murina (Keely et al. Reference Keely, Fischer, Cushion and Stringer2004) is the sole species described in laboratory mice (Mus musculus). Pneumocystis jirovecii (Frenkel, Reference Frenkel1999) infects the lungs of humans. Finally, P. oryctolagi (Dei-Cas et al. Reference Dei-Cas, Chabé, Moukhlis, Durand-Joly, Aliouat, Stringer, Cushion, Noël, Sybren De Hoog, Guillot and Viscogliosi2006) has been described in Old World rabbits (Oryctolagus cuniculus). These Pneumocystis species are characterized by marked genetic divergence at several mitochondrial and nuclear loci and by differences in their ultrastructural morphology, growth rate and infectivity (Dei-Cas et al. Reference Dei-Cas, Chabé, Moukhlis, Durand-Joly, Aliouat, Stringer, Cushion, Noël, Sybren De Hoog, Guillot and Viscogliosi2006; Aliouat-Denis et al. Reference Aliouat-Denis, Chabé, Demanche, Aliouat, Viscogliosi, Guillot, Delhaes and Dei-Cas2008).
The aim of this study was to investigate the genetic diversity and host specificity of Pneumocystis organisms infecting wild Southeast Asian murid rodents belonging to the subfamilies Rhizomyinae and Murinae and to test the co-phylogeny hypothesis among these fungal parasites and their rodent hosts. Murid rodents are the most diverse mammalian family, including more than 700 species (Musser and Carleton, Reference Musser, Carleton, Wilson and Reeder2005). Southeast Asia is considered to be the centre of origin and diversification of Murinae rodents from where they dispersed to other Old World regions (Schenk et al. Reference Schenk, Rowe and Steppan2013). The exceptionally high diversification of these rodents in Southeast Asia is the result of several significant radiations during the last 15 million years (Chaimanee and Jaeger, Reference Chaimanee and Jaeger2001; Rowe et al. Reference Rowe, Aplin, Baverstock and Moritz2011; Schenk et al. Reference Schenk, Rowe and Steppan2013). Murid rodents display various life histories and collectively inhabit a wide range of ecological niches where they occupy diversified habitats, from cities to agricultural fields to primary forests. They are also the reservoirs and vectors of many pathogens of zoonotic importance (Meerburg et al. Reference Meerburg, Singleton and Kijlstra2009; Blasdell et al. Reference Blasdell, Bordes, Chaisiri, Chaval, Claude, Cosson, Latinne, Michaux, Morand, Pagès and Tran2015). Due to their high taxonomic and ecological diversity and the high prevalence of Pneumocystis among wild rodents (Mazars et al. Reference Mazars, Guyot, Fourmaintraux, Renaud, Petavy, Camus and Dei-Cas1997; Palmer et al. Reference Palmer, Settnes, Lodal and Wakefield2000; Chabé et al. Reference Chabé, Herbreteau, Hugot, Bouzard, Deruyter, Morand and Dei-Cas2010; Demanche et al. Reference Demanche, Deville, Michaux, Barriel, Pinçon, Aliouat-Denis, Pottier, Noël, Viscogliosi, Aliouat, Dei-Cas, Morand and Guillot2015; Danesi et al. Reference Danesi, da Rold, Rizzoli, Hauffe, Marangon, Samerpitak, Demanche, Guillot, Capelli and de Hoog2016), Southeast Asian murid rodents represent highly relevant models to understand the evolutionary interactions of Pneumocystis species and their mammalian hosts.
Material and methods
Sampling
A total of 445 Southeast Asian wild murid rodents (Rodentia, Myomorpha, Muroidea, Muridae) from 18 species belonging to the subfamilies Rhizomyinae (genus Cannomys) and Murinae (genera Mus belonging to the Murini tribe and Maxomys, Leopoldamys, Niviventer, Berylmys, Bandicota and Rattus belonging to the Rattini tribe) were tested for the presence of Pneumocystis in their lungs. These specimens were collected in 12 localities corresponding to several habitat types (human settlements, forests and cultivated areas) in Thailand, Lao P.D.R. and Cambodia (Fig. 1). Sample collection has spanned more than 10 years (1998–2009). The lung samples were collected immediately after euthanasia and stored in RNAlater (QIAGEN, France) at −20 °C. Rodent species included in the study are neither on the CITES list, nor the Red List (IUCN). Animals were treated in accordance with the guidelines of the American Society of Mammalogists, and within the European Union legislation guidelines (Directive 86/609/EEC). Each sampling campaign was validated by the national, regional and local health authorities. Approval notices for trapping and investigation of rodents were provided by the Ministry of Health Council of Medical Sciences, National Ethics Committee for Health Research (NHCHR) Lao PDR, number 51/NECHR, and by the Ethical Committee of Mahidol University, Bangkok, Thailand, number 0517.1116/661.
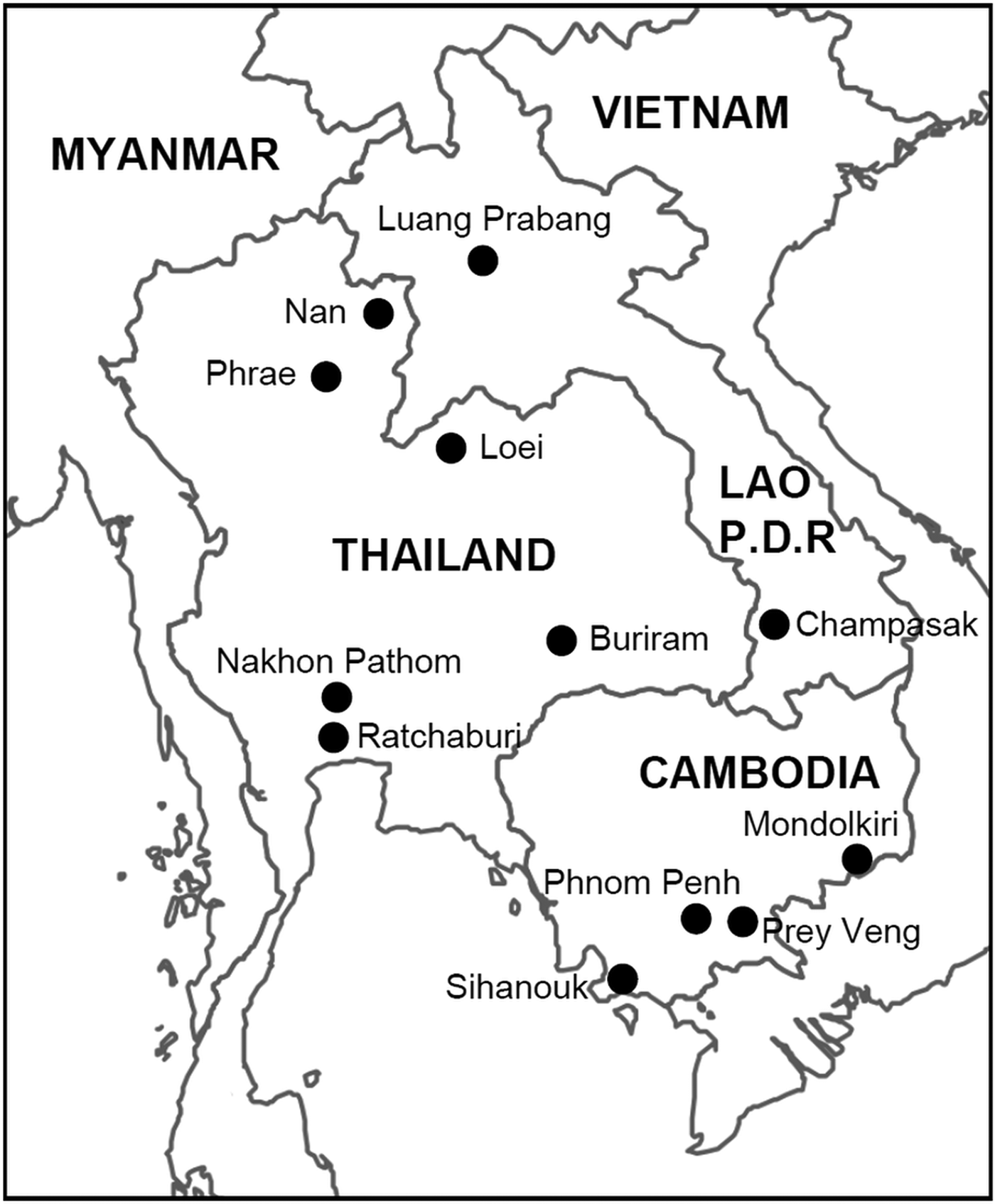
Fig. 1. Map of rodent sampling localities.
