Published online by Cambridge University Press: 19 January 2004
Sex ratio theory posits that the adaptive proportion of male to female gametocytes of a malaria parasite within the vertebrate host depends on the degree of inbreeding within the vector. Gametocyte sex ratio could be phenotypically flexible, being altered based on the infection's clonal diversity, and thus likely inbreeding. This idea was tested by manipulating the clonal diversity of infections of Plasmodium mexicanum in its lizard host, Sceloporus occidentalis. Naive lizards were inoculated with infected blood from a single donor or 3 pooled donors. Donors varied in their gametocyte sex ratios (17–46% male), and sex ratio theory allowed estimation of the clonal diversity within donor and recipient infections. Phenotypic plasticity would produce a correlation between donor and recipient infections for infections initiated from a single donor, and a less female-biased gametocyte sex ratio in recipients that received a mixed blood inoculum (with predicted higher clonal diversity) than recipients receiving blood from a single donor. Neither pattern was observed. Gametocyte sex ratio of most infections ranged from 35 to 42% male, expected if clonal diversity was high for all infections. Alternative explanations are suggested for the observed variation of gametocyte sex ratio among P. mexicanum infections.
Malaria parasites (Plasmodium and related apicomplexan genera) replicate asexually within their vertebrate host, and produce sexual stages, or gametocytes, in the host's blood cells. When the vector takes a bloodmeal, the female gametocytes develop into a single female gamete and the male gametocytes produce 1 to several motile male gametes. The potential fecundity of male gametocytes thus depends on the number of gametes they produce (termed here c). Joining of male and female gametes initiates sexual reproduction of the parasite (Bruce-Chwatt, 1985).
The gametocyte sex ratio, or proportion of male to female gametocytes in the vertebrate host, varies among species of malaria parasite (Schall, 1989, 1996), among geographical regions for the same parasite species (Read et al. 1995), and among infections of a parasite species at a local site (Read et al. 1992; Schall, 1996; Osgood, Eisen & Schall, 2002; Osgood et al. 2003). An explanation for this variation is offered by the evolutionary theory of sex ratios which concludes that the adaptive sex ratio depends on the degree of selfing, or mating between genetically identical gametes, within the vector (Read et al. 1992; Dye & Godfray, 1993). High clonal diversity of the parasites (a measure of both the number of clones present and their relative abundance) will result in low selfing and selection will favour an equal proportion of male to female gametocytes. In contrast, low clonal diversity, and thus high selfing in the vector, will favour a female-biased sex ratio. For example, if a single clone exists in an infection, that genotype will experience highest fitness if just enough males are produced to mate with all the females (c females[ratio ]1 male). Additional clones added to the infection would result in competition among males for access to females and thus each clone should produce additional males. The predicted relationship between inbreeding and sex ratio is shown in Fig. 1. Two additional factors, however, will select for an unbiased sex ratio even if inbreeding is high. First, if male fecundity is low, a higher proportion of male gametocytes will be necessary (Paul et al. 2000). The most extreme example would be male gametocytes that produce but a single gamete (c=1); the expected sex ratio would be 1[ratio ]1 even when only a single clone is present. This effect is illustrated in Fig. 1. Second, if few gametocytes are carried into the vector's blood meal, male gametes would rarely meet a female gamete and each gametocyte would have low fecundity. Additional males would thus be required to insure all female gametes encounter a male gamete during the brief period available for mating. These 2 effects have been termed ‘fertility insurance’ by West et al. (2002).

