STRUCTURE, FUNCTION AND SPECIFICITY OF CHOLINESTERASES
Cholinesterases are serine hydrolases which react preferentially with choline esters at close to diffusion-controlled rates (Bazelyansky, Robey & Kirsch, 1986). Vertebrate enzymes can be distinguished by their substrate specificity: acetylcholinesterases (AChEs) hydrolyse acetylcholine (ACh) most efficiently, whereas butyrylcholinesterases (BuChEs) act preferentially on choline esters such as butyrylcholine (BuCh). The latter enzymes also hydrolyse ACh, but have broader substrate specificity and can act on a range of other esters, including alkaloids such as cocaine (Sun et al. 2002). Cholinesterases can be distinguished from related enzymes by their sensitivity to the alkaloid physostigmine (eserine). AChEs are potently inhibited by the bis quaternary ligand 1,5-bis(4-allyldimethylammoniumphenyl) pentan-3-one dibromide (BW284C51), whereas BuChEs are highly sensitive to the organophosphate tetramonoisopropylpyrophosphortetramide (iso-OMPA) (Austin & Berry, 1953). AChEs can be further distinguished from BuChEs by excess substrate inhibition. The basis for this effect is still debated, but it has been suggested that excess substrate binding to a region of the enzyme termed the ‘peripheral’ site induces an allosteric effect which alters the conformation of the active site (Masson et al. 2002). Invertebrates appear to exclusively express AChEs rather than BuChEs, but these enzymes frequently exhibit considerable activity against BuCh (Toutant, 1989). It has been proposed that the evolution of AChE enzymes which have no activity against BuCh coincided with the appearance of the vertebrates (Sanders et al. 1996), although nematode secreted enzymes provide an exception to this rule, as will be discussed later.
AChEs hydrolyse ACh to choline and acetate. Nucleophilic attack of the carbonyl carbon of ACh generates choline and an acylated form of the enzyme, followed by hydrolysis of the acyl-enzyme intermediate to liberate acetic acid (Wilson, Bergmann & Nachmansohn, 1950). By hydrolysing ACh, AChEs terminate transmission of cholinergic signals, and are therefore primarily associated with synaptic contacts between neurones and at the neuromuscular junction. In the vertebrate central nervous system, the enzyme is thought to contribute to neuromodulation (Descarries, 1998), whereas expression on the surface of haematopoietic cells probably serves to hydrolyse any ACh which makes its way into the bloodstream, or may serve to regulate cholinergic signalling in the immune system (Tracey, 2002). The function(s) of BuChE are still unclear, as it is not essential, but this soluble enzyme is found at high concentrations in the plasma, liver, lungs and intestine, and is generally thought to play a protective role in hydrolysing orally ingested toxic compounds (Lockridge & Masson, 2000).
The three-dimensional structure of AChE has been determined from a variety of sources: initially from the electric ray Torpedo californica (Sussman et al. 1991), and subsequently from mammals (Bourne, Taylor & Marchot, 1995) and Drosophila (Harel et al. 2000). The protein is composed of central strands of beta sheets surrounded by alpha helices, and a similar assembly (the alpha/beta fold) is shared by a group of lipases, carboxypeptidases and adhesion molecules (Cygler et al. 1993). A remarkable feature of the enzyme is the position of the catalytic triad (S200, H440, E327), which is located at the base of a deep narrow gorge extending approximately halfway (20 Å) into the enzyme (Sussman et al. 1991). The enzyme has a strong electrostatic dipole aligned with the catalytic gorge, so that a positively charged substrate such as ACh can be attracted to the active site by the electrostatic field (Ripoll et al. 1993). The gorge is lined with aromatic residues which are thought to shield ACh from direct interaction with the negatively charged residues which contribute to the dipole, and possibly contribute to substrate guidance through the affinity of quaternary ammonium compounds for aromatic rings (Ripoll et al. 1993). ACh is oriented in the active site by interaction of the quaternary nitrogen of choline with a tryptophan residue (W84), and other aromatic residues form the acyl pocket, which determines substrate specificity and sensitivity to active site inhibitors (Harel et al. 1992; Radic et al. 1993; Vellom et al. 1993; Kraut et al. 2000). It is difficult to reconcile the extremely high turnover rate of AChE with the position of the active site at the base of such a restricted gorge, particularly when considering both access of substrate and clearance of reaction products, and this conceptual problem has led to the suggestion of an alternative ‘back door’ route for the latter (Gilson et al. 1994).
STRUCTURAL DIVERSITY OF ACETYLCHOLINESTERASES IN VERTEBRATES
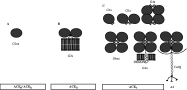
Vertebrate AChEs exist in multiple molecular forms distinguished by their subunit interactions and hydrodynamic properties (Massoulié, 2002), and these different quaternary associations are illustrated in Fig. 1. Catalytic subunits may be assembled into asymmetric (A) or globular (G) forms, the latter consisting of monomers (G1), dimers (G2) and tetramers (G4). These may be hydrophilic or amphiphilic, the latter associating with cell membranes via glycolipid anchors or a non-catalytic, proline-rich membrane anchor (PRiMA). Asymmetric forms are composed of one to three tetramers (A4, A8 and A12) linked to collagen Q (ColQ) subunits which associate with the basal lamina of neuromuscular junctions (Massoulié, 2002). For clarity, only the form with a single tetramer is shown in Fig. 1.

Fig. 1. Molecular diversity of AChEs. A: Soluble monomer. These hydrophilic, or non-amphiphilic (na) enzymes have a truncated C-terminus. They are represented by read-through or R forms in vertebrates due to lack of splicing downstream of the common catalytic exons, and soluble S forms found in Elapid snakes such as Bungarus and Naja. Soluble AChEs are also notably represented by a distinct family in parasitic nematodes. They have been identified in several nematode species, and their primary structures determined in N. brasiliensis and D. viviparus. B: GPI-anchored dimer. Represented by vertebrate hydrophobic (H) form, and analogous enzymes identified in C. elegans (ACE-2 and ACE-3), and D. viviparus (ACE-2). C: Tailed forms. These proteins adopt a variety of homo-oligomeric or heteromeric associations in vertebrates. The C-terminal T peptide, or WAT, forms an amphipathic alpha helix which is assumed to be exposed in amphiphilic (a) forms. This can associate with a Proline Rich Association Domain (PRAD) found on non-catalytic sub-units such as PRiMA or ColQ. The latter is known as an asymmetric form, and may involve ColQ associating with a single tetramer as shown (A4), but also two (A8) or three (A12) tetramers. Analogous protein subunits have been identified in nematodes, and are represented by C. elegans (ACE-1), Meloidogyne and D. viviparus (ACE-1), although the identity of the subunit which mediates membrane attachment is not known in any of these species.