Field identifications of captured rodents were made based on geographical and morphological criteria according to Lekagul and McNeely (Reference Lekagul and McNeely1988), Corbet and Hill (Reference Corbet and Hill1992), Aplin et al. (Reference Aplin, Brown, Jacobs, Krebs and Singleton2003) and Francis (Reference Francis2008). These field identifications were then confirmed using molecular barcoding implemented in a web tool (http://data.ceropath.org/) (Pages et al. Reference Pages, Chaval, Herbreteau, Waengsothorn, Cosson, Hugot, Morand and Michaux2010; Galan et al. Reference Galan, Pagès and Cosson2012; Latinne et al. Reference Latinne, Waengsothorn, Rojanadilok, Eiamampai, Sribuarod and Michaux2013). In Southeast Asia, Rattus tanezumi is characterized by two divergent and paraphyletic mitochondrial lineages but these lineages are undistinguishable according to nuclear markers and morphological data (Pages et al. Reference Pages, Chaval, Herbreteau, Waengsothorn, Cosson, Hugot, Morand and Michaux2010, Reference Pages, Bazin, Galan, Chaval, Claude, Herbreteau, Michaux, Piry, Morand and Cosson2013). These two mitochondrial lineages belonging to R. tanezumi were included in this study and referred to as R. tanezumi R2 and R3 in accordance with Pages et al. (Reference Pages, Chaval, Herbreteau, Waengsothorn, Cosson, Hugot, Morand and Michaux2010).
DNA extraction, polymerase chain reaction (PCR) and sequencing
DNA was extracted from lung tissue using the QIAamp DNA mini kit (QIAGEN, France) according to the manufacturer's protocol. Two mitochondrial genes of Pneumocystis, the large subunit rRNA (mtLSU rRNA) and the small subunit rRNA (mtSSU rRNA), were amplified by nested PCR using a high-fidelity DNA polymerase (QIAGEN HotStarTaq). The first round of PCR was performed using the external primers pAZ102-H and pAZ102-E for mtLSU rRNA (Wakefield et al. Reference Wakefield, Pixley, Banerji, Sinclair, Miller, Moxon and Hopkin1990) and the external primers pAZ112-10F and pAZ112-10R for mtSSU rRNA (Tsolaki et al. Reference Tsolaki, Beckers and Wakefield1998). The second round of PCR was performed when the first-round PCR was negative using the internal primers pAZ102-X and pAZ102-W for mtLSU rRNA (Wakefield, Reference Wakefield1996; Chabé et al. Reference Chabé, Herbreteau, Hugot, Bouzard, Deruyter, Morand and Dei-Cas2010) and the internal primers pAZ112-13 and pAZ112-14 for mtSSU rRNA (Tsolaki et al. Reference Tsolaki, Beckers and Wakefield1998). PCR mixtures and conditions for both mtLSU rRNA and mtSSU rRNA were as described in Chabé et al. (Reference Chabé, Dei-Cas, Creusy, Fleurisse, Respaldiza, Camus and Durand-Joly2004) except that a touchdown PCR cycling program with decreasing annealing temperatures from 65 °C to 55 °C (−1 °C/cycle) for the ten first cycles, was used for the mtLSU rRNA first-round PCR. DNA of P. murina was used as a positive control for each PCR round. Moreover, several negative controls were included in each series of DNA extraction and PCR to detect possible cross-contamination. Amplification products were visualized using electrophoresis. Samples were considered as positive for Pneumocystis infection when a PCR product of the expected size (250–350 bp) was amplified either at mtLSU rRNA or mtSSU rRNA or at both loci. When non-specific bands were obtained, the QIAEX II Gel Extraction Kit (QIAGEN) was used to extract and purify amplification products of the expected size from agarose gel. Sequencing reactions were performed from both ends by GenoScreen (Pasteur Institute of Lille, France) on an ABI 3730 XL automated DNA sequencer.
Pneumocystis species-specific PCR
To estimate the proportion of Rattus specimens co-infected by both P. carinii and P. wakefieldiae, a randomly selected subset of 91 infected Rattus specimens belonging to R. nitidus, R. norvegicus, R. exulans, R. andamanensis, R. sakeratensis, R. tanezumi R2 and R. tanezumi R3 were tested with species-specific primers. First-round mtLSU rRNA PCR products obtained with the universal primers pAZ102-H and pAZ102-E were used as templates for the second round of PCR using primers RC1/RC2 and RR1/RR2. These primer pairs developed by Palmer et al. (Reference Palmer, Cushion and Wakefield1999) were used to amplify a portion of the mtLSU rRNA gene of P. carinii and P. wakefieldiae, respectively. Amplification products were then visualized using electrophoresis.
Sequence alignments and phylogenetic reconstructions
Sequence alignments were performed in BioEdit 7·0·9·0 (Hall, Reference Hall1999) using ClustalW algorithm and subsequently refined by eye. The mtLSU rRNA (395 bp including indels) and mtSSU rRNA (872 bp including indels) genes were then concatenated in a combined dataset. Two concatenated alignments were created, one including the hypervariable and ambiguous regions of mtSSU rRNA and one excluding these regions. Gaps were coded as a fifth state.