Fig. 1. Equilibrium gametocyte sex ratio (proportion of male gametocytes=r*) predicted as a function of selfing rate, the proportion of female gametes fertilized by male gametes of the same genotype (=s). The outcome depends on c, the number of male gametes produced by a male gametocyte, a measure of male fecundity. Selfing rate is determined by the clonal diversity (CD) in the vertebrate host's blood. Note that the relationship between selfing rate and CD is not linear. Thus, an increase in the CD from 1 to 3 leads to a major change in selfing rate, and an associated large change in expected sex ratio, whereas an increase from 5 to 9 clones only slightly alters selfing rate and expected sex ratio. The lower limit for sex ratio is determined by c (relationship given). Adapted from Read et al. (1992).
Natural selection could produce an adaptive gametocyte sex ratio in 2 ways. First, sex ratio could be genetically fixed, and selection would produce a locally adaptive sex ratio based on the prevailing degree of selfing within infections. Second, a phenotypically plastic response could allow each infection to monitor cues that allow it to reach its optimal sex ratio based on the selfing likely for that infection. Phenotypic plasticity would thus explain the observed variation in sex ratio among infections at a local site. These 2 mechanisms reflect a venerable issue in evolutionary biology, the relative importance of local adaptation versus adaptive phenotypic plasticity. Here we present an experimental test of the phenotypic plasticity hypothesis. Infections of P. mexicanum were experimentally induced in the parasite's natural lizard host using a protocol that should have generated different clonal diversity among the infections (high vs low clonal diversity), and the sex ratio of those infections was compared to expectations drawn from the theory. Except for a small, but highly suggestive, experiment with a laboratory rodent malaria model (Taylor, 1997), this is the first experimental study on the response of gametocyte sex ratio to manipulation of clonal diversity.
The study site was the Hopland Research and Extension Center located in southern Mendocino County, California, USA, where P. mexicanum and its vertebrate host, the western fence lizard, Sceloporus occidentalis, have been under study for many years (Schall, 1996, 2002). Fifteen naturally infected male donor lizards were collected from sites where P. mexicanum infection has been common in the lizards. The sites were at least 2 km apart to increase the likelihood of clonal diversity among the infections. Another 150 uninfected recipient male lizards were collected from sites where P. mexicanum has been rare or absent in the lizards over a 20-year period (Schall & Marghoob, 1995). Thin blood smears were made for each lizard, stained with Giemsa's stain (pH 7·0, 50 min), and scanned for 6 min at 1000× magnification to confirm that they were not already infected. Use of a highly sensitive PCR-based technique revealed that weak infections, not detectable under the light microscope, are rare at the Hopland study area (Perkins, Osgood & Schall, 1998).
Parasitaemia was quantified for donor smears by counting the number of asexual parasites per 1000 erythrocytes at 1000× magnification. Sex ratio was determined for each donor infection by scoring 100 mature gametocytes based on colour, size of the nucleus, and the distribution of pigment granules (Schall, 1989). Some infections of P. mexicanum reveal an unstable sex ratio within the first 10 days after patency in the blood, but reach a stable point within a week or two (Osgood et al. 2002). The naturally infected donor lizards were collected in early spring and these infections contained both mature gametocytes and asexuals, indicating they were long established infections that overwintered, and thus should have had a stable sex ratio (Eisen, 2000).
Five experiments were conducted, each using 3 donors and 25 (Exps 1–4) or 50 (Exp. 5) recipients. For each experiment, recipients were randomly assigned to 1 of 4 treatment groups. Lizards in the first 3 treatment groups within an experiment were each inoculated with blood from a single donor (Donors 1–3 for that experiment). These recipients (5 per treatment for Exps 1–4 and 10 for Exp. 5) are termed the ‘single donor infections’. Blood from the 3 donors used in each experiment was combined to initiate infections in 10 (Exps 1–4) or 20 (Exp. 5) recipients. These recipient infections are termed ‘multiple donor infections’. For each donor lizard, the density of erythrocytes per μl of blood was determined using a counting chamber (Hausser Scientific). Parasitaemia of asexual P. mexicanum and erythrocyte density of each donor were used to calculate the quantity of blood containing 2×105 asexual parasites, which was then mixed with PBS to a total volume of 20 μl for intraperitoneal injection into each recipient lizard. This method readily initiates P. mexicanum infections in fence lizards (Osgood et al. 2002, 2003). The multiple donor infections received blood that contained an equal number of parasites from each of the donors (total of 2×105 asexual parasites).
Recipients were housed in large outdoor, vector-proof enclosures (2·44×2·44×1·83 m) from mid-May until mid-September. Lizards were fed each day with crickets and mealworms, and once each week a drop of blood was drawn from a toe clip to produce a thin blood smear for microscopical examination. Sex ratio was determined over the entire course of infection for 10 lizards, 1 from among the single donor infections and 1 from the multiple donor infections for each of the 5 experiments. For the other recipients, parasitaemia and sex ratio were scored for the last 2 smears for each recipient as described above. The last 2 smears were used because sex ratio presumably had reached a stable equilibrium by this time (Osgood et al. 2002). Of the 150 lizards, 5 died too early to determine their equilibrium sex ratio and 2 never produced any gametocytes in their infections.
To determine the potential fecundity (c in Fig. 1) of male gametocytes, exflagellation was observed in vitro. A drop of blood was taken from an infection with high numbers of gametocytes, and mixed with an equal amount of an exflagellation medium (20 mM sodium bicarbonate, 20 mM glucose, and PBS to pH 7·5) on a slide. The slide was examined under 1000× magnification, and the number of flagella counted for each exflagellating gametocyte.
Because the data are proportions and sample sizes are small for each treatment (5, 10, or 20), non-parametric tests were used to compare sex ratio of single and multiple donor treatment groups in each experiment. Large numbers of gametocytes were scored for each infection (100), so no adjustment by sample size was made.
Inbreeding of gametes will depend on the number and relative abundance of the parasite clones present in the vertebrate's blood. No direct measure of this clonal diversity was possible. Instead, the observed sex ratio of each donor was used to calculate the clonal diversity (CD), which is a measure of both the number of genetically distinct clones in an infection and their relative abundance. Table 1 presents this value, calculated as CD=1/1−2r, where r is the proportion of male gametocytes. For the mixed donor infections, a minimum and a maximum CD was calculated. If all parasites from the donors were genetically identical, the minimum CD would be equal to the largest CD for the 3 donors. The maximum possible CD is simply the sum of the CD for each of the 3 donors, and assumes that the donors do not share clones of parasites. Predicted sex ratio was calculated as r=1/2(1−1/CD). The theory predicts that the sex ratio for multiple donor infections will fall between the value calculated using the minimum and maximum CD.
Table 1. Design of 5 experiments that manipulated the clonal structure of Plasmodium mexicanum infections (For each experiment, 3 lizards infected with P. mexicanum were used as donors of blood to initiate infections in naive lizards (single donor infections vs multiple donor infections). The sex ratio of each donor (percentage male gametocytes) is given, as well as an estimate of the clonal diversity (CD) of the parasite in those infections. The possible range of CD in the multiple donor recipient infections and resulting range in predicted sex ratios are also given.)