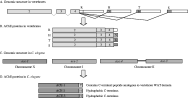
All these variants are generated from a catalytic subunit which is encoded by a single gene, transcripts of which are alternatively spliced at the 3′ end and translated to generate proteins with a common catalytic core domain. This is presented in Fig. 2: constitutive exons 2–4 encode the catalytic core. H (‘hydrophobic’) transcripts are generated by splicing to exon 5 in the figure, giving rise to AChEH forms, which dimerise and are membrane bound via glycosylphosphatidylinositol (GPI) anchors. In mammals these are found predominantly on haematopoietic cells. Alternative splicing to exon 6 generates T (‘tailed’) forms. AChET subunits are the basis of monomeric, dimeric and tetrameric globular enzymes, in addition to the heteromeric forms which associate with PRiMA and ColQ molecules (see Fig. 1). A third type of enzyme, AChER results from a lack of splicing downstream of exon 4, generating R (‘readthrough’) transcripts. AChER enzymes are soluble monomers which are found in embryonic tissues and are variably expressed in haematopoietic cells, in addition to being induced by stress (Kaufer et al. 1998). Finally, S (‘soluble’) transcripts have been found in some species of Elapid snakes (Cousin et al. 1998). These enzymes are also soluble and monomeric, and are highly expressed in venom glands, although they do not appear to be major determinants of the toxicity of venom in these species (Cousin et al. 1996b). In some ways, the latter two types of enzymes are similar to the AChEs secreted by parasitic nematodes (discussed later), and their function is equally obscure.

Fig. 2. Organisation of ace genes in vertebrates and C. elegans. The molecular diversity of AChEs in vertebrates is generated via alternative splicing of AChE transcripts at the 3′ end (A), generating proteins which contain a common catalytic core but distinct C terminal peptides (B). See text for details. Constitutive exons encoding the catalytic core of the enzyme are numbered 2–4 according to convention (exon 1 is variable). Adapted from Massoulié (2002). Four distinct genes encoding AChEs have been identified in C. elegans (C). Each gene is composed of multiple exons, but with no evidence of alternative cis-splicing of transcripts. Ace-3 and ace-4 are found in tandem on chromosome II and are transcribed as a bicistronic messenger unit. Mature transcripts for ace-4 are found at very low levels and the corresponding enzyme has not been detected in vivo. ACE-1 contains a C-terminal region analogous to mammalian T peptides, whereas ACE-2 and ACE-3 contain a hydrophobic C-terminus which is post-translationally modified with the addition of a GPI anchor (D).
ACETYLCHOLINESTERASES INARE ENCODED BY MULTIPLE GENES
In contrast to vertebrates, different forms of AChE may be encoded by separate genes in invertebrate species (Baxter & Barker, 2002; Li & Han, 2004). This is certainly the case for nematodes, in which ACh is the major excitatory neurotransmitter controlling motor activities (Segerberg & Stretton, 1993; Rand & Nonet, 1997a). Application of classical genetics led to the definition of three genes in Caenorhabditis elegans which encoded kinetically distinct classes of AChE. The two major classes, A and B, are encoded by ace-1 on chromosome X and ace-2 on chromosome I respectively (Culotti et al. 1981; Johnson et al. 1981). Class C AChE, encoded by ace-3 on chromosome II, accounts for less than 5% of the total enzyme activity in the worm, and is characterized by a low Km for ACh and an unusual insensitivity to eserine (Kolson & Russell, 1985; Johnson et al. 1988). Homozygous mutants in ace-1 or ace-2 have only slight alterations in locomotion, and in ace-3 have no visible phenotype, whereas ace-1−/ace-2− mutants are severely uncoordinated, and the triple mutation is lethal (Culotti et al. 1981; Johnson et al. 1981; Johnson et al. 1988). The existence of a fourth type of enzyme, class D, representing less than 0·5% of total AChE activity, had previously been suggested (Stern, 1986), but in the absence of any mutants was largely ignored until closer examination of the ace-3 locus revealed two closely linked genes (Grauso et al. 1998).
The first gene to be cloned in C. elegans was ace-1, which was isolated by PCR using primers to regions conserved in vertebrate and Drosophila AChEs (Arpagaus et al. 1994). Analysis by sucrose density centrifugation indicated that ACE-1 is assembled into an amphiphilic tetramer, formed by association with a hydrophobic non-catalytic subunit, analogous to vertebrate T AChEs, as illustrated in Figs 1 and 2 (Combes et al. 2000). Notably, the C-terminus of ACE-1 is homologous to that of vertebrate T subunits (Combes et al. 2000). In vertebrate AChEs, the T peptide is 40 amino acids in length, containing seven highly conserved aromatic residues and a cysteine at position -4 from the C-terminus. T peptides form elongated alpha helices, with aromatic side chains clustered into a hydrophobic patch (Bon et al. 2004). The cysteine residue allows formation of dimers by disulphide bonding, whereas the hydrophobic patch facilitates the formation of tetramers and association with the Proline Rich Attachment Domain (PRAD) on the non-catalytic subunits ColQ or PriMA (Simon, Krejci & Massoulié, 1998; Belbeoc'h et al. 2004). Of the aromatic residues in the T peptide, three tryptophans which are evenly spaced seven residues apart (W10, W17 and W24) are particularly crucial for assembly of tetramers, and thus the T peptide is also called the Tryptophan Amphiphilic Tetramerisation (WAT) domain (Belbeoc'h et al. 2004). Synthetic peptides corresponding to the WAT domain and PRAD spontaneously assemble into a complex with a 1[ratio ]4 molecular ratio, thus mimicking the natural assembly of T AChEs (Simon, Krejci & Massoulié, 1998; Bon et al. 2004; Dvir et al. 2004). The C-terminus of C. elegans ACE-1 contains alternating charged and hydrophobic residues which could form an amphiphilic alpha helix, notably with the three tryptophan residues crucial for tetramerisation present with intervening stretches of seven and eight residues (Fig. 3). It is therefore highly likely that assembly into an amphiphilic tetramer involves a similar type of association with a PRAD on a non-catalytic structural subunit which anchors the molecule to membranes, although the identity of such a subunit in C. elegans or any other nematode is not known as yet.

Fig. 3. C-terminal sequences in nematode AChEs. Alignment of C-terminal sequences in nematode secreted AChEs and those analogous to hydrophobic (H) and tailed (T) enzymes in vertebrates. Cysteine residues likely to be involved in intramolecular disulphide bonding are shown in bold, whereas those likely to be involved in intermolecular associations are indicated with an asterisk. The hydrophobic regions of H forms are underlined, as is the upstream predicted ω cleavage site to which the GPI anchor is added. All three ‘tailed’ sequences show features characteristic of the WAT domain of vertebrate T subunits (see text for details). The eight conserved aromatic residues in these sequences are highlighted in bold.