Molecular phylogenies were estimated by Bayesian inference (BI) and Maximum Likelihood (ML) approaches on the mtLSU rRNA and mtSSU rRNA datasets separately and on the two concatenated datasets (with and without mtSSU rRNA hypervariable regions) including all distinct sequence types. We also added to our dataset reference sequences from P. murina, P. carinii and P. wakefieldiae as well as Pneumocystis sequences available on GenBank and isolated from other Muroid rodents belonging to Muridae and Cricetidae (Table 1). Sequences of Pneumocystis isolated from Ctenodactylus gundi (Rodentia, Hystricomorpha, Ctenodactylidae) were used as outgroups in our phylogenetic trees (Table 1). The most suitable model of DNA substitution (GTR + gamma for each dataset) was determined for each dataset using jMODELTEST 0·1 (Posada, Reference Posada2008). Bayesian analyses were performed with MrBayes 3.1.1 (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003). Metropolis-coupled Markov chain Monte Carlo (MCMC) sampling was performed with five chains run for five million generations with one tree sampled every 1000 generations, using default parameters as starting values. A 50% majority-rule consensus tree was then generated in PAUP 4.0b10 (Swofford, Reference Swofford1998) with burn-in values of 300 000 generations. ML analyses were performed using PhyML 3.0 (Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). The transition/transversion ratio, the proportion of invariable sites and the gamma distribution parameter were estimated. The starting tree was determined by BioNJ analysis of the datasets. Robustness of the tree was assessed by 1000 bootstrap replicates.
Table 1. Pneumocystis reference and outgroup sequences used in phylogenetic analyses and their GenBank accession number

The net genetic distance among the main Pneumocystis lineages recovered in the phylogenetic trees was computed for the mtLSU rRNA dataset in Mega 4.1 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007) under Jukes-Cantor model with complete deletion of gaps or with pairwise-deletion of gaps.
Co-phylogenetic analyses between Pneumocystis and Southeast Asian Rattini
In order to test the congruence between phylogenies of Pneumocystis and their rodent hosts, we first used two global-fit methods: ParaFit (Legendre et al. Reference Legendre, Desdevises and Bazin2002) and PACo (Procrustean Approach to Cophylogeny) (Balbuena et al. Reference Balbuena, Míguez-Lozano and Blasco-Costa2013) implemented in R 3.2.2 (R Core Team, 2013) using the packages ape and vegan. These methods assess the degree of congruence between host and parasite trees using matrices of patristic distances and test its significance against a random distribution (999 permutations for Parafit and 100 000 for PACo). ParaFit also identifies the host–parasite associations significantly contributing to the co-phylogenetic structure.
We then used the heuristic approach with a genetic algorithm implemented in Jane 4.0 (Conow et al. Reference Conow, Fielder, Ovadia and Libeskind-Hadas2010). This event-based method assigns different costs to five evolutionary events (i.e. co-speciation, duplication, host switch, loss and failure to diverge) used to map the parasite phylogeny to the host one and finds a mapping that minimizes the total cost. The least cost solution is considered as the best solution and is then statistically tested by comparison with the costs obtained after randomization of the parasite tree and tip mappings. The two phylogenies are considered as significantly congruent if the cost of the best solution is lower than the costs expected by chance. We tested four different sets of costs for each type of evolutionary event: (1) co-speciation = 0, duplication = 1, host switch = 2, loss = 1, failure to diverge = 1 (default cost scheme of Jane); (2) co-speciation = 0, duplication = 1, host switch = 1, loss = 1, failure to diverge = 1; (3) co-speciation = 0, duplication = 1, host switch = 2, loss = 2, failure to diverge = 1; and (4) co-speciation = −1, duplication = 0, host switch = 0, loss = 0, failure to diverge = 0, this cost scheme maximizes the number of inferred co-speciation events. The analyses were performed with 500 generations and population size of 300. The cost of the best solution was compared with the costs found in 1000 randomizations of both tip mapping and parasite tree topologies.
These co-phylogenetic analyses were limited to the Rattini tribe, the most diverse Murinae tribe in Southeast Asia. This tribe includes, among others, the genera Maxomys, Leopoldamys, Niviventer, Berylmys, Bandicota and Rattus and a robust phylogeny is already available and well accepted (Pages et al. Reference Pages, Chaval, Herbreteau, Waengsothorn, Cosson, Hugot, Morand and Michaux2010; Fabre et al. Reference Fabre, Pagès, Musser, Fitriana, Fjeldså, Jennings, Jønsson, Kennedy, Michaux, Semiadi, Supriatna and Helgen2013). The Pneumocystis tree was compared to the phylogeny of the Rattini tribe obtained by Fabre et al. (Reference Fabre, Pagès, Musser, Fitriana, Fjeldså, Jennings, Jønsson, Kennedy, Michaux, Semiadi, Supriatna and Helgen2013) on the basis of one mitochondrial (cytb) and two nuclear (IRBP and GHR) genes. The Pneumocystis input tree was therefore pruned to include only the six main lineages identified in Southeast Asian Rattini (lineages 5, 7, 9, 10, 11, 12).
Results
Pneumocystis genetic diversity in murid rodents in Southeast Asia
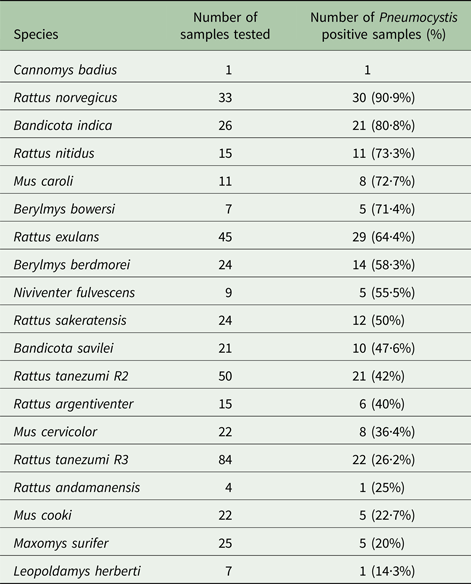
Pneumocystis DNA was detected in 215 out of 445 (48.3%) wild murid rodents in Southeast Asia. Of these 215 positive individuals, 24% were positive after PCR mtLSU1, 29% after PCR mtSSU1, 41% after PCR mtLSU2 and 23% after PCR mtSSU2. We observed an important variation in the frequency of Pneumocystis infection among Muridae species, it varied from 90·9% in R. norvegicus to only 14·3% in Leopoldamys herberti (Table 2).