Nineteen exflagellation events were witnessed in vitro (Fig. 2). A mode of 5 flagella was observed (mean=3·7, S.E.=0·33), with a range of 1–6, produced by the male gametocytes. For subsequent discussion c=5 based on these results.

Fig. 2. Distribution of number of observed potential male gametes (number of flagella seen) during exflagellation of Plasmodium mexicanum male gametocytes in vitro.
Sex ratio of donor infections is given in Table 1. None of the gametocyte sex ratios of donor infections fell below the minimum predicted by the theory (16·7% for c=5; Fig. 1). Sex ratios of recipient infections that receive parasites from a single donor are shown in Fig. 3. Again, none produced a sex ratio lower than the minimum predicted by the theory. Under the phenotypic plasticity hypothesis, the sex ratio of single donor recipients is expected to mirror that of their donor infection because the clonal diversity of the donor would be replicated in the recipient infection. Fig. 4 shows the predicted and observed sex ratio of all single donor infections, and reveals no significant relationship between the two (Spearman rank correlation, P>0·05). Instead, sex ratio fell around a mean of 40% males (Figs 3 and 5).

Fig. 3. Histogram of observed recipient sex ratio in single (top) and multiple (bottom) donor recipient Plasmodium mexicanum infections. Each infection was scored twice for sex ratio and both results are included here. The total number of counts was reduced because 7 infections did not produce gametocytes.

Fig. 4. Observed mean recipient sex ratio versus sex ratio of their donor infections for the single donor experiments. The sex ratio of these Plasmodium mexicanum infections expected under the hypothesis of a genetic basis for sex ratio (recipient=donor sex ratio) is indicated.