Subsequently, three other ace genes were isolated from C. elegans (Grauso et al. 1998). The predicted proteins encoded, ACE-2, ACE-3 and ACE-4, all possess hydrophobic C-termini, and are thus more similar to vertebrate H enzymes (Figs 2 and 3). They contain signals for peptide cleavage and GPI addition, and ACE-2 and ACE-3 have been shown to assemble into GPI-linked amphiphilic dimers (Combes et al. 2000). ACE-2 contains a cysteine residue just upstream of the predicted cleavage site, which by analogy with mammalian enzymes is likely to contribute to an intramolecular disulphide bond. This residue is absent in both ACE-3 and ACE-4, which are encoded by genes present in tandem on chromosome II, with just 356 nucleotides separating the stop codon of ace-4 and the putative initiation codon of ace-3. In terms of sequence similarity, ACE-1, ACE-2 and ACE-3 are relatively divergent, with approximately 35% identity in amino acid sequence. On the other hand, ACE-3 and ACE-4 are more similar (54% identity), indicative of a recent duplication event (Combes et al. 2000). A similar arrangement of ace genes is found in Caenorhabditis briggsae (Grauso et al. 1998; Combes et al. 2000), suggesting that this duplication event occurred prior to separation of these species, which is thought to have occurred about 40 million years ago (Kennedy et al. 1993).
Despite these data, it is still not clear whether ACE-4 is produced as a functional protein. It has been estimated that ACE-1 and ACE-2 comprise approximately 95% of the total enzyme activity in worm extracts, whereas ACE-3 accounts for the remaining 5% (Culotti et al. 1981; Johnson et al. 1981; Kolson & Russell, 1985; Johnson et al. 1988). More recently, Northern blot analysis revealed that levels of mRNA in mixed stage preparations of worms decreased from ace-2 and ace-3 to ace-1, and that ace-4 was almost undetectable (Combes et al. 2003). The relative abundance of ace-3 mRNA is surprising considering the low levels of enzyme detected, and suggests that ACE-3 may have a low catalytic efficiency (Combes et al. 2000). The ace-1 transcript is trans-spliced by SL1, but those of ace-2, -3 and -4 are trans-spliced by both SL1 and SL2 (Combes et al. 2000). Trans-splicing by SL2 in C. elegans generally indicates that a gene lies downstream of other gene(s) in a polycistronic operon (Blumenthal, 1995), however no gene was found in close proximity to ace-2, or within 8·5 kb upstream of ace-4 and 1·8 kb downstream of ace-3 (Combes et al. 2000). Subsequent analysis demonstrated the presence of a bicistronic mRNA for ace-3/ace-4 (Combes et al. 2003). Trans-splicing by SL2 of the upstream gene (ace-4) in the operon is quite unusual, and splicing of ace-3 by both SL1 and SL2 suggests the existence of internal promoters. It is unclear why the level of mature ace-4 mRNA is so low. The number of synonymous and non-synonymous nucleotide substitutions between C. elegans and C. briggsae is the same for all four coding sequences of the ace genes, indicating that there has been no accumulation of mutations in ace-4, as would generally be expected for a non-functional gene. In the same study, however, the authors found a higher percentage of defective cis-splicing in ace-4 than in ace-3, and suggested that maturation of the ace-4 portion of the pre-mRNA may be defective (Combes et al. 2003).
Unlike vertebrates, there is no alternative splicing in the C. elegans ace genes, or indeed in any invertebrate ace genes examined to date. The exon structure is more complicated however: ace-1 is composed of 10 exons, ace-2 has 9, ace-3 has 8 and ace-4 has 17. Only two cis-splicing sites are conserved between C. elegans and vertebrate ace genes, located at the 5′ and 3′ splice sites of exon 4 in Fig. 2. Otherwise, splice sites vary considerably between C. elegans ace genes, although three sites at the 3′ end are conserved. In general, introns are positioned such that they do not interrupt areas of secondary structure in the proteins, but are located in loops or unstructured regions, although ace-4 is an exception to this rule (Combes et al. 2000).
All four C. elegans ACE proteins encode residues known to be critical for enzyme activity, such as S200, H440 and E327 which make up the catalytic triad, and W84 in the substrate choline binding site (note that Torpedo numbering is quoted for ease of comparison and by convention). The six cysteine residues which define the position of the three intramolecular disulphide bonds in cholinesterases are also present in all four ACEs. Of the fourteen aromatic residues which line the wall of the active site gorge in the Torpedo enzyme (Sussman et al. 1991), twelve are either conserved or show conservative substitutions in ACE-1, -3 and -4, and eleven in ACE-2.
Vertebrate AChEs are distinguished from BuChEs by their preference for ACh as a substrate rather than larger choline esters. On the other hand, invertebrate AChEs tend to exhibit intermediate substrate specificities, and this is true for the C. elegans enzymes. ACE-1 and ACE-2 show considerable activity with propionylcholine (PCh) and butyrylcholine (BuCh), whereas ACE-3 hydrolyses all substrates with equal efficiency (Combes et al. 2000). The molecular basis for acceptance of larger substrates by the C. elegans enzymes is likely to lie in the architecture of the acyl-binding pocket. Mutagenesis studies on Torpedo and human AChE have shown that F288 and F290 dictate substrate specificity, most probably via steric occlusion. Replacement of F288 in Torpedo and human AChE by smaller, non-aromatic residues greatly enhanced the ability of these enzymes to hydrolyse BuCh, in addition to conferring sensitivity to inhibition by iso-OMPA, a bulky compound which typically inhibits BuChE but not AChE (Austin & Berry, 1953; Harel et al. 1992; Ordentlich et al. 1993; Vellom et al. 1993). F288 is substituted by non-aromatic residues in all four C. elegans sequences (glycine in ACE-1, methionine in ACE-2, and leucine in both ACE-3 and ACE-4), corresponding to similar substitutions in the Drosophila melanogaster AChE (Hall & Spierer, 1986) and mammalian BuChE (Lockridge et al. 1987).
One particular point of note in the C. elegans enzymes lies in the amino acids surrounding the active site serine. AChEs generally have the consensus sequence FGESAG. This is changed to VGESAG in ACE-3, and FGQSAG in ACE-4. Whether the former substitution affects enzyme activity is unclear, but the latter is likely to have adverse effects. Site directed mutagenesis of a number of vertebrate AChEs has demonstrated that the substitution E199Q almost completely ablated enzyme activity (reduced to 2% of control values; Radic et al. 1992). ACE-4 is therefore likely to be non-functional, or alternatively may have another, non-enzymatic role.