Table 2. Numbers of tested and Pneumocystis positive murid rodent samples
After sequencing, we obtained 130 valid mtLSU rRNA sequences and 77 valid mtSSU rRNA sequences. In total, valid sequences were obtained at both mtLSU rRNA and mtSSU rRNA for 77 specimens. A total of 69 distinct sequence types were identified among our dataset for mtLSU rRNA and 43 for mtSSU rRNA (Tables 3 and 4). The concatenated dataset (mtLSU rRNA + mtSSU rRNA) included 97 distinct sequence types (92 when the hypervariable regions of mtSSU rRNA were excluded) (Supplementary Tables S1 and S2). Most sequence types of both loci are specific to one Muridae species with the exception of HLSU17, HLSU20, HLSU62, HLSU64, HLSU68, HSSU32, HSSU33 and HSSU43 that are shared by several Rattus species, HLSU27 and HLSU29 that are shared by two Berylmys species and HSSU6 that is shared by both Maxomys surifer and L. herberti (Tables 3 and 4). These shared sequence types have been isolated at different time periods in various localities (Supplementary Tables S3 and S4).
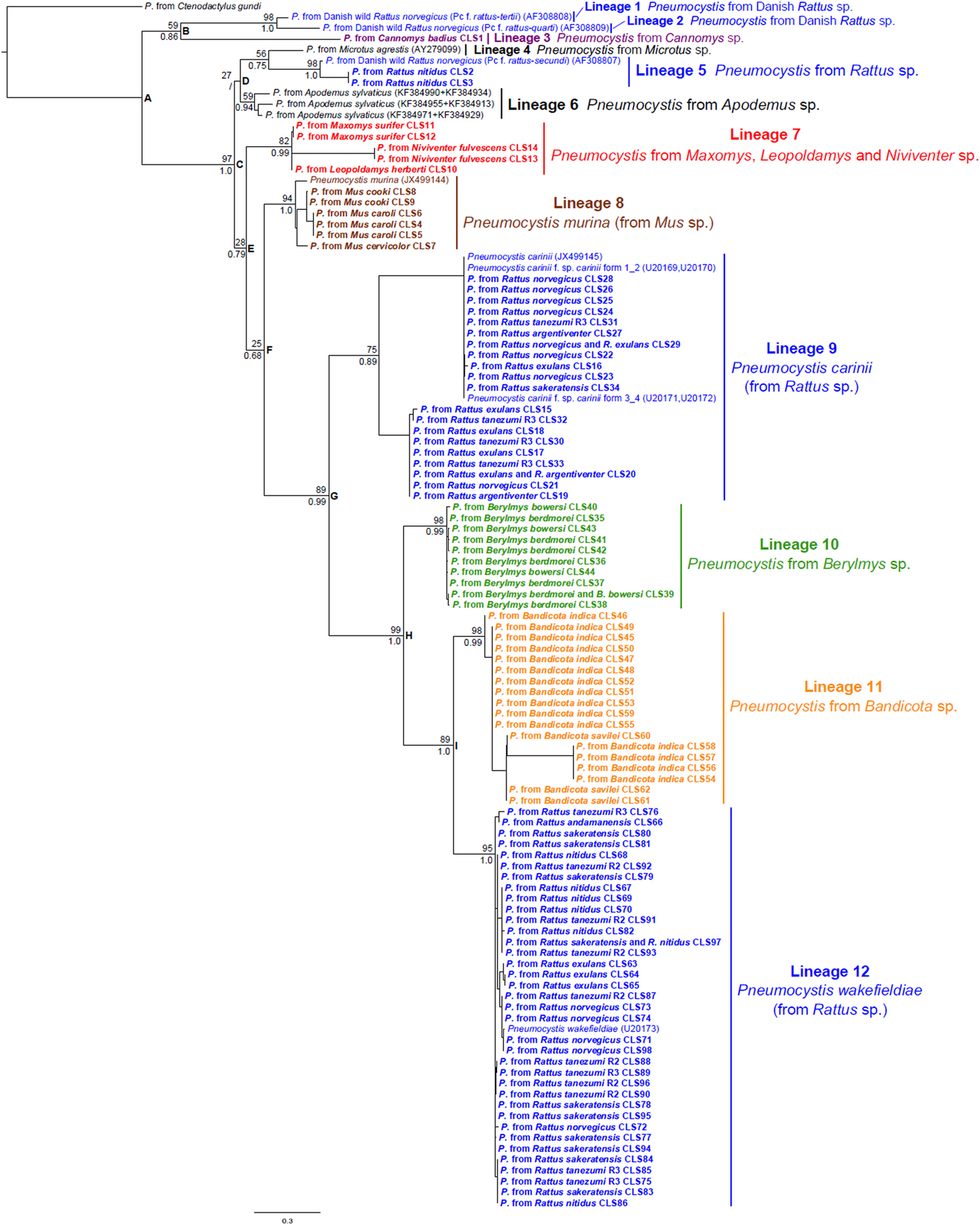
Fig. 2. Maximum Likelihood (ML) tree (GTR + G) depicting phylogenetic relationships among Pneumocystis from murid rodents inferred from concatenated mtLSU rRNA and mtSSU rRNA sequences including mtSSU rRNA hypervariable regions. Bootstrap support (%, 1000 replicates) and posterior probabilities of nodes are indicated above and below the branches, respectively. Node supports from within lineages were removed for clarity of presentation. Sequences from Southeast Asian murid rodents are in bold, other sequences are from GenBank (accession numbers in brackets). Pneumocystis lineages from murid rodent genera distributed in Southeast Asia are coloured according to their host genus.
Table 3. Host range, phylogenetic lineage (numbers refer to Fig. 2) and GenBank accession number of mtLSU rRNA sequence types isolated in Southeast Asian murid rodents

Table 4. Host range, phylogenetic lineage (numbers refer to Fig. 2) and GenBank accession number of mtSSU rRNA sequence types isolated in Southeast Asian murid rodents

Pneumocystis co-infection within the genus Rattus
Positive PCR amplification using the Pneumocystis species-specific primers was obtained in only 56 out of the 91 infected Rattus specimens that were tested. Pneumocystis wakefieldiae was found alone in 43 specimens (76·8%), P. carinii in seven specimens (12·5%) while six individuals (10·7%) were positive for both P. wakefieldiae and P. carinii.
Phylogenetic reconstructions
The two mitochondrial loci used in this study were first used separately to reconstruct phylogenetic trees. They yielded poorly-resolved phylogenies but they mostly recovered similar lineages with the exception of taxa not represented in the mtSSU rRNA matrix (data not shown). The mtLSU rRNA and mtSSU rRNA genes were then concatenated in a single matrix.