Fig. 5. Results from 5 experiments in which infections of Plasmodium mexicanum were initiated in lizard hosts from blood from a single infected donor or multiple infected donors. For each experiment, 3 donors were each used to initiate groups of single donor infections (left 3 lines for each experiment's results on the Fig.). The gametocyte sex ratio means (horizontal line) and ranges (vertical lines) are given for each group. Under the hypothesis, the sex ratio of recipient infections should match that of the individual donor; this expected sex ratio is given as an open circle for each group. For each experiment, a fourth group of mixed donor infections is shown with mean sex ratio shown as an open rectangle and range with a vertical line. The predicted range of gametocyte sex ratio for the mixed donor infections is given as a closed box.
Fig. 1 reveals that the predicted sex ratio is not linearly related to CD. A 3-fold increase in CD in the lower range of selfing would not substantially alter the expected sex ratio, whereas small increases in CD when selfing is high would result in a clear change in sex ratio. In Exps 1, 2 and 3, donor sex ratios were high (33–46% male gametocytes), suggesting CD was also high (>3). The sex ratio of single donor versus mixed donor infections would not be expected to differ substantially (range expected for mixed infection would be 35–48% male). Fig. 5 displays the observed and predicted sex ratios for all experiments and treatments. As predicted, there was no significant difference between single and multiple donor recipient infections for Exps 1, 2 and 3 (Kruskal–Wallis tests, P>0·05). A contrasting prediction emerges for Exps 4 and 5 in which donor sex ratios were female biased (17–29% male) and CD was calculated to be low. Sex ratio for the single donor and mixed donor recipient infections was predicted to differ substantially (29–43% male for mixed recipient infections). This prediction based on theory was not supported because there were no differences between sex ratio of single donor or mixed donor infections (Kruskal–Wallis tests, P>0·05). Fisher's method of combined probability was also used to test for differences between treatment groups; results were not significant. For all experiments and treatments, the sex ratio of recipients varied rather little, with a mean of 40% male (Fig. 5).
A significant positive correlation existed between recipient gametocyte sex ratio and gametocytaemia (number of gametocytes per 1000 erythrocytes) (Spearman rank correlation, r=0·26, P<0·0001). Experiment 5, in which donor sex ratios were most female biased, was the only experiment to show a significant donor effect on gametocytaemia (Kruskal–Wallis Test, P=0·011).
Sex ratio was followed for 10 infections. All 10 showed no significant differences in sex ratio over time (comparing initial and final sex ratio; χ2 tests, P>0·05).
Sex ratio theory is among the most successful programmes in evolutionary biology (Charnov, 1982), and is a potentially powerful tool in the study of the biology of malaria parasites (Read et al. 1992; West, Smith & Read, 2000). Several studies support the lead argument of the theory as applied to malaria parasites, that inbreeding drives selection to favour female-biased gametocyte sex ratios, but these studies do not allow a clear distinction between a locally adaptive sex ratio versus phenotypic plasticity.
Read et al. (1995) found that gametocyte sex ratio tends to be less female-biased at sites where parasite prevalence is high. This would be expected if higher prevalence is a result of greater transmission intensity leading to a greater clonal diversity within most infections. Either local adaptation or phenotypic plasticity of gametocyte sex ratio would yield the observed pattern. Osgood et al. (2002) demonstrated that gametocyte sex ratio is heritable from donor to recipient infections of P. mexicanum, and Osgood et al. (2003) found that experimentally altering testosterone levels, and thus the overall physiology of recipients, had no effect on the sex ratio of induced infections. This argues that sex ratio is driven by a genetic component of the infections, but this could be either passage of the diversity of clones from donor to recipient infection, or the presence of genetic polymorphism for specific sex ratio among infections. Last, a high rate of parasite increase and high maximal parasitaemia are associated with a less female-biased gametocyte sex ratio (Osgood et al. 2002; Pickering et al. 2000; Schall, 2000). This association is expected if high clonal diversity leads to competition within the vertebrate host among parasite genotypes, higher rates of replication as the clones compete, and the expected higher proportion of male gametocytes (Schall, 2000). These results are supportive of phenotypic plasticity, but not conclusive.
The most convincing evidence for an adaptive phenotypic plasticity in sex ratio would come from experiments that manipulate the clonal diversity of infections such as the one presented by Taylor (1997). Our experiment took that track, to determine if sex ratio of P. mexicanum was phenotypically plastic and altered based on the assumed clonal diversity within the induced infection.
In the experiment, clonal diversity of the donor and recipient infections was not determined directly. Variable genetic markers, such as the microsatellite loci described for P. falciparum are useful in determining clonal diversity (Anderson et al. 1999; Ferdig & Su, 2000) but are not known for P. mexicanum. Instead, we used the theory itself to estimate clonal diversity in the donors, then again using the theory, calculated the sex ratios expected in the single versus mixed donor recipient infections. Thus, the experiment was an onerous test of the hypothesis.