TISSUE EXPRESSION PATTERNS SUGGEST NON-REDUNDANT FUNCTIONS IN
The tissue expression pattern of specific ace genes has been investigated in C. elegans by microinjection of Green Fluorescent Protein (GFP) reporter constructs, and was first examined for ace-1:GFP, using 2·4 kb of 5′ flanking sequence containing promoter elements in five blocks of sequence (CS1-CS5) conserved between C. elegans and C. briggsae (Culetto et al. 1999; Combes et al. 2003). GFP expression was observed in all body wall muscle cells, in the pharyngeal muscle cells pm5 and in three pairs of sensory neurons in head ganglia, and is illustrated in Fig. 4 (panel A). CS4 acted as an enhancer for expression in body wall muscle, anal sphincter and vulval muscle cells, whereas another block of conserved sequence directed expression in pm5 and the cephalic neurons (Culetto et al. 1999). This pattern of expression differs from histochemical staining for ace-1, which has been reported to highlight the nerve ring, dorsal and ventral nerve cords, but not muscle cells (Culotti et al. 1981). This discrepancy could be due to the different sensitivities of the techniques used, or may indicate synthesis of ACE-1 in muscle cells followed by transport and concentration at synapses along nerve cords (Culetto et al. 1999). The pharyngeal muscle cells are essential for feeding. Pharyngeal pumping is regulated via cholinergic innervation through neuron M5 (Rand & Nonet, 1997b), and is defective in ace-1−/ace-2− double mutants (Culetto, 1998).

Fig. 4. Anatomical patterns of expression of ace:GFP in C. elegans adult worms. Ace-1, -2 and -3 show distinct, non-redundant anatomical patterns of expression. This was determined by GFP reporter constructs for each gene, as detailed in the text. The major sites of tissue expression are illustrated here. Panel A: ace-1:GFP. Strong fluorescence is observed in body wall (mc) and pharyngeal (pm5) muscle cells, in addition to head mesoderm (hm) and anal muscles (am). The muscle quadrants are twisted, characteristic of the rol-6 phenotype. Panel B: ace-2:GFP. Expression is almost exclusively detected in neurons. Fluorescence is observed in sensory endings (s) and cephalic neurons around the pharyngeal bulb (n), in addition to neurons in the anal ganglion (n) and hypodermal cells (h). Note co-expression with other ace:GFP constructs in the pharyngeal muscle cells (pm5). Arrows indicate autofluorescence of intestinal cells (i) and twisting of an axon in the ventral nerve cord in the middle of the worm due to the roller phenotype. Panel C: ace-3;ace-4:GFP. Prominent expression in pharyngeal muscle cells (pm) and the two medial canal-associated neurons (CAN). These data are reproduced from Culetto et al. (1999) and Combes et al. (2003) with permission from the authors and the publishers. Please refer to these publications for a more detailed analysis.
A similar approach was taken to examine tissue sites of expression of ace-2:GFP, following definition of a 5′ regulatory region by rescue of ace-1−/ace-2− mutants (Combes et al. 2003). This region contains just three blocks of sequence conserved between C. elegans and C. briggsae, two of which are homologous to CS1 and CS2 in the 5′ regulatory region of ace-1. These lie close to the initiator ATG, and are thought to represent basal transcription elements (Combes et al. 2003). In contrast to ace-1:GFP, ace-2:GFP is expressed almost exclusively in neurons. Fig. 4, (panel B) shows that it is particularly prominent in cephalic sensory neurons around the anterior and posterior of the pharyngeal bulb, in pm5 cells, and motor neurons in the anal ganglion and hypodermal cells. Examination of the cephalic region in finer detail revealed expression in the inner labial neurons, and possibly the amphidial neurons AWB and AWC. Histochemical staining of ace-1- worms (which defines sites of expression of ace-2) highlighted the nerve ring, the pharyngo-intestinal valve, the tail ganglion and the ventral nerve cord (Combes et al. 2003). Interestingly, ace-2:GFP fluorescence was detected in the anterior portion of embryos by the one-fold stage, and the complete pattern of expression seen in adult worms was present in first stage larvae.
Expression of ace-3 was examined by fusing GFP to a 4 kb 5′ region of the ace-3/ ace-4 operon, i.e. ace-3;ace-4:GFP, as expression could not be driven by the 356 bp intervening sequence (Combes et al. 2003). Fluorescence was observed in pharyngeal muscle cells, 12–15 neurons in the head and some in the anal ganglion, and notably in the two medial canal-associated neurons (Fig. 4, panel C). Conventional histochemical staining did not reveal AChE activity in ace-1−/ace-2− mutants (Culotti et al. 1981), although by using a higher substrate concentration and longer incubation time, Combes et al. (2003) reported weak activity in the pharynx and vulval region, a result which again suggests that the catalytic activity of ACE-3 may be low relative to that of ACE-1 and -2. The strong fluorescence in pharyngeal muscle was very specifically restricted to pm3, pm4, pm5 and pm7 cells. Considerable differences in ace-3;ace-4:GFP expression were observed during development, with intense fluorescence observed at the one-fold embryo stage, and expression in two rows of dorsal body wall muscle cells extending from the posterior bulb of the pharynx right through to the anus at the L1 stage, which progressively decreased to almost undetectable levels in adult worms (Combes et al. 2003). This is interesting, as most genes in C. elegans muscle are ubiquitously expressed, or follow an expression gradient along the anterior-posterior axis (Moerman & Fire, 1997).
In summary, the different ace genes of C. elegans show distinct anatomical patterns of expression, implying non-redundant functions. A few examples of coordinated expression were found: pm5 pharyngeal muscle cells express ace-1, ace-2 and ace-3/ace-4 GFP constructs, whereas an anal neuron thought to be PDA expressed ace-2:GFP and ace-3;ace-4:GFP (Combes et al. 2003). Despite the overall non-redundant pattern of expression of ace-1 and ace-2 constructs, phenotype rescue experiments showed that both genes could restore coordinated motility to the uncoordinated ace-1−/ace-2− double mutants (Culetto et al. 1999; Combes et al. 2003). Although ace-4 is transcribed along with ace-3, the extremely low levels of mature processed mRNA, the failure to detect enzymatic activity in vivo and the E199Q substitution adjacent to the active site serine residue suggest that ACE-4 is non functional, at least in terms of enzymatic activity.