The ML tree topology of the concatenated dataset, including the hypervariable regions of mtSSU rRNA retrieved 12 main Pneumocystis lineages (Fig. 2). A weakly-supported group (node B, BS = 59, BP = 0·86) including two Pneumocystis sequences derived from Danish wild R. norvegicus and referred to as Pc f. sp. rattus-tertii (lineage 1) and Pc f. sp. rattus-quarti (lineage 2) in Palmer et al. (Reference Palmer, Settnes, Lodal and Wakefield2000) and Pneumocystis from Cannomys badius (lineage 3) was the first to diverge within the ingroup (Fig. 2). Then a well-supported group (node C, BS = 97, BP = 1·0) included Pneumocystis sequence types derived from Murinae rodents with the exception of one sequence from Microtus agrestis (Rodentia, Cricetideae). Several weakly-supported nodes (nodes D, E and F) leading to five well-supported lineages were also identified, corresponding to Pneumocystis from Microtus agrestis (lineage 4), Pneumocystis from Danish wild R. norvegicus (Pc f. sp. rattus-secundi) and two Southeast Asian R. nitidus (lineage 5, BS = 98, BP = 1·0), Pneumocystis from Apodemus sylvaticus (lineage 6, BS = 59, BP = 0·94), Pneumocystis from Maxomys, Leopoldamys and Niviventer (lineage 7, BS = 82, BP = 0·99), and Pneumocystis from Mus (P. murina, lineage 8, BS = 94, BP = 1·0). Then a well-supported node (node G, BS = 89, BP = 0·99) included a lineage corresponding to P. carinii from Rattus (lineage 9, BS = 75, BP = 0·89) and a well-supported group (node H, BS = 99, BP = 1·0) encompassing Pneumocystis from Berylmys (lineage 10, BS = 98, BP = 0·99), Bandicota (lineage 11, BS = 98, BP = 0·99) and Rattus (P. wakefieldiae, lineage 12, BS = 95, BP = 1·0). The ML and BI tree topologies were congruent for all the main internal nodes except one (node D). Branching topologies within each of the 12 main lineages are poorly-resolved and only two monophyletic sublineages specific to one host species (i.e. a sublineage within lineage 7 including Pneumocystis from Niviventer fulvescens and a sublineage within lineage 8 including Pneumocystis from Mus caroli) were retrieved in both ML and BI trees.
The tree topology of the concatenated dataset excluding the hypervariable regions of mtSSU rRNA recovered the same 12 main lineages but differed in some internal nodes (nodes B, D, F, and I) that are weakly-supported in both trees (Supplementary Fig. S1).
Genetic distance among the main Pneumocystis lineages
The percentage of net genetic distance is 11·6% (15·7% after complete deletion of gaps) between P. carinii and P. wakefieldiae, 10·9% (16.5%) between P. carinii and P. murina and 12·3% (18·7%) between P. wakefieldiae and P. murina (Table 5). Similar or slightly lower levels of genetic distance were observed between lineages 4, 5, 6, 7 and these Pneumocystis species while the genetic distances among Pc f. sp. rattus-tertii (lineage 1), Pc f. sp. rattus-quarti (lineage 2), Pneumocystis from C. badius (lineage 3) and all other lineages were much higher (Table 5). Low levels of genetic distance were observed among lineage 10 (Pneumocystis from Berylmys), lineage 11 (Pneumocystis from Bandicota) and P. wakefieldiae (Table 5).
Table 5. Net genetic distance (percentages) among the main Pneumocystis lineages recovered in the phylogenetic tree (Fig. 2) computed under Jukes–Cantor model with complete deletion of gaps (above the grey cells) or with pairwise-deletion of gaps (below the grey cells)
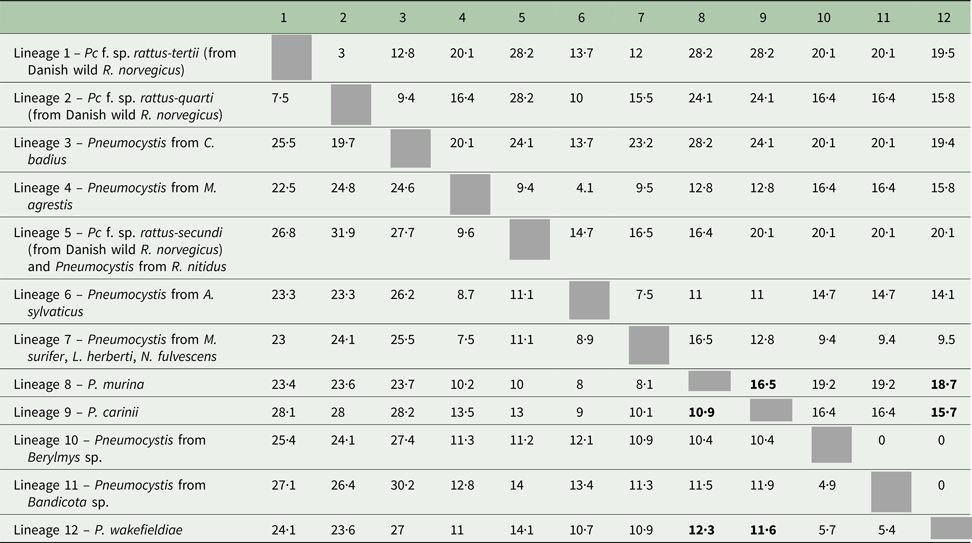
Distances among recognized Pneumocystis species (P. murina, P. carinii and P. wakefieldiae) are in bold.
Phylogenetic congruence between Pneumocystis and Rattini species in Southeast Asia
Both ParaFit (ParaFitGlobal = 11·73, P value = 0·009) and PACo (m 2 = 8·15, P value = 0·004) provided evidence for significant global congruence between the topologies of Pneumocystis and Southeast Asian Rattini trees. However, only six of the 19 host–parasite associations were significant according to ParaFit1 values (P ⩽ 0·05) (Fig. 3A).