Three predictions were made. (1) For the experimental infections initiated from a single donor, the donor and recipient infections should be correlated. No donor effect was seen on recipient sex ratios, but instead all sex ratios tended toward 40% male gametocytes. This conflicts with the results of Osgood et al. (2002), but their donor infections harboured a greater range of gametocyte sex ratios which may have allowed a weak trend to be visible. (2) Sex ratio of the mixed donor infections should not differ significantly from single donor recipient infections if the donors had a high proportion of male gametocytes. This prediction was upheld, but the same result would be expected if no phenotypic plasticity in sex ratio exists. (3) Sex ratio of mixed donor recipient infections would be significantly less female biased than single donor mixed infections when the donor infections were female biased. This was a central prediction emerging from the hypothesis, yet sex ratio of single and mixed donor infections were similar, again around 40% male. In summary, the hypothesis was not supported by the results of the experiment.
Infections of P. mexicanum may not facultatively adjust the sex ratio to match the clonal diversity of the infection. Instead, the most commonly observed sex ratio may reflect a genetically fixed phenotype that matches the prevailing degree of selfing within the vectors. Sex ratios of 35–42% observed in P. mexicanum infections suggest a prevailing selfing rate of 0·2 to 0·3, and a clonal diversity of 5 or greater for most infections. This contrasts with the almost always strongly female-biased gametocyte sex ratios of P. falciparum infections in humans (Read et al. 1992). For example, the mean sex ratio of P. falciparum infections from Madang Province, Papual New Guinea was 0·18, with an estimated clonal diversity <2 (Read et al. 1992). Direct measures of clonal diversity of P. falciparum infections from many sites suggest single-clone infections often predominate (Day et al. 1992). Such comparisons suggest that the clonal diversity of P. mexicanum infections may typically be much higher than those of human malaria. This would be surprising considering the strongly seasonal transmission of P. mexicanum in California and the apparent low density of vectors (Schall & Marghoob, 1995). However, infections of P. mexicanum are long lived in the lizard host (Eisen, 2000), which may allow addition of parasite genotypes as vectors bite over time.
A rejection of the phenotypic plasticity hypothesis leaves unresolved the origin of the observed variation in sex ratios among natural and induced infections of P. mexicanum. Three factors unrelated to clonal diversity could lead to variation in sex ratio among infections. (1) Even if there is a locally adapted fixed sex ratio, there will always be some variation due to developmental error. Gametocyte sex ratio should, however, be a life-history trait under strong selection in part because of its influence on the transmission success of parasites as they move from vertebrate to insect vector (Robert et al. 1996; Schall, 2000), so we would expect selection to reduce such maladaptive developmental errors. (2) The number of gametocytes taken into a feeding vector could vary among infections. Low numbers of gametocytes would select for a higher proportion of male gametocytes as fertility insurance (West et al. 2002). We observed, however, a positive relationship between gametocyte density in the blood and sex ratio, rather than the negative relationship suggested by the fertility insurance hypothesis. (3) The potential fecundity of male gametocytes could vary among infections. Burkot, Williams & Schneider (1984) found variation in gametocyte production, sex ratio, and ability to exflagellate among clones of P. falciparum isolated from a single infection. We estimated c=5 from observations of the modal number of flagella emerging from male gametocytes. However, fully 26% of male gametocytes produced 1 or 2 flagella, and the actual viability of the male gametes was not measured. If only one or two gametes are often produced by P. mexicanum gametocytes, the sex ratio should be close to 40% even if selfing is complete. The results of Paul et al. (2000) suggest that Plasmodium infections produce more male gametocytes when cues within the vertebrate host indicate that male gametes will suffer low mating success in the vector. Perhaps the variation in gametocyte sex ratio seen in P. mexicanum is not an indication of adaptive phenotypic plasticity based on clonal diversity but, instead, on the likely fecundity of the male gametocytes. The developmental feedbacks that would control this mechanism are difficult to postulate.
We are particularly intrigued by the consistent finding of a relationship between sex ratio and parasite density in the vertebrate's blood (results presented here and by Osgood et al. 2002; Pickering et al. 2000; Schall, 2000). This pattern originally suggested to us that both sex ratio and the infection's growth rate were adjusted based on clonal diversity. Again, a quite different mechanism could be at play. Infections with a high rate of replication may be associated with a stronger immune response, which could reduce the fecundity of male gametocytes as noted above, and favour a higher production of male gametocytes. Unfortunately, the nature of a lizard's immune response to malaria infection is completely unknown.
Resolution of the enigmatic results that have emerged from studies on the sex ratio of P. mexicanum should cast light on the ecological factors that influence the population structure of malaria parasites and how selection responds to shape the parasite's life-history traits. A deeper understanding of the problem requires direct measures of clonal diversity within infections, and will be possible only with the discovery of variable genetic markers within the parasite's genome.
We thank T. Smith and E. Glesmann for helping in the field, and I. Jones for his assistance in the lab. The staff of the Hopland Research and Extension Center provided logistical support. C. Goodnight suggested the calculation of clonal diversity (CD). The study benefited from discussions on gametocyte sex ratio with R. Paul, S. West, A. Read, I. Paperna, and S. Reece. The experiment was conducted under a protocol approved by the University of Vermont Institutional Animal Care and Use Committee. Funding was provided by the US-NSF.