NEUROMUSCULAR ENZYMES IN PARASITIC NEMATODES
Neuromuscular AChEs from parasitic nematodes are much less well defined than those of C. elegans. A dimeric amphiphilic (G2a) form has been described in Parascaris equorum (Talesa et al. 1997), two classes of AChE have been detected in somatic extracts of Trichinella spiralis which are most likely dimeric and tetrameric forms (deVos & Dick, 1992), and a tetrameric amphiphilic (G4a) enzyme has been reported from Nippostrongylus brasiliensis (Hussein, Grigg & Selkirk, 1999). As these enzymes were biochemically defined from limiting material, it is likely that they represent the major forms expressed in these organisms, and there is no reason to doubt that homologues of ACE-1, ACE-2 and ACE-3 will be present in most parasitic species. As 95% of AChE activity in C. elegans extracts can be accounted for by ACE-1 and ACE-2, it is understandable that ACE-3 homologues have been difficult to define in parasites. The C. elegans ace genes were determined initially via genetic analysis, and latterly with the aid of the genome project, so we expect that the full repertoire of ace genes will be defined in a number of parasitic nematodes in the near future.
Where examined, multiple forms of AChEs have been characterized in plant-parasitic nematodes: three have been defined in Heterodera glycines (Chang & Opperman, 1992), and five in Meloidogyne incognita and M. arenaria, although the latter were refined to three separate classes (A, B and C) based on substrate affinity, sensitivity to inhibitors and detergent, and thermal inactivation (Chang & Opperman, 1991). Analysis by sucrose density gradient centrifugation indicates that ACE-A is predominantly tetrameric, whereas ACE-B and ACE-C are dimeric, analogous to ACE-1, -2 and -3 in C. elegans. To further illustrate this comparison, Meloidogyne ACE-C has a high affinity for ACh, yet is extremely resistant to inhibition with carbamates and organophosphates (Chang & Opperman, 1991), much like ACE-3 of C. elegans (Kolson & Russell, 1985). The practical significance of this observation is that carbamates have long been used as nematicides for plant-parasitic species, and expression of a resistant AChE may confer a degree of tolerance to these agents. Chang & Opperman (1991) pointed out that adult Meloidogyne are sedentary endoparasites which, once a feeding site has been established, have a limited capacity or need for movement, and suggested that if control of the pharyngeal musculature relied on ACE-C, then the parasite could survive and feed in the presence of high concentrations of nematicide. As illustrated above, recent analysis indicates that C. elegans ace-3 is expressed primarily in pharyngeal muscle in adult worms (Combes et al. 2003), and if this pattern of tissue expression is conserved in plant-parasitic nematodes, then this may indeed confer a degree of resistance to nematicides.
Since this analysis of the Meloidogyne native enzymes, genes have been cloned for AChEs from M. incognita and M. javanica which are clear homologues of C. elegans ACE-1 (Piotte et al. 1999). Interestingly, ace-1 is expressed in eggs, pre-parasitic juveniles and males of Meloidogyne, but is undetectable by RT-PCR in adult female worms, which are sedentary in plant root tissue. It would be particularly informative to analyse the anatomical expression pattern of this gene in Meloidogyne, because if the analogy can again be drawn with C. elegans, one would expect a homologue of ace-1 to be predominantly expressed in body wall muscle cells. Pharyngeal pumping continues essentially unaffected in C. elegans ace-1 mutants (Johnson et al. 1981), and therefore expression of ace-1 may not be necessary for survival of adult female Meloidogyne.
We have very recently isolated partial cDNAs homologous to C. elegans ace-1 from the bovine lungworm Dictyocaulus viviparus (Lazari et al. unpublished data). The C-terminus of the predicted protein is shown in Fig. 3, in which it is aligned with the M. javanica ACE and C. elegans ACE-1. All three sequences show features characteristic of the WAT domain of vertebrate T subunits, notably eight conserved aromatic residues, six of which align with those in Torpedo ACET, including the three tryptophan residues crucial for tetramerisation (Belbeoc'h et al. 2004). All three nematode sequences contain a cysteine residue at position -3 or -4 from the C-terminus, again analogous to vertebrate T peptides. It is reasonable to assume that these proteins adopt a similar quaternary structure, as discussed above.
This is the second neuromuscular AChE gene to be isolated from D. viviparus. The first is clearly homologous to C. elegans ACE-2 (65% identity in amino acid sequence) and was cloned by PCR using a primer to the conserved region surrounding W84 in combination with the SL1 primer (Lazari et al. 2004). Although originally designated ACE-3 due to the order in which it was cloned, we have now re-named the D. viviparus enzymes in order to be consistent with those first described in C. elegans. Like the other parasitic neuromuscular enzymes, Dv-ACE-2 contains conserved residues associated with known properties of AChEs, such as the residues which constitute the catalytic triad, W84 in the choline binding site, and the six cysteine residues known to contribute to intra-molecular disulphide bonds in Torpedo AChE. Of the 14 aromatic residues which line the catalytic gorge of vertebrate AChEs, 11 are conserved or show conservative substitutions. Dv-ACE-2 has the same two potential N-linked glycosylation sites as C. elegans ACE-2, and also has a predicted consensus site for GPI addition at the C-terminus. This is illustrated in Fig. 3, along with similar sequences from the C. elegans enzymes with analogous hydrophobic C-termini. Just proximal to the predicted site for GPI addition, a cysteine residue aligns with that of C. elegans ACE-2 which is most likely responsible for intermolecular disulphide bonding (Combes et al. 2000). The Dv-ace-2 gene is composed of twelve exons compared to nine in C. elegans ace-2. In contrast to C. elegans ace-2, several splice sites are located in areas predicted to interrupt secondary structure in the enzyme, but the position of the site for the exon encoding the hydrophobic C-terminal sequence is conserved (Lazari et al. 2004).
SECRETED ACETYLCHOLINESTERASES
In addition to expression of neuromuscular AChEs, many parasitic nematodes secrete variants of these enzymes from specialised amphidial and secretory glands. This was initially discovered via cytochemical staining in N. brasiliensis, a parasite which colonises the jejunum of rats and is commonly used as a laboratory model of infection (Lee, 1970). Secretion of AChE was confirmed by analysis of products released in vitro by adult N. brasiliensis (Sanderson, 1972), and rapidly extended to a broad range of nematode species (Ogilvie et al. 1973). Although AChE secretion is largely restricted to enteric parasites, there are some exceptions. D. viviparus, which inhabits the trachea and main stem bronchi of bovine lungs, is known to secrete several AChE isoforms (McKeand et al. 1994), and Stephanurus dentatus, which forms cysts in porcine ureters, also secretes the enzyme (Rhoads, 1981). Hence, secretion of AChE appears to be associated with those nematode parasites which colonise mucosal surfaces rather than the alimentary tract per se. In addition, not all enteric parasites secrete AChE, and there appears to be no obvious discrimination between AChE secretors and non-secretors based upon colonisation of compartments such as the abomasum, small intestine and colon, or whether they reside in the lumen, are anchored to, or invade mucosal tissue.