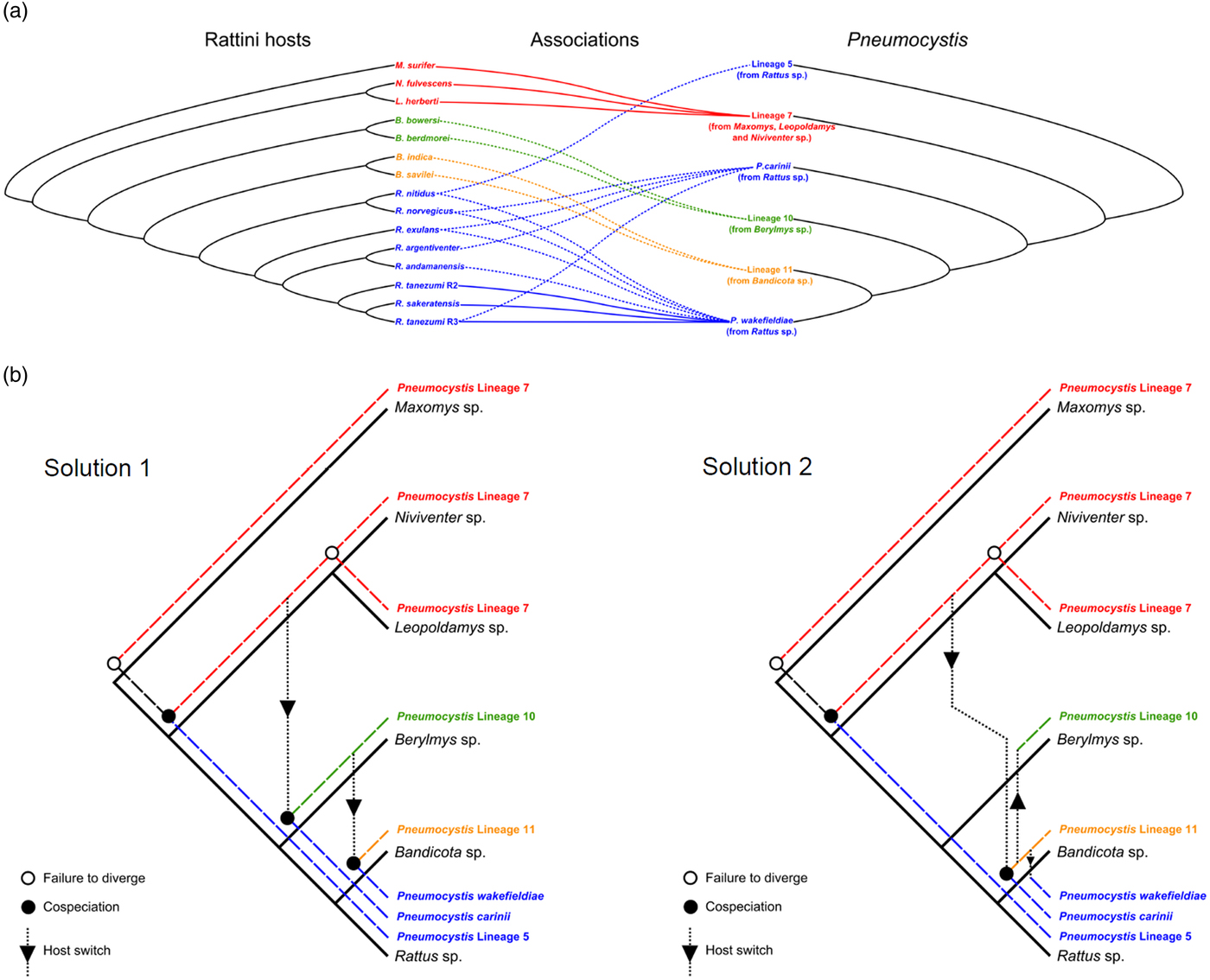
Fig. 3. Phylogenetic congruence between Pneumocystis and Rattini phylogenies. (A) Tanglegram depicting the co-phylogenetic pattern among Pneumocystis and Rattini. Lines connecting taxa indicate Rattini-Pneumocystis associations. Plain lines correspond to significant associations as indicated by ParaFit (P ⩽ 0·05, 999 permutations) while dotted lines correspond to non-significant associations. (B) Reconstructions of the two optimal solutions recovered from Jane analysis under different cost schemes. Plain black lines and dashed coloured lines represent the host and Pneumocystis trees, respectively. The Rattini tree is limited to the genus level for clarity of presentation.
Jane inferred three co-speciation events, 0 duplication, two host switches, 10 losses and 13 failures to diverge (=solution 1) between Pneumocystis and Rattini phylogenies under the four tested cost schemes (Fig. 3B). However, an alternative solution of similar cost (two co-speciation events, 0 duplication, three host switches, nine losses and 13 failures to diverge = solution 2) was also suggested under cost scheme 3 (Fig. 3B). For both solutions and whichever the cost scheme applied, the number of co-speciation events inferred by Jane was always significantly greater than expected by chance.
According to the solution 1, the first co-speciation event occurred between lineage 5 and lineage 7, followed by a host switch occurring from lineage 7, which led to a co-speciation event between lineage 10 and P. carinii. A second host switch occurred from lineage 10 and led to a co-speciation event between lineage 11 and P. wakefieldiae. The solution 2 inferred the first co-speciation event between lineage 5 and lineage 7 followed by host switch from lineage 7 leading to co-speciation between lineage 11 and P. carinii. Then two host switches occurred from lineage 11 to lineage 10 and from lineage 11 to P. wakefieldiae. All loss events occurred within the genus Rattus for both solutions (Fig. 3B).
Discussion
Species boundaries in Pneumocystis infecting murid rodents
Due to the complexity of the in vitro culture of Pneumocystis organisms, the Phylogenetic Species Concept (PSC) has been widely used to recognize distinct species of this fungal parasite (Stringer et al. Reference Stringer, Cushion and Wakefield2001; Keely et al. Reference Keely, Fischer, Cushion and Stringer2004). A phylogenetic species is an independent evolutionary lineage having a unique combination of DNA sequences (Taylor et al. Reference Taylor, Jacobson, Kroken, Kasuga, Geiser, Hibbett and Fisher2000). The phylogenetic concordance of multiple unlinked genes indicates a lack of genetic exchange and the evolutionary distinctiveness of these lineages and therefore allows the recognition of distinct fungal species (Giraud et al. Reference Giraud, Refrégier, Le Gac, de Vienne and Hood2008). Standard loci should be used for this purpose and Stringer et al. (Reference Stringer, Cushion and Wakefield2001) suggested that one of these loci should be mtLSU rRNA. The level of genetic distance between lineages is also useful to delimit species boundaries among Pneumocystis (Keely et al. Reference Keely, Fischer, Cushion and Stringer2004; Dei-Cas et al. Reference Dei-Cas, Chabé, Moukhlis, Durand-Joly, Aliouat, Stringer, Cushion, Noël, Sybren De Hoog, Guillot and Viscogliosi2006). Stringer et al. (Reference Stringer, Cushion and Wakefield2001) indicated that ‘if the genetic distance at the mtLSU rRNA locus between two Pneumocystis organisms equals or exceeds that seen between P. carinii and P. carinii f. sp. ratti (the older name of P. wakefieldiae), the two test organisms are recognizable as different species, even if they are found in the same host species.… A lower divergence calls for more analysis’.