Fig. 1. Equilibrium gametocyte sex ratio (proportion of male gametocytes=r*) predicted as a function of selfing rate, the proportion of female gametes fertilized by male gametes of the same genotype (=s). The outcome depends on c, the number of male gametes produced by a male gametocyte, a measure of male fecundity. Selfing rate is determined by the clonal diversity (CD) in the vertebrate host's blood. Note that the relationship between selfing rate and CD is not linear. Thus, an increase in the CD from 1 to 3 leads to a major change in selfing rate, and an associated large change in expected sex ratio, whereas an increase from 5 to 9 clones only slightly alters selfing rate and expected sex ratio. The lower limit for sex ratio is determined by c (relationship given). Adapted from Read et al. (1992).

Table 1. Design of 5 experiments that manipulated the clonal structure of Plasmodium mexicanum infections

Fig. 2. Distribution of number of observed potential male gametes (number of flagella seen) during exflagellation of Plasmodium mexicanum male gametocytes in vitro.

Fig. 3. Histogram of observed recipient sex ratio in single (top) and multiple (bottom) donor recipient Plasmodium mexicanum infections. Each infection was scored twice for sex ratio and both results are included here. The total number of counts was reduced because 7 infections did not produce gametocytes.

Fig. 4. Observed mean recipient sex ratio versus sex ratio of their donor infections for the single donor experiments. The sex ratio of these Plasmodium mexicanum infections expected under the hypothesis of a genetic basis for sex ratio (recipient=donor sex ratio) is indicated.
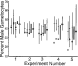
Fig. 5. Results from 5 experiments in which infections of Plasmodium mexicanum were initiated in lizard hosts from blood from a single infected donor or multiple infected donors. For each experiment, 3 donors were each used to initiate groups of single donor infections (left 3 lines for each experiment's results on the Fig.). The gametocyte sex ratio means (horizontal line) and ranges (vertical lines) are given for each group. Under the hypothesis, the sex ratio of recipient infections should match that of the individual donor; this expected sex ratio is given as an open circle for each group. For each experiment, a fourth group of mixed donor infections is shown with mean sex ratio shown as an open rectangle and range with a vertical line. The predicted range of gametocyte sex ratio for the mixed donor infections is given as a closed box.