All nematode secretory AChEs examined to date are hydrophilic (non-amphiphilic or na), although both monomeric (G1na) and dimeric (G2na) forms have been documented. Analysis of the AChE activity secreted by Necator americanus indicated that it existed as a single G2na form (Pritchard, Brown & Toutant, 1994), whereas both monomeric and dimeric hydrophilic secreted AChEs have been described for Trichostrongylus colubriformis (Griffiths & Pritchard, 1994). The three secreted AChE isoforms of N. brasiliensis are all monomeric, between 69 and 74 kDa in apparent mass, and analysis of substrate specificity and sensitivity to inhibitors indicates that they can all be classified as true AChEs rather than BuChEs (Grigg et al. 1997). A similar conclusion was drawn for the enzymes secreted by D. viviparus. Adult parasites of this species secrete five isoforms of AChE, although it is unknown how many gene products these correspond to, as post-translational modifications affect electrophoretic mobilities used to define the isoforms (McKeand et al. 1994).
In the last few years we have cloned genes for all three secreted AChEs (sACEs) of N. brasiliensis, sACE-A, -B and -C (Hussein et al. 1999, 2000; Hussein, Harel & Selkirk, 2002) and two secreted enzymes from D. viviparus, Dv-sACE-1 and -2 (Lazari et al. 2003). These studies indicate that, as for nematode neuromuscular enzymes, expression of multiple secreted AChEs is accounted for by separate genes rather than differential splicing of a common gene. The five secreted AChEs characterized thus far show common features which clearly distinguish them from their neuromuscular counterparts. The C-terminus of all enzymes is truncated, and they therefore do not possess peptide sequences which define H or T subunits, consistent with their secretion as hydrophilic proteins. Moreover, they all lack a free cysteine residue at the C-terminus, explaining their monomeric nature. These features are illustrated in Fig. 3. In this respect the nematode secreted AChEs are similar to those found in venoms from Elapid snakes such as Bungarus and Naja (Cousin et al. 1996a,b; Frobert et al. 1997).
Another prominent feature which is characteristic of the secreted AChEs is an insertion at the molecular surface, although the precise position differs between nematode species. Thus, all three N. brasiliensis enzymes possess a unique stretch of 17 amino acids between residues 258 and 259 in the Torpedo sequence, which can be modelled as forming part of a 30 residue surface loop. The insertion in the two Dictyocaulus secreted enzymes is somewhat larger (32 amino acids), and is located between residues 55 and 61 in the Torpedo sequence, depending on alignment, but again predicted to lie on the molecular surface of the enzyme (Lazari et al. 2003). Whilst insertions of this nature are not necessarily meaningful in terms of protein function, there are reasons to assume that there may be some biological significance in this case. Firstly, they are present in secreted AChEs of both species, but missing in their neuromuscular enzymes. Secondly, although we have not found any identity to known sequence motifs, they both contain cysteine residues which probably stabilise their structure. All five secreted AChEs (three for N. brasiliensis and two for D. viviparus) have the six cysteine residues characteristic of AChEs which are responsible for formation of intramolecular disulphide bonds, but also possess a variable number of cysteine residues in the novel insertions. Thus, all three N. brasiliensis secreted AChEs have two conserved cysteine residues at positions 232 and 263. These enzymes appear to have no free sulphydryl groups by biochemical analysis, indicating that the two additional cysteines most probably contribute to the formation of a fourth disulphide bond (Hussein, Harel & Selkirk, 2002), and this has been confirmed by mass spectrometric analysis of peptide fragments from sACE B. Both D. viviparus secreted AChEs have four cysteine residues in the short peptide insertion (Lazari et al. 2003). Despite the lack of any obvious sequence motif, it is tempting to speculate that these insertions play some biological role specific to the secreted enzymes, such as cytophilic binding or interaction with an unknown molecular target.
The genes which encode the Dictyocaulus secreted enzymes consist of 16 exons. All the splice sites in the neuromuscular AChE gene Dv-ace-2 are conserved in the secreted AChE genes, with the exception of the most 3′ exon, which is missing, and the five additional splice sites in the latter, which are located near the 5′ end. Two of these sites flank the 32 amino insertion mentioned above, suggesting that it is encoded by a dedicated exon (Lazari et al. 2004).
As mentioned above, invertebrate AChEs generally exhibit the property of hydrolysing both ACh and larger choline esters, and this has been explained by the substitution of F288 in the acyl pocket by smaller non-aromatic residues such as glycine, methionine and leucine (Arpagaus et al. 1994). All three N. brasiliensis secreted AChEs have the substitution F288M, yet nevertheless still show minimal activity against BuCh. Visual inspection of the sequence revealed that in comparison to Torpedo AChE, the nematode enzymes had two substitutions of amino acids with bulkier side chains which might restrict access of substrate to the binding site, F290W and F331W. A double mutation of tryptophan to phenylalanine at these positions had little effect on substrate specificity, whereas the triple mutant M288G/W290F/W331F showed good activity with PCh and BuCh (Hussein et al. 2000). We subsequently built a model of sACE A which helped to interpret these data. The nematode AChE contains an insertion of two residues in its acyl binding pocket, and hence the trajectory of residues in this region is different from that of the Torpedo enzyme. Docking of BuCh indicated a clash of the butyryl moiety of the substrate with W331, and an alleviation of the steric tightness by mutation of M288 to glycine (Hussein, Harel & Selkirk, 2002). This was consistent with the mutagenesis data which indicated that the single mutants W331F or M288G by themselves do not enhance BuCh hydrolysis, whereas the triple mutant W331F/M288G/W290F does. The Dictyocaulus enzymes have preferential activity against ACh, and also possess a methionine residue at position 288 and a tryptophan at position 331 (Lazari et al. 2003). One anomaly with this analysis is that the mutant N. brasiliensis AChEs are still insensitive to the BuChE-specific inhibitor iso-OMPA, in contrast to analogous mutants of vertebrate AChEs and the invertebrate enzymes (Hussein et al. 2000).
The key features which distinguish nematode secreted AChEs from their neuromuscular counterparts are thus (1) their hydrophilic nature, (2) the presence of an insertion of unknown function, and (3) their substrate specificity for ACh rather than other choline esters. Other properties are consistent with AChEs from diverse sources. Whilst we have insufficient information to know whether this is the rule for all secreted enzymes, one remarkable feature of N. brasiliensis sACE-B is its stability. This enzyme is being developed for use in biosensors to detect the presence of organophosphates in food and environmental samples (Toutant et al. 2004). During development, very little loss in enzymatic activity has been recorded following prolonged storage at room temperature over 15 months (Schulze et al. personal communication). These properties, and the robust nature of such an enzyme may be an adaptation for functionality in the mammalian host following secretion by the parasite.