If we apply this phylogenetic criterion to our dataset, several new species may be recognized among the Pneumocystis infecting murid rodents. We estimated the level of net genetic distance between P. carinii and P. wakefieldiae, between P. carinii and P. murina and between P. wakefieldiae and P. murina at around 11·6, 10·9 and 12·3%, respectively (pairwise gap deletion) (Table 5). The roughly similar or higher levels of genetic distance among lineages 1 (Rattus), 2 (Rattus), 3 (Cannomys), 4 (Microtus), 5 (Rattus), 6 (Apodemus), 7 (Maxomys, Leopoldamys, Niviventer), P. murina (Mus), P. carinii (Rattus) and P. wakefieldiae (Rattus) as well as the phylogenetic independence of all these lineages at both mtLSU rRNA and mtSSU rRNA (for the lineages for which mtSSU rRNA sequences were available) confirm the absence of gene flow among them and are indicative of phylogenetic species recognition. However, due to the particular and distinct evolutionary history of the mitochondrial genome, mitochondrial markers alone are not sufficient for the delineation of new Pneumocystis species. The use of nuclear genes is needed to confirm the phylogenetic concordance of mitochondrial and nuclear genes and validate the phylogenetic species status of these seven Pneumocystis lineages. Evidence of morphological or biological differences (e.g. ultrastructure, growth rate, etc.) among these lineages would also reinforce their taxonomic distinctiveness and allow us to describe new Pneumocystis species using the Morphological Species Concept (MSC) or the Biological Species Concept (BSC). However morphological and biological studies of Pneumocystis organisms require a large amount of fungal material, which cannot be obtained in wild rodents. Natural cases of severe Pneumocystis infection in wild rodents are scarce and Pneumocystis infections in wild rodents are mild compared with those of immunosuppressed laboratory animals that develop Pneumocystis pneumonia (Chabé et al. Reference Chabé, Herbreteau, Hugot, Bouzard, Deruyter, Morand and Dei-Cas2010). Inducing immunosuppression in wild rodents might be the answer, but this seems difficult to perform for wild specimens of animal species that cannot be routinely kept in laboratory facilities. The lower genetic distances among lineages 10, 11 and P. wakefieldiae preclude the recognition of Pneumocystis infecting Berylmys and Bandicota rodents as distinct phylogenetic species. The analysis of additional genes is needed to confirm their taxonomic status.
Pneumocystis host specificity and diversity in Southeast Asian murid rodents
Narrow specificity at the host-species level has usually been reported for Pneumocystis parasites based on genetic and phenotypic data and cross-infection experiments (Aliouat et al. Reference Aliouat, Mazars, Dei-Cas, Cesbron and Camus1993, Reference Aliouat, Mazars, Dei-Cas, Delcourt, Billaut and Camus1994; Gigliotti et al. Reference Gigliotti, Harmsen, Haidaris and Haidaris1993; Demanche et al. Reference Demanche, Berthelemy, Petit, Polack, Wakefield, Dei-Cas and Guillot2001; Hugot et al. Reference Hugot, Demanche, Barriel, Dei-Cas and Guillot2003; Akbar et al. Reference Akbar, Pinçon, Aliouat-Denis, Derouiche, Taylor, Pottier, Carreto-Binaghi, González-González, Courpon, Barriel, Guillot, Chabé, Suarez-Alvarez, Aliouat, Dei-Cas and Demanche2012). However, the Pneumocystis monoxenism may not systematically occur at the host intra-generic level and several exceptions to this host-species specificity have been described in the literature when a single Pneumocystis species/lineage infected at least two closely related host species in primates (Macaca mulatta and M. fascicularis) (Guillot et al. Reference Guillot, Demanche, Norris, Wildschutte, Wanert, Berthelemy, Tataine, Dei-Cas and Chermette2004) and rodents (Apodemus flavicollis and A. sylvaticus) (Danesi et al. Reference Danesi, da Rold, Rizzoli, Hauffe, Marangon, Samerpitak, Demanche, Guillot, Capelli and de Hoog2016; Demanche et al. Reference Demanche, Deville, Michaux, Barriel, Pinçon, Aliouat-Denis, Pottier, Noël, Viscogliosi, Aliouat, Dei-Cas, Morand and Guillot2017). Our study demonstrates that murid rodent Pneumocystis host specificity is mostly limited to the generic level rather than the species level as several mtLSU rRNA and mtSSU rRNA sequence types are shared among several host species belonging to the same genus (Rattus, Berylmys) or even among two well-differentiated Muridae genera (Maxomys and Leopoldamys) (Tables 3 and 4). Most of the Pneumocystis lineages/species retrieved in our phylogenetic tree infect several rodent species (lineage 5, P. murina, P. carinii, lineage 10, lineage 11 and P. wakefieldiae) or genera (lineage 7). The considerable temporal and geographical range of our sampling demonstrate that this Pneumocystis sharing across rodent species/genera is a long-term and large-scale process across the Indochinese region and is not limited only to a particular geographical location or to rodents sharing the same environment.
These results suggest that the host species (and genus in some cases) is not a barrier to Pneumocystis transmission in wild rodent populations, indicating a weaker host specificity of Pneumocystis species infecting Southeast Asian murid rodents than that of Pneumocystis infecting primates and bats (Demanche et al. Reference Demanche, Berthelemy, Petit, Polack, Wakefield, Dei-Cas and Guillot2001; Guillot et al. Reference Guillot, Demanche, Hugot, Berthelemy, Wakefield, Dei-Cas and Chermette2001; Akbar et al. Reference Akbar, Pinçon, Aliouat-Denis, Derouiche, Taylor, Pottier, Carreto-Binaghi, González-González, Courpon, Barriel, Guillot, Chabé, Suarez-Alvarez, Aliouat, Dei-Cas and Demanche2012). We assume that the weaker host specificity of this Pneumocystis is possible because of physiological, cellular and/or immunological similarities among these closely related rodent species that diverged quite recently.
Rattus species are the only ones among the Southeast Asian Muridae rodents that we tested to be infected by several Pneumocystis species. Palmer et al. (Reference Palmer, Settnes, Lodal and Wakefield2000) described five highly divergent Pneumocystis species/lineages infecting wild Danish R. norvegicus, sometimes in co-infection. We identified three of them [P. carinii, P. wakefieldiae and Pc f. sp. rattus-secundi (lineage 5)] in Southeast Asian Rattus specimens, each Rattus species hosting a maximum of two Pneumocystis species/lineages. According to the results of our species-specific PCR, P. carinii and P. wakefieldiae were mostly found alone but some instances of co-infection were also detected (10·7%). As the sensitivities of these species-specific PCR are similar (Chabé et al. Reference Chabé, Herbreteau, Hugot, Bouzard, Deruyter, Morand and Dei-Cas2010), these results revealed the higher prevalence of P. wakefieldiae (76.8%) in wild Rattus populations. As illustrated previously by Chabé et al. (Reference Chabé, Herbreteau, Hugot, Bouzard, Deruyter, Morand and Dei-Cas2010), these findings are in discrepancy with studies performed on laboratory rats (R. norvegicus) where P. wakefieldiae was almost always found in co-infection with P. carinii and rarely alone (Cushion, Reference Cushion1998; Icenhour et al. Reference Icenhour, Arnold, Medvedovic and Cushion2006). In our study, P. wakefieldiae was found to infect all Rattus species except R. argentiventer. However, this could be due to the low number of R. argentiventer specimens tested in our study (15), examining a larger number of R. argentiventer is required before confirming the absence of P. wakefieldiae in this species. Moreover Sanger sequencing is not optimal for identifying Pneumocystis mixed infections and minority alleles might not have been detected using this method. The use of other methods able to detect a mixture of distinct sequences, such as next-generation sequencing, is needed to accurately investigate the Pneumocystis co-infection pattern among murid rodents.
With 66 described species, Rattus is among the most speciose mammal genera (Musser and Carleton, Reference Musser, Carleton, Wilson and Reeder2005). This genus likely originated in Southeast Asia, the centre of Rattus diversity, from where they dispersed to continental Asia and the Sahul region (Rowe et al. Reference Rowe, Aplin, Baverstock and Moritz2011). The origin of the genus is relatively recent, estimated at the Plio-Pleistocene boundary (around 2–3 Mya) and its diversification rate is more than 3 times higher than for other Murinae rodents (Rowe et al. Reference Rowe, Aplin, Baverstock and Moritz2011). This exceptionally high species richness of the genus Rattus is one of the hypotheses that may explain the higher diversity of Pneumocystis in Rattus compared with other Muridae genera. Several studies found a positive correlation between the taxonomic richness of hosts and that of their parasites, which may be explained by the role of both host availability and evolutionary co-diversification (Kamiya et al. Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014). The host social behavior has also been suggested as a variable that may explain parasite richness, with gregarious species living closely together having a higher parasite richness than solitary species (Desdevises et al. Reference Desdevises, Morand, Jousson and Legendre2002). Several Rattus species are known to live communally with a strong hierarchical social system (Aplin et al. Reference Aplin, Brown, Jacobs, Krebs and Singleton2003) but the inter-specific interactions of Rattus species and the social behaviour of other Muridae genera remain poorly known, which currently prevents the further assessment of the relevance of this hypothesis.
Pneumocystis and Southeast Asian Rattini rodent evolutionary history
Our co-phylogenetic analyses revealed a complex evolutionary history among Pneumocystis and their murid rodent hosts. Even if a significant global signal of co-speciation has been detected, co-speciation alone is not sufficient to explain the observed co-phylogenetic pattern. These results conflict with the traditional view of a prolonged process of co-evolution and co-speciation of Pneumocystis and their hosts. The most striking findings of this study are that the most basal Rattini genera (Maxomys, Leopoldamys, Niviventer) are infected by the less diversified Pneumocystis lineage (lineage 7 which failed to diverge) while the evolutionarily young genus Rattus host several paraphyletic and highly divergent Pneumocystis species/lineages, including the most basal ones (lineages 1, 2 and 5). These findings contradict two of the principles traditionally proposed to explain host–parasite evolution, the Fahrenholz's rule: ‘parasite phylogeny mirrors that of its host’ (Brooks, Reference Brooks1985) and Szidat's rule: ‘evolutionarily primitive (basal) hosts harbour evolutionarily primitive (basal) parasites’ (Brooks, Reference Brooks1979). According to our Jane's analysis, several host switches are the most likely reason to explain this incongruence between Pneumocystis and Rattini phylogenies. These host switches that were inferred in the deep Pneumocystis phylogeny are macro-evolutionary processes and they did not involve contemporary rodent and Pneumocystis species but their ancestors. From an ecological point of view, a host switch should be considered as the colonization of the new host by parasites. Simulation and empirical studies have shown that parasite species with various levels of ecological specialization are able to colonize new hosts, even those that are highly specialized (Hoberg and Klassen, Reference Hoberg and Klassen2002; Janz and Nylin, Reference Janz, Nylin and Tilmon2008; Araujo et al. Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015). Opportunity and compatibility (ecological fitting) are major determinants of host switch success but evolutionary changes (i.e. acquisition of novel genetic information) are not necessarily required (Agosta and Klemens, Reference Agosta and Klemens2008; Hoberg and Brooks, Reference Hoberg and Brooks2008; Araujo et al. Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015). Pneumocystis ancestors may have come into contact with new rodent hosts through changes in rodent geographic distributions and/or ecological structure during past periods of environmental changes. They may also have been able to colonize distantly related hosts through ‘stepping-stone process’, sequentially colonizing several more closely related hosts (Araujo et al. Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015). Studying Pneumocystis diversity in murid rodents in other regions of the world would allow to better understand the historical biogeography of these host–parasite associations at the global scale. The use of nuclear genes in the future would also help to confirm these evolutionary hypotheses.
Estimation of Pneumocystis speciation time could be a useful tool to better understand the evolutionary history of this fungal parasite and confirm that speciation of both host and parasite occurred simultaneously when co-speciation events are inferred. Indeed, testing that reciprocal speciation is temporally plausible is essential to confirm true co-speciation events rather than host switches followed by parasite speciation (‘pseudo co-speciation’) (Percy et al. Reference Percy, Page and Cronk2004; De Vienne et al. Reference De Vienne, Giraud and Shykoff2007, Reference De Vienne, Refrégier, López-Villavicencio, Tellier, Hood and Giraud2013). However, estimating divergence times in Pneumocystis is particularly difficult due to the lack of Pneumocystis fossil records (Leung, Reference Leung2015). Several attempts have been made in the literature using fungi nucleotide substitution rates. Keely et al. (Reference Keely, Fischer, Cushion and Stringer2004) estimated that P. carinii and P. murina diverged 30–40 Mya while Cushion et al. (Reference Cushion, Keely and Stringer2004) estimated that P. carinii and P. wakefieldiae diverged 15–22 Mya. However, these dates seem implausible as they are much older than the divergence times estimated for their rodent hosts: the Mus/Rattus split is estimated to be around 12 Mya, while the first divergences within the genus Rattus occurred around 2–3 Mya (Rowe et al. Reference Rowe, Aplin, Baverstock and Moritz2011; Fabre et al. Reference Fabre, Pagès, Musser, Fitriana, Fjeldså, Jennings, Jønsson, Kennedy, Michaux, Semiadi, Supriatna and Helgen2013; Kimura et al. Reference Kimura, Hawkins, McDonough, Jacobs and Flynn2015).
Conclusion
This study revealed a complex evolutionary history among Pneumocystis and murid rodents, far from the traditional simple picture of strict host specificity and ancient co-evolution of Pneumocystis and their mammalian hosts. According to our results, the specificity of Pneumocystis infecting murid rodents is weaker than previously thought and mainly limited to the host genus level. Pneumocystis from wild rodents thus appears to be stenoxenous (narrow range of closely related hosts) rather than strictly monoxenous parasites (single host species). An important Pneumocystis diversity, which does not only result from a process of co-speciation but also from several host switches, has been evidenced within the genus Rattus. Several new Pneumocystis phylogenetic species could be recognized among the Pneumocystis lineages that we identified in murid rodents. Additional genetic and phenotypic data are now needed to confirm their taxonomic status.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001883
Acknowledgements
We thank the CERoPath team and drivers for help during fieldwork. We also thank Pr Ali Ayadi and Dr Mohamed Ali Jarboui (University of Sfax, Tunisia) for providing us with the lung samples of Ctenodactylus gundi used in this study.
Financial support
This study was funded by the ‘CERoPath project’, ANR Biodiversity ANR07 BDIV012, and the ‘BiodivHealthSEA project’, ANR CPandES11 CPEL002, funded by the French National Agency for Research. This work was also supported by a Marie Curie COFUND postdoctoral fellowship to A. Latinne and by the French Ministry of Research (Lille 2 University and Pasteur Institute of Lille).