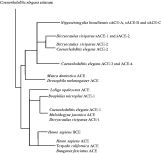
The relationship of nematode AChEs sequenced thus far is shown schematically in Fig. 5. All secreted enzymes are most closely related to C. elegans ACE-2, but both the Nippostrongylus and the Dictyocaulus enzymes form independent clusters, suggesting that they evolved independently after separation of the species (Hussein, Harel & Selkirk, 2002; Lazari et al. 2004). The neuromuscular enzymes Dv-ACE-2 and Dv-ACE-1 cluster separately with C. elegans ACE-2 and ACE-1 respectively, rather than with the secreted enzymes. No homologues of C. elegans ACE-3/ACE-4 have yet been described in parasitic nematodes.

Fig. 5. Schematic representation of the phylogeny of AChEs. Adapted from Lazari et al. (2004). See this publication for details. Bars are not drawn to scale.
PHYSIOLOGICAL FUNCTIONS FOR SECRETED ACETYLCHOLINESTERASES
The unusual phenomenon of AChE secretion by parasitic nematodes has naturally provoked several hypotheses as to their possible physiological function. As this issue is focused on parasite neuromusculature, we will not discuss this in any detail, but several reviews specifically address this subject (Rhoads, 1984; Lee, 1996; Selkirk et al. 2001). It is worthwhile noting, however, that the function of vertebrate AChEs expressed on haematopoietic cells is still unresolved. One interesting point to note is that the amount of AChE produced by adult N. brasiliensis appears to be positively regulated by immune pressure from the mammalian host (Jones & Ogilvie, 1972; Sanderson, Jenkins & Ogilvie, 1972; Sanderson, Jenkins & Phillipson, 1976). Whether this reflects a general stress response, or is indicative of specific functions for the enzyme in countering immune effector mechanisms is still unclear.
The original suggestion was that AChE might regulate peristalsis or intestinal spasm (Lee, 1970; Edwards, Burt & Ogilvie, 1971), and whilst the first proposition seems unlikely, a role for regulation of local spasm via inhibition of the muscularis mucosa may be feasible. Another possibility is that these enzymes act to limit fluid and mucus secretion by hydrolysing ACh released from the enteric nervous system, which is known to control these transport processes in enterocytes (Cooke, 2000). ACh also stimulates exocytosis in Paneth cells, epithelial granulocytes located at the base of the crypts of Lieberkühn (Satoh et al. 1992). These cells release a variety of antimicrobial products, such as pore-forming proteins termed defensins (Ouellette & Selsted, 1996), although the effect of this response on macroparasites is unknown.
An attractive role for secreted AChEs which is unrelated to host physiology is that they may protect parasites against enzyme inhibitors ingested in the host diet, as detailed in the following section. Secreted enzymes could form a protective barrier by binding to inhibitors in the environment immediately external to the parasite, thus protecting neuromuscular AChEs, which if affected could lead to paralysis and subsequent expulsion. Analysis of resistance to organophosphates in Drosophila indicated that one of the factors involved in protection was an overproduction of AChE outside the central nervous system, and flies which produced a soluble AChE secreted into the haemolymph were resistant to insecticides (Fournier et al. 1993). The analogy here with nematode secreted enzymes is quite compelling.
A possible route to examine the role of secreted AChEs is through functional silencing by RNA interference (RNAi). We recently managed to suppress synthesis of these enzymes in adult Nippostrongylus by direct soaking of worms in double stranded RNA (dsRNA). The use of a small dsRNA of 240 bp, corresponding to the 5′ region of the coding sequence of sACE-B, resulted in over 90% suppression of AChE synthesis which persisted throughout a culture period of six days. Secretion of all three AChE isoforms was suppressed, yet parasite motility was unaffected, suggesting that the secreted enzymes do not perform additional functions in the parasite neuromuscular system. Expression of neuromuscular enzymes appeared unaffected, although the method used for analysis (visualisation of enzyme activities in non-denaturing PAGE) was not amenable to quantitation of inhibition (Hussein, Kichenin & Selkirk, 2002). A single experiment suggested that adult worms in which secreted AChEs were silenced were compromised in their ability to maintain their position in the small intestine of rats (unpublished results), but recoveries were low and we are working to modify the procedures in order to generate more rigorous data.
CHOLINESTERASE INHIBITORS
An extensive literature has been compiled on inhibition of AChEs, due in part to the development of neurotoxic agents (nerve gases) which target the mammalian enzyme, but also in relation to compounds utilised as broad-spectrum pesticides. More recently, AChE inhibitors have been used to treat Alzheimer's disease, as a decrease in the levels of ACh at cholinergic nerve terminals in the brain has been suggested to be associated with some of the disease symptoms (Giacobini, 2003). Treatment of patients with reversible AChE inhibitors such as rivastigmine, galanthamine and donepezil have been reported to improve cognitive function, although this is generally modest, and may not have enduring effects (Doody, 2003).
Compounds which mediate irreversible inhibition (e.g. organophosphates) form covalent adducts with the active site serine, and the acute toxicity of these compounds is due to the stability of the phosphoryl-enzyme complex. Reversible inhibitors may act in a competitive manner, effecting blockade of substrate at the active site (e.g. edrophonium), or non-competitive, binding to the ‘peripheral’ site, thought to reside near the entrance to the active site gorge (e.g. propidium). Peripheral site ligands can be highly selective (Eichler et al. 1994). Other compounds such as the bis quaternary ligands decamethonium and BW284C51 bind across both active and peripheral sites.
A number of natural inhibitors of AChE have been isolated, notably from venomous snakes. Fasciculins from mamba venoms are the only known peptide inhibitors of AChE. They act in a highly selective manner on vertebrate and eel AChEs, but are weak inhibitors of avian and invertebrate AChEs or BuChEs. The crystal structure of the enzyme-inhibitor complex showed that fasciculin interacts with residues at the entrance to the active site gorge, blocking access of substrate with potential additive allosteric effects (Bourne et al. 1995). The calabar bean (seeds of an African climbing plant) is a natural source of the alkaloid physostigmine, which carbamylates the active site serine residue of ChEs, and has a long history of use in medicinal extracts (Taylor & Radic, 1994). Galanthamine, one of the drugs approved for treatment of Alzheimer's disease, is a tertiary alkaloid AChE inhibitor which is derived from bulbs of the snowdrop and related plants, and has also has also been used in anaesthetics to reverse neuromuscular paralysis induced by turbocurarine-like muscle relaxants (Sramek, Frackiewicz & Cutler, 2000).
Numerous plant species in the Solanaceae contain glycoalkaloid toxins important in defence against pathogens such as viruses, bacteria, fungi and insects (Culliney, Pimentel & Pimentel, 1992). Potatoes and aubergines are a major source, with the major glycoalkaloids α-solanine and α-chaconine (Schreiber, 1968). These are acetylcholinesterase inhibitors which can lead to poisoning in animals and humans. They are present at high concentrations in the leaves and stems of potatoes, and although normally at very low levels in tubers, produce elevated amounts on exposure to light and in tuber sprouts (van Gelder, Vinke & Scheffer, 1988). A number of plant products have been used in traditional medicines for their anthelmintic properties, such as pumpkin seeds (Sharma, Bahga & Srivastava, 1971), the active ingredient of which has been identified as the tetracyclic triterpene cucurbitacin (Forgacs, Provost & Tiberghion, 1970), and unripe fruits of the Chinese medicinal plant Evodia rutaecarpa, from which the anthelmintic alkaloid 3-dimethylallyl-4-methoxy-2-quinolone, or atanine, was isolated (Perrett & Whitfield, 1995). It is thus possible that eating plant products with anti-cholinesterase activities has evolved in animals as a means of expulsion of intestinal helminths, providing selective pressure for AChE secretion by parasites.
Although AChE inhibitors have been used to control animal-parasitic and plant-parasitic nematodes, these have been restricted to organophosphates (OPs) and carbamates, which act by phosphorylating or carbamylating the active site serine residue of the enzyme. Inhibition of AChE results in accumulation of ACh at neuromuscular junctions leading to paralysis via sustained contraction (Martin, 1997). OPs currently listed for use in domestic animals include dichlorvos, metrifonate, haloxon, naphfhalofos, coumaphos, fenthion, cythioate, phosmet, diazinon and propetamphos, although most of these are used to control ectoparasites such as mites, lice, ticks and fleas (Bishop, 2004). Dichlorvos was one of the first broad-spectrum anthelmintics to be used in pigs, whereas metrifonate shows good activity against ascarids and oxyurids in horses. Haloxon has generally been the safest OP anthelmintic for use in ruminants, particularly for parasites of the abomasum and small intestine, and has been used with some success against adult Haemonchus, Trichostrongylus, Cooperia and Strongyloides (Aiello, 1998).
There appear to be very few reports of resistance to OPs in nematode parasites of livestock, probably due to their limited use in the field (Sangster, 1999). Resistance to naphfhalofos has been reported in H. contortus, but the same isolate was also resistant to levamisole and benzimidazoles (Green et al. 1981). It is therefore possible that levamisole (which acts on nicotinic ACh receptors) induced alterations in these receptors which resulted in reduced responsiveness to ACh. Both these parasites and levamisole-resistant C. elegans were also shown to be resistant to eserine (physostigmine) (Lewis et al. 1980; Sangster, Davis & Collins, 1991). Experimentally, a wide variety of genes have been described in C. elegans which, when mutated, confer resistance to OP (trichlorfon) and carmabate (aldicarb) inhibitors (Nguyen et al. 1995). The different mutants displayed a wide range of defects, from mild coordination to almost complete paralysis. None of the mutants appeared to result from direct alterations in AChE however, but rather showed altered metabolic compensation for AChE inhibition (Nguyen et al. 1995). Similarly, no direct alterations in AChE have been linked to OP resistance in parasitic nematodes, in contrast to insects. For example, a number of specific mutations in Drosophila AChE result in a different pattern of resistance to OPs, and specific combinations of mutations lead to highly resistant enzymes (Fournier et al. 1993). In the mosquitoes Culex pipiens and Anopheles gambiae, resistant strains have been described which all show mutation of G119 in the active site gorge of ACE-1 to a serine residue (Weill et al. 2003). Two mutations have been consistently observed in all OP-resistant aphids, namely A302S in ACE-1 and F139L in ACE-2 (Li & Han, 2004), whereas S431F in ACE-1 also confers selective resistance to pyrimicarb (Andrews et al. 2004). Interestingly, residue 431 corresponds to F331 in Torpedo AChE, discussed above in relation to substrate specificity in the Nippostrongylus AChEs.
As a general rule, OPs are non-selective towards nematode AChEs with a low therapeutic index, and variable levels of toxicosis may result in treated animals. Over the last 30 years, the introduction of safer drugs such as the benzimidazoles, imidathiazoles and macrocyclic lactones has led to the progressive withdrawal of OPs from the market for treatment of endoparasites. They are still used as general purpose insecticides and for the control of ectoparasites, although the UK sales for application to crops are approximately 10 times those for use in livestock. Recent years have seen intensified concerns over long term exposure of people to low levels of OPs, notably in agricultural workers engaged in sheep dipping, where exposure through skin contact can result in acute symptoms commonly referred to as dipper's flu. Numerous studies have highlighted possible links between low-level exposure to OPs and chronic neuropsychiatric effects such as depression, mood swings and anxiety (Jamal, 1997). This may not simply be due to inhibition of AChE but linked to interaction with other esterases and a variety of other target proteins. Neuropathy Target Esterase (NTE) is involved in neural development and is the target for neurodegeneration induced by specific OPs (Glynn et al. 1999).
The toxicity of OPs is thus due to their non-selective nature and the fact that they bind semi-irreversibly to AChEs and to a variety of other molecular targets. Despite these concerns, it may still be possible to design selective inhibitors of nematode AChEs, although the anti-cholinesterases used to date have had a limited spectrum of activity against parasitic helminths (McKellar & Jackson, 2004), and high profit margins are a major driver in product development in the animal health industry (Geary, Conder & Bishop, 2004). Structures of Torpedo AChE complexed with inhibitors in use or under development for the treatment of Alzheimer's disease have recently been determined. The authors noted that these compounds varied greatly in their structures and bound non-covalently to different sites of the enzyme, offering many different starting points for future drug design, suggesting that this may indeed be a fruitful area for future research (Greenblatt et al. 2003). Investment in pharmaceuticals for human health far outweighs that for animal health, and it may be possible to take advantage of this interest in human AChE as a target. We are therefore attempting to gain structural information on the nematode enzymes with this objective in mind.
ACKNOWLEDGEMENTS
This work was supported by the Biotechnology and Biological Sciences Research Council, the Wellcome Trust, and the European Commission under Framework 5. We thank Martine Arpagaus and Jean-Pierre Toutant for helpful advice. Fig. 4, panel A is reprinted from The Journal of Molecular Biology, Vol. 290, Culetto, E. et al. Structure and promoter activity of the 5′ flanking region of ace-1, the gene encoding acetylcholinesterase of class A in Caenorhabditis elegans, pp. 951–966, copyright (1999) with permission from Elsevier. Fig. 4, panels B and C are reprinted from The European Journal of Neuroscience, Vol. 18, Combes, D. et al. Multiple ace genes encoding acetylcholinesterases of Caenorhabditis elegans have distinct tissue expression, pp. 497–512, copyright (2003) with permission from Blackwell Publishing Ltd